Preliminary Study of the Occurrence of Microplastics in the Sediments of the Rzeszów Reservoir Using the Laser Direct Infrared (LDIR) Method
Abstract
:1. Introduction
2. Methods
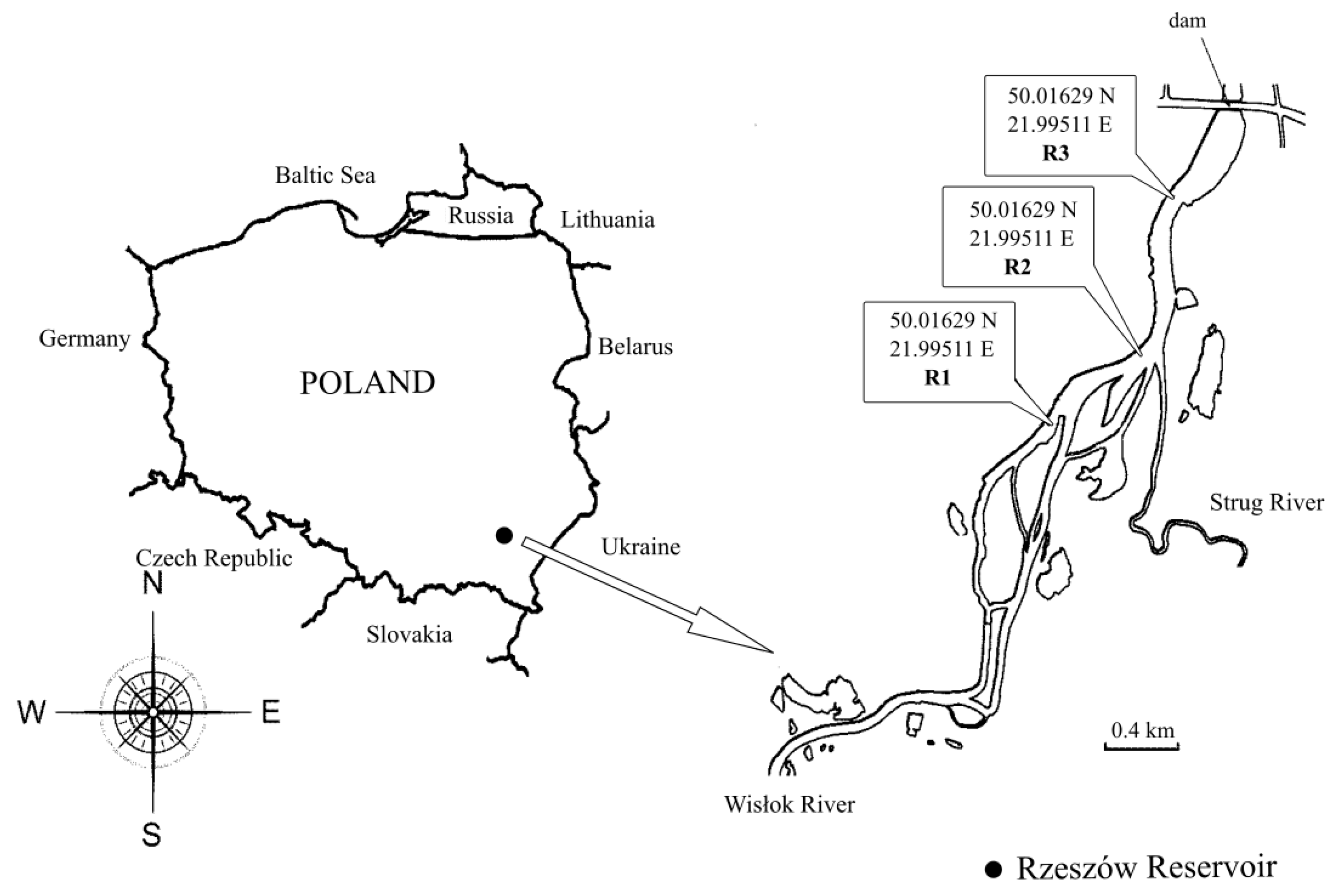
2.1. Study Area
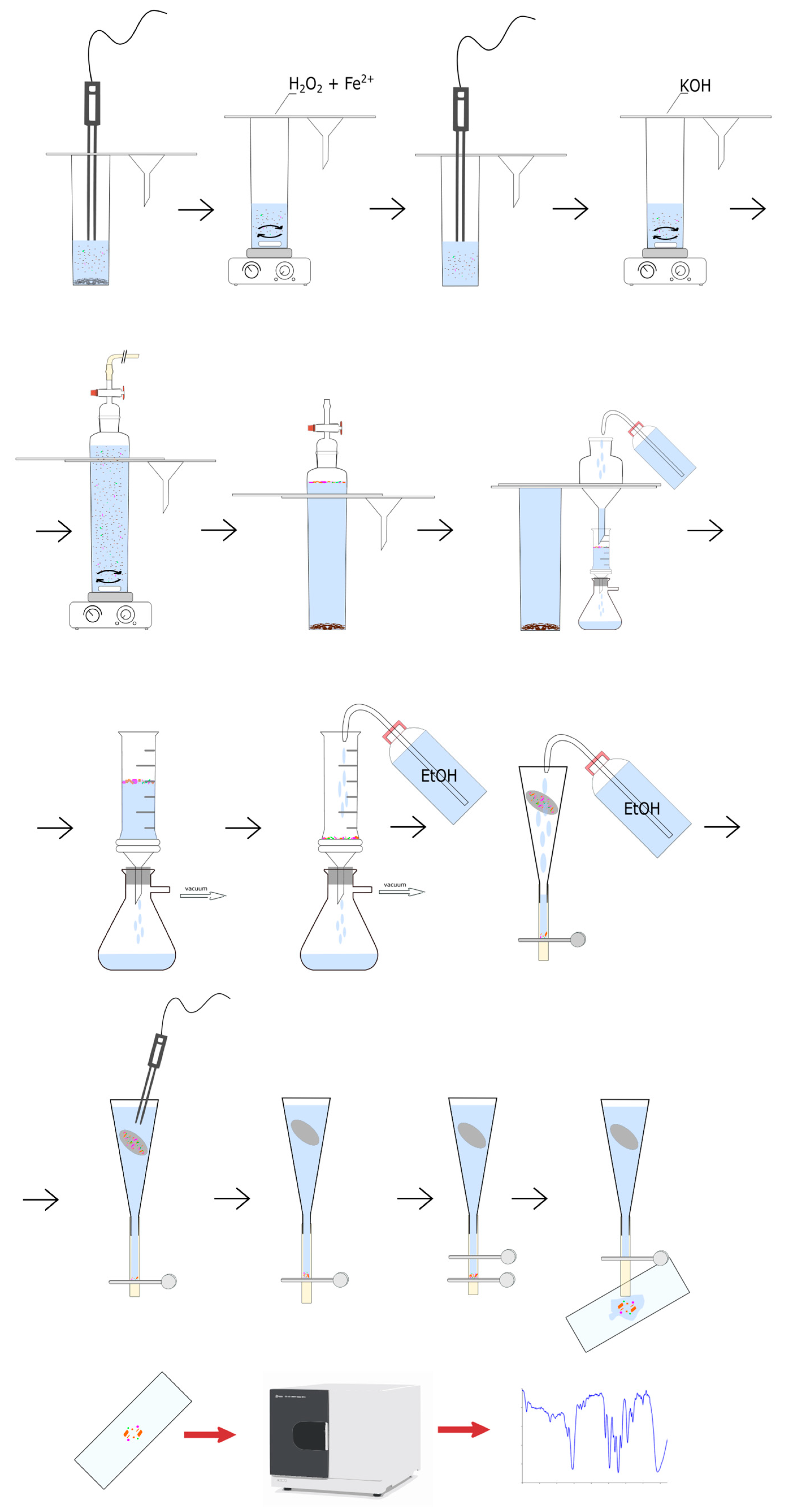
2.2. Methodology of the Studies
2.3. Assessment of Ecotoxicological Risk
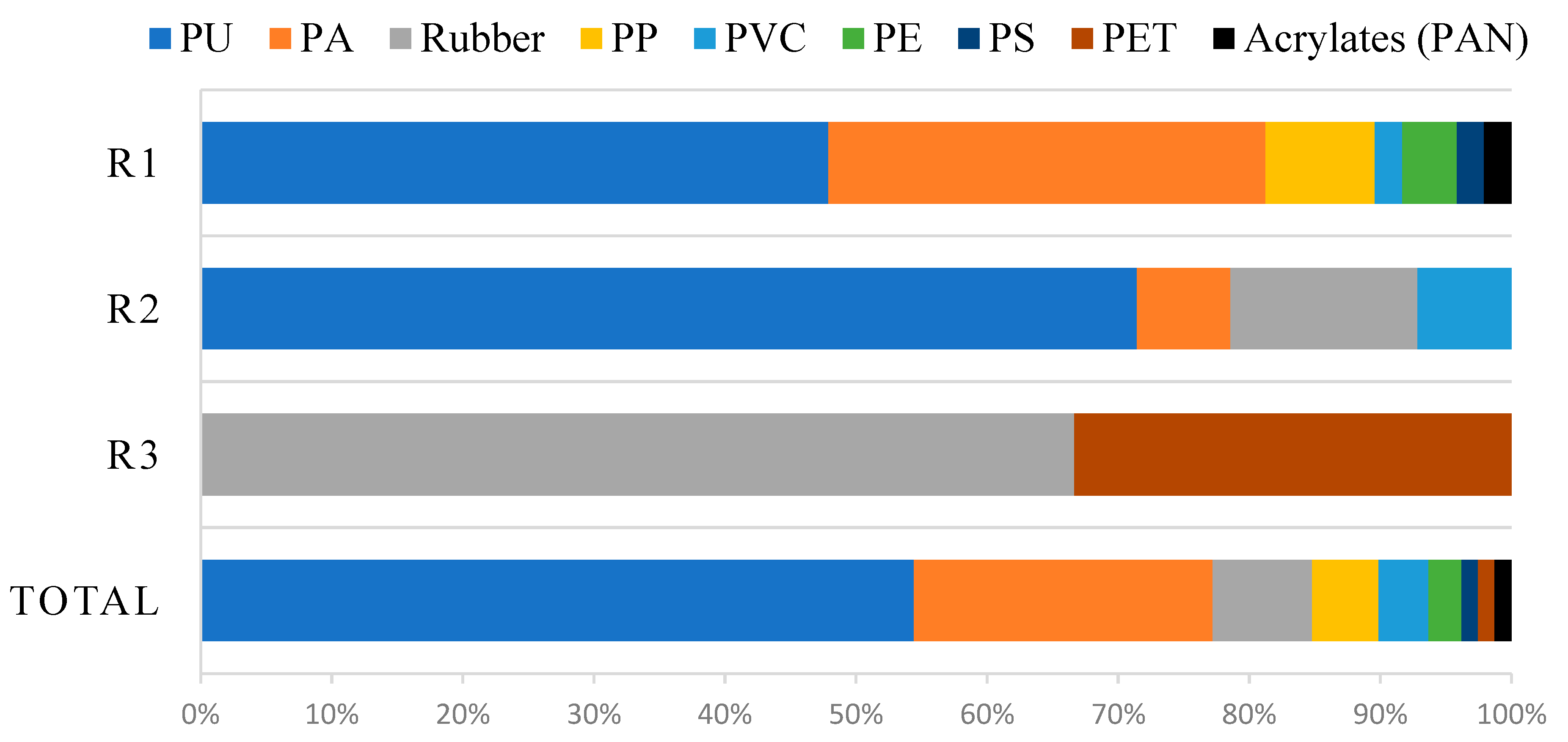
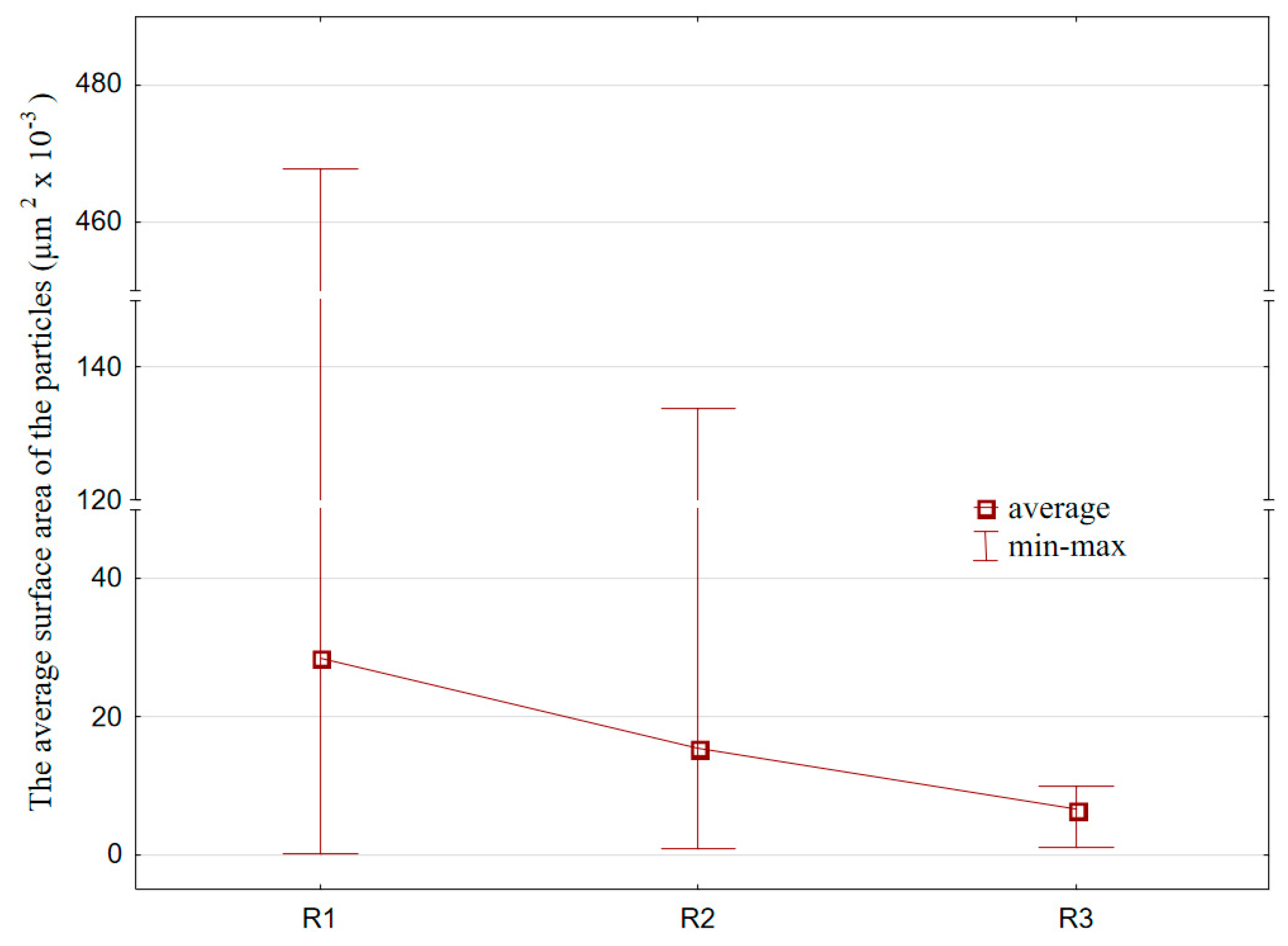
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, T.R.; Fequet, L. Current trends of unsustainable plastic production and micro (nano) plastic pollution. TrAC—Trends Anal. Chem. 2023, 160, 116984. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Madden, B.; Jazbeck, M.; Florin, N. Increasing packaging grade recovery rates of plastic milk bottles in Australia: A material flow analysis approach. Sustain. Prod. Consum. 2023, 37, 65–77. [Google Scholar] [CrossRef]
- Soni, S.; Pradhan, S.K. Improving crash worthiness and dynamic performance of frontal plastic automotive body components. Mater. Today Proc. 2020, 27, 2308–2313. [Google Scholar] [CrossRef]
- Gayer, R.; Jambeck, J.R.; Lavender Law, K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Kida, M.; Ziembowicz, S.; Pochwat, K.; Koszelnik, P. Experimental and computational hazard prediction associated with reuse of recycled car tire material. J. Hazard. Mater. 2022, 438, 129489. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ahn, Y.; Cho, H.; Kim, J. Optimal strategy to sort plastic waste considering economic feasibility to increase recycling efficiency. Process Saf. Environ. Prot. 2021, 165, 420–430. [Google Scholar] [CrossRef]
- Jiao, M.; Cao, S.; Ren, L.; Li, R. Analysis of composite microplastics in sediment using 3D Raman spectroscopy and imaging method. J. Hazard. Mater. Adv. 2021, 3, 100016. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Khoo, K.S.; Rawindran, H.; Lim, J.W.; Ng, H.S.; Shahid, M.K.; Tong, W.Y.; Hatshan, M.R.; Sun, Y.M.; Lan, J.C.W.; et al. Environmental Sustainability of Solvent Extraction Method in Recycling Marine Plastic Waste. Sustainability 2023, 15, 15742. [Google Scholar] [CrossRef]
