Micro- and Nano-Bubbles Enhanced the Treatment of an Urban Black-Odor River
Abstract
:1. Introduction
2. Materials and Methods
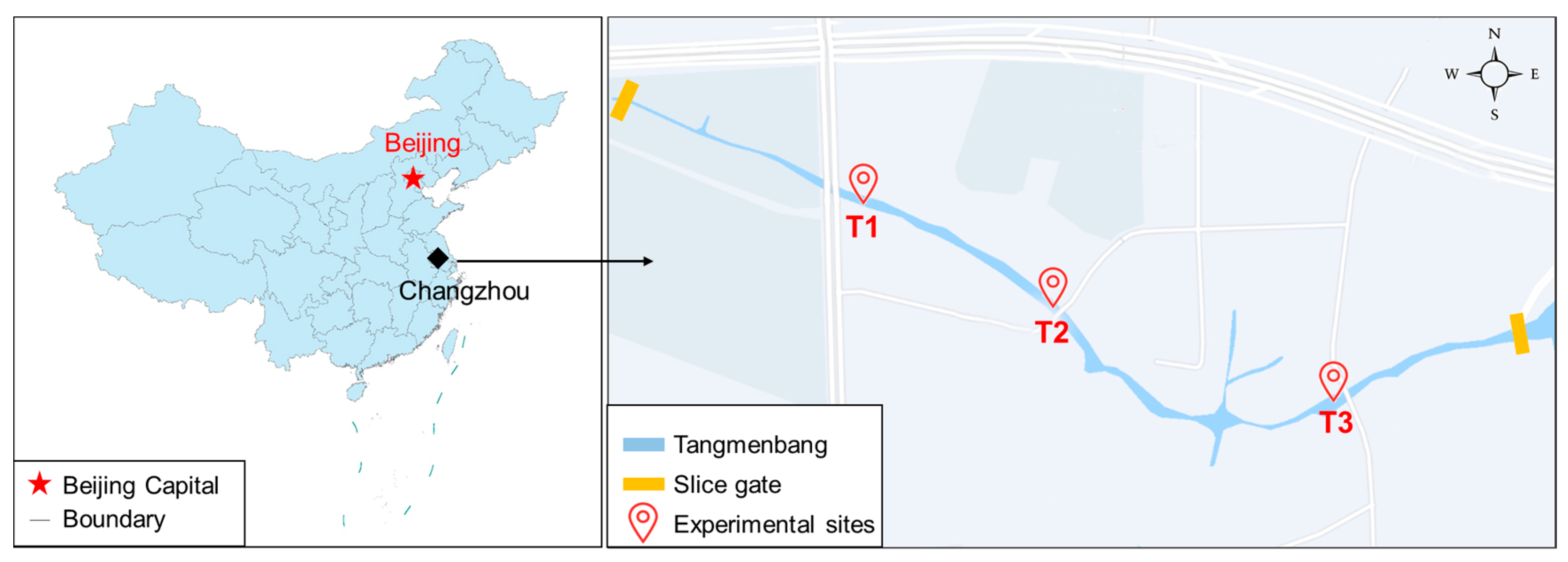
2.1. Study Area
2.2. Experiment Settings and Sample Collection
2.3. Analytical Methods
2.3.1. Physicochemical Parameters of Water and Sediment
2.3.2. Plankton Analysis
2.3.3. DNA Extraction, Q-PCR Amplification and High-Throughput Sequencing
2.4. Statistical Analysis
3. Results and Discussion