- Reimonn, G.; Lu, T.; Gandhi, N.; Chen, W.T. Review of Microplastic Pollution in the Environment and Emerging Recycling Solutions. J. Renew. Mater. 2019, 7, 1251–1268. [Google Scholar] [CrossRef]
- Erekes-Merdano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Russell, M.; Webster, L. Microplastics in sea surface waters around Scotland. Mar. Pollut. Bull. 2021, 166, 112210. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Smith, M.; Egodawatta, P.; Ayoko, G.A.; Rintoul, L.; Goonetilleke, A. Dispersal and transport of microplastics in river sediments. Environ. Pollut. 2021, 279, 116884. [Google Scholar] [CrossRef] [PubMed]
- Malla-Pradhan, R.; Pradhan, B.L.; Phoungthong, K.; Joshi, T.P. Occurrence and Distribution of Microplastics from Nepal’s Second Largest Lake. Water Air Soil Pollut. 2022, 233, 423. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, S.; Zhang, C.; Zhou, Y.; Qin, W. Soil microplastic characteristics and the effects on soil properties and biota: A systematic review and meta-analysis. Environ. Pollut. 2022, 313, 120183. [Google Scholar] [CrossRef] [PubMed]
- Croxatto Vega, G.; Gross, A.; Birkved, M. The impacts of plastic products on air pollution—A simulation study for advanced life cycle inventories of plastics covering secondary microplastic production. Sustain. Prod. Consum. 2021, 28, 848–865. [Google Scholar] [CrossRef]
- Guan, Q.; Jiang, J.; Huang, Y.; Wang, Q.; Liu, Z.; Ma, X.; Yang, X.; Li, Y.; Wang, S.; Cui, W.; et al. The landscape of micron-scale particles including microplastics in human enclosed body fluids. J. Hazard. Mater. 2023, 442, 130138. [Google Scholar] [CrossRef]
- Baj, J.; Dring, J.C.; Czeczelewski, M.; Kozyra, P.; Forma, A.; Flieger, J.; Kowalska, B.; Buszewicz, G.; Teresiński, G. Derivatives of Plastics as Potential Carcinogenic Factors: The Current State of Knowledge. Cancers 2022, 14, 4637. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelley, F.J. Plastic and Human Health: A Micro Issue. Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef] [PubMed]
- Chatziparaskeva, G.; Papamichael, I.; Zorpas, A.A. Microplastics in the coastal environment of Mediterranean and the impact on sustainability level. Sustain. Chem. Pharm. 2022, 29, 100768. [Google Scholar] [CrossRef]
- La Cecilia, D.; Philipp, M.; Kaegi, R.; Schrimer, M.; Moeck, C. Microplastics attenuation from surface water to drinking water: Impact of treatment and managed aquifer recharge—And identification uncertainties. Sci. Total Environ. 2023, 908, 168378. [Google Scholar] [CrossRef] [PubMed]
- Maw, M.M.; Boonatanon, N.; Fujii, S.; Boontanon, S.K. Rapid and efficient removal of organic matter from sewage sludge for extraction of microplastics. Sci. Total Environ. 2022, 853, 158642. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Sequeira, I.F.; Monteiro, S.S.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Preparation of biological samples for microplastic identification by Nile Red. Sci. Total Environ. 2021, 783, 147065. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiang, J.; Islam, M.A. Elastomers and plastics for resisting erosion attack of abrasive/erosive slurries. Wear 2019, 426–427, 612–619. [Google Scholar] [CrossRef]
- Tsering, T.; Sillanpää, M.; Viitala, M.; Reinikainen, S. Variation of microplastics in the shore sediment of high-altitude lakes of the Indian Himalaya using different pretreatment methods. Sci. Total Environ. 2022, 849, 157870. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Y.; Kleinmeyer, Z.; Habil, G.; Yang, Q.; Zhao, L.; Rosso, D. Microplastics separation using stainless steel mini-hydrocyclones fabricated with additive manufacturing. Sci. Total Environ. 2022, 840, 156697. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ. Pollut. 2022, 297, 118773. [Google Scholar] [CrossRef]
- Dellisanti, W.; Ming-Lok Leung, M.; Wing-Kei Lam, K.; Wang, Y.; Hu, M.; Lo, H.S.; Fang, J.K.H. A short review on the recent method development for extraction and identification of microplastics in mussels and fish, two major groups of seafood. Mar. Pollut. Bull. 2023, 186, 114221. [Google Scholar] [CrossRef]
- Bartoszek, L.; Koszelnik, P.; Gruca-Rokosz, R.; Kida, M. Assessment of Agricultural Use of the Bottom Sediments from Eutrophic Rzeszów Reservoir. Annu. Set Environ. Prot. 2015, 17, 396–409. [Google Scholar]
- Ranjani, M.; Veerasingam, S.; Venkatachalapathy, R.; Mugilarasan, M.; Bagaev, A.; Mukhanov, V.; Vethamony, P. Assessment of potential ecological risk of microplastics in the coastal sediments of India: A meta-analysis. Mar. Pollut. Bull. 2021, 163, 111969. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Bonacci, O.; Tadic, Z.; Trninic, D. Effects of dams and reservoirs on the hydrological characteristics of the lower drava river. Regul. Rivers Res. Manag. 1992, 7, 349–357. [Google Scholar] [CrossRef]
- Cieśla, M.; Gruca-Rokosz, R.; Bartoszek, L. Significance of organic matter in the process of aggregation of suspended sediments in retention reservoirs. Sci. Total Environ. 2022, 815, 152850. [Google Scholar] [CrossRef] [PubMed]
- Järlskog, I.; Jaramillo-Vogel, D.; Rausch, J.; Gustafsson, M.; Strömvall, A.M.; Andersson-Sköld, Y. Concentrations of tire wear microplastics and other traffic-derived non-exhaust particles in the road environment. Environ. Int. 2022, 170, 107618. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s something in the air: A review of sources, prevalence and behaviour of microplastics in the atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Dry and wet deposition of microplastics in a semi-arid region (Shiraz, Iran). Sci. Total Environ. 2021, 786, 147358. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Z.; Zhu, Z.-R.; Fu, W.; Dai, X.; Ni, B.-J. The atmospheric microplastics deposition contributes to microplastic pollution in urban waters. Water Res. 2022, 225, 119116. [Google Scholar] [CrossRef]
- Ouillon, S. Why and How Do We Study Sediment Transport? Focus on Coastal Zones and Ongoing Methods. Water 2018, 10, 390. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Maggi, F. A mesocosm experiment of suspended particulate matter dynamics in nutrient- and biomass-affected waters. Water Res. 2016, 89, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Marques Mendes, A.; Golden, N.; Bermejo, R.; Morrison, L. Distribution and abundance of microplastics in coastal sediments depends on grain size and distance from sources. Mar. Pollut. Bull. 2021, 172, 112802. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Haave, M.; Lorenz, C.; Primpke, S.; Gerdts, G. Different stories told by small and large microplastics in sediment—First report of microplastic concentrations in an urban recipient in Norway. Mar. Pollut. Bull. 2019, 141, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; Rojo, D.; Olmos, S. Weathering indices of microplastics along marine and coastal sediments from the harbor of Cartagena (Spain) and its adjoining urban beach. Mar. Pollut. Bull. 2022, 178, 113647. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Desender, M.; Wilkinson, T.; Van Hoytema, N.; Amos, R.; Airahui, S.; Graham, J.; Maes, T. Occurrence and abundance of meso and microplastics in sediment, surface waters, and marine biota from the South Pacific region. Mar. Pollut. Bull. 2020, 160, 111572. [Google Scholar] [CrossRef] [PubMed]
- Ghayebzadeh, M.; Aslani, H.; Taghipour, H.; Mousavi, S. Contamination of the Caspian Sea Southern coast sediments with microplastics: A marine environmental problem. Mar. Pollut. Bull. 2020, 160, 111620. [Google Scholar] [CrossRef]
- Zobkov, M.; Belkina, N.; Kovalevski, V.; Zobkova, M.; Efremova, T.; Galakhina, N. Microplastic abundance and accumulation behavior in Lake Onego sediments: A journey from the river mouth to pelagic waters of the large boreal lake. J. Environ. Chem. Eng. 2020, 8, 104367. [Google Scholar] [CrossRef]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef]
- Fischer, E.K.; Paglialonga, L.; Czech, E.; Tamminga, M. Microplastic pollution in lakes and lake shoreline sediments—A case study on Lake Bolsena and Lake Chiusi (central Italy). Environ. Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Oni, A.B.; Ayeni, O.A.; Agboola, O.; Oguntade, T.; Obanla, O. Comparing microplastics contaminants in (dry and raining) seasons for Ox- Bow Lake in Yenagoa, Nigeria. Ecotoxicol. Environ. Saf. 2020, 198, 110656. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.O.; Abrantes, N.; Goncalves, F.J.M.; Nogueira, H.; Marques, J.C.; Goncalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Saad, D.; Ndlovu, M.; Ramaremisa, G.; Tutu, H. Microplastics in freshwater environment: The first evaluation in sediment of the Vaal River, South Africa. Heliyon 2022, 8, e11118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, P.; Zhang, G.; Wang, S.; Cai, Y.; Wang, H. Vertical microplastic distribution in sediments of Fuhe River estuary to Baiyangdian Wetland in Northern China. Chemosphere 2021, 280, 130800. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Shang, Y.; Zheng, Y.; Jia, Y.; Wang, F. Temporal and spatial variation of microplastics in Baotou section of Yellow River, China. J. Environ. Manag. 2023, 338, 117803. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef]
- Reineccius, J.; Bresien, J.; Waniek, J.J. Separation of microplastics from mass-limited samples by an effective adsorption technique. Sci. Total Environ. 2021, 788, 147881. [Google Scholar] [CrossRef]
- Mári, Á.; Bordós, G.; Gergely, S.; Büki, M.; Háhn, J.; Palotai, Z.; Besenyő, G.; Szabó, É.; Salgó, A.; Kriszt, B.; et al. Validation of microplastic sample preparation method for freshwater samples. Water Res. 2021, 202, 117409. [Google Scholar] [CrossRef]
- Devriese, L.I.; De Witte, B.; Dick Vethaak, A.; Hostens, K.; Leslie, H.A. Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere 2017, 186, 10–16. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Li, Y. Toxicity inhibition strategy of microplastics to aquatic organisms through molecular docking, molecular dynamics simulation and molecular modification. Ecotoxicol. Environ. Saf. 2021, 226, 11287. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Nabi, G.; Ahmad, S.; Ullah, S.; Zada, S.; Sarfraz, M.; Guo, X.; Ismail, M.; Wanghe, K. The adverse health effects of increasing microplastic pollution on aquatic mammals. J. King Saud Univ. Sci. 2022, 34, 102006. [Google Scholar] [CrossRef]
- Yu, H.; Qi, W.; Cao, X.; Hu, J.; Li, Y.; Peng, J.; Hu, C.; Qu, J. Microplastic residues in wetland ecosystems: Do they truly threaten the plant-microbe-soil system? Environ. Int. 2021, 156, 106708. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Talsness, C.E.; Andrade, J.M.A.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Phil. Trans. R. Soc. B 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Dick Vethaak, A.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Bashir, A.; Hashmi, I. Detection in influx sources and estimation of microplastics abundance in surface waters of Rawal Lake, Pakistan. Heliyon 2022, 8, e09166. [Google Scholar] [CrossRef]
- Petroody, A.S.S.; Hashemi, S.H.; van Gestel, C.A.M. Transport and accumulation of microplastics through wastewater treatment sludge processes. Chemosphere 2021, 278, 130471. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization) Search Site. Available online: https://www.iso.org/search.html?q=microplastic (accessed on 21 April 2022).
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493. [Google Scholar] [CrossRef]
- Choi, D.; Hwang, J.; Bang, J.; Han, S.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis. Sci. Total Environ. 2021, 752, 142242. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Bergmann, M.; Hildebrandt, N.; Krumpen, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Microplastic ingestion in zooplankton from the Fram Strait in the Arctic. Sci. Total Environ. 2022, 831, 154886. [Google Scholar] [CrossRef]


| PHI | Hazard Category |
|---|---|
| 0–1 | I |
| 1–10 | II |
| 10–100 | II |
| 100–1000 | IV |
| >1000 | V |
| Type of Plastic | Station | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| Rubber | - | 4 | 2 |
| PET | - | - | 1 |
| PU | 23 | 20 | - |
| PA | 16 | 2 | - |
| PP | 4 | - | - |
| PE | 2 | - | - |
| PS | 1 | - | - |
| PVC | 1 | 2 | - |
| Acrylates (PAN) | 1 | - | - |
| Station | Polymer | Score (Sn) | PHI Value | Risk Category |
|---|---|---|---|---|
| R1 | Polyurethane (PU) | 13,844 | 7133 | V |
| Polyamide (PA) | 63 | |||
| Polypropylene (PP) | 1 | |||
| Polyethylene (PE) | 11 | |||
| Polystyrene (PS) | 30 | |||
| Polyvinyl chloride (PVC) | 10,551 | |||
| Polyacrylonitrile (PAN) | 12,379 | |||
| R2 | Rubber | 1628 | 10,879 | V |
| Polyurethane (PU) | 13,844 | |||
| Polyamide (PA) | 63 | |||
| Polyvinyl chloride (PVC) | 10,551 | |||
| R3 | Rubber | 1628 | 1086 | V |
| Polyethylene terephthalate (PET) | 4 |
| Objects | Min | Max | Method | References | |
|---|---|---|---|---|---|
| Marine | Beibu Gulf (China) | 264 ± 2 * | 3D Raman spectroscopy | (Jiao et al., 2021) [8] | |
| Coastline of Ireland | 548 | 3D Raman Spectroscopy | (Marques Mendes et al., 2021) [42] | ||
| Lagoon of Venice (Italy) | 672 | 2175 | ESEM-EDS | (Vianello et al., 2013) [43] | |
| Byfjorden (Norway) | 12,000 | 200,000 | FTIR | (Haave et al., 2019) [44] | |
| Harbor of Cartagena (Spain) | 16.57 ± 2.96 * | 23.78 ± 4.59 * | Microscopy | (Bayo et al., 2022) [45] | |
| Mele Bay (Vanuatu) | 7300 ± 33,300 * | Microscopy, ATR-FTIR | (Bakir et al., 2020) [46] | ||
| Caspian Sea (Iran) | 28 | 542 | SEM, FTIR | (Ghayebzadeh et al., 2020) [47] | |
| Lake/Reservoir | Lake Onega (Russia) | 2188.7 ± 1164.4 * | Microscopy | (Zobkov et al., 2020) [48] | |
| Edgbaston Pool (UK) | 250 | 300 | Microscopy | (Vaughan et al., 2017) [49] | |
| Lake Ontario (Canada) | 500 | 28,000 | Microscopy | (Ballent et al., 2016) [50] | |
| Lake Chiusi (Italy) | 112 | 234 | UV-microscope, SEM | (Fisher et al., 2016) [51] | |
| Ox-Bow Lake (Nigeria) | 347 | 7593 | Microscopy | (Oni et al., 2020) [52] | |
| River | Antuã River (Portugal) | 18 | 629 | Microscopy | (Rodrigues et al., 2018) [53] |
| Vaal River (South Africa) | 29.63 * | 1028.57 * | Microscopy | (Saad et al., 2022) [54] | |
| Fuhe River (China) | 1049± 462 * | Microscopy | (Zhou et al., 2021) [55] | ||
| Yellow River (China) | 6166 ± 2914 * | LDIR | (Qian et al., 2023) [56] | ||
| Rhine River (Germany) | 68 | 3763 | FTIR | (Klein et al., 2015) [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strojny, W.; Gruca-Rokosz, R.; Cieśla, M. Preliminary Study of the Occurrence of Microplastics in the Sediments of the Rzeszów Reservoir Using the Laser Direct Infrared (LDIR) Method. Sustainability 2023, 15, 16653. https://doi.org/10.3390/su152416653
Strojny W, Gruca-Rokosz R, Cieśla M. Preliminary Study of the Occurrence of Microplastics in the Sediments of the Rzeszów Reservoir Using the Laser Direct Infrared (LDIR) Method. Sustainability. 2023; 15(24):16653. https://doi.org/10.3390/su152416653
Chicago/Turabian StyleStrojny, Wojciech, Renata Gruca-Rokosz, and Maksymilian Cieśla. 2023. "Preliminary Study of the Occurrence of Microplastics in the Sediments of the Rzeszów Reservoir Using the Laser Direct Infrared (LDIR) Method" Sustainability 15, no. 24: 16653. https://doi.org/10.3390/su152416653
APA StyleStrojny, W., Gruca-Rokosz, R., & Cieśla, M. (2023). Preliminary Study of the Occurrence of Microplastics in the Sediments of the Rzeszów Reservoir Using the Laser Direct Infrared (LDIR) Method. Sustainability, 15(24), 16653. https://doi.org/10.3390/su152416653








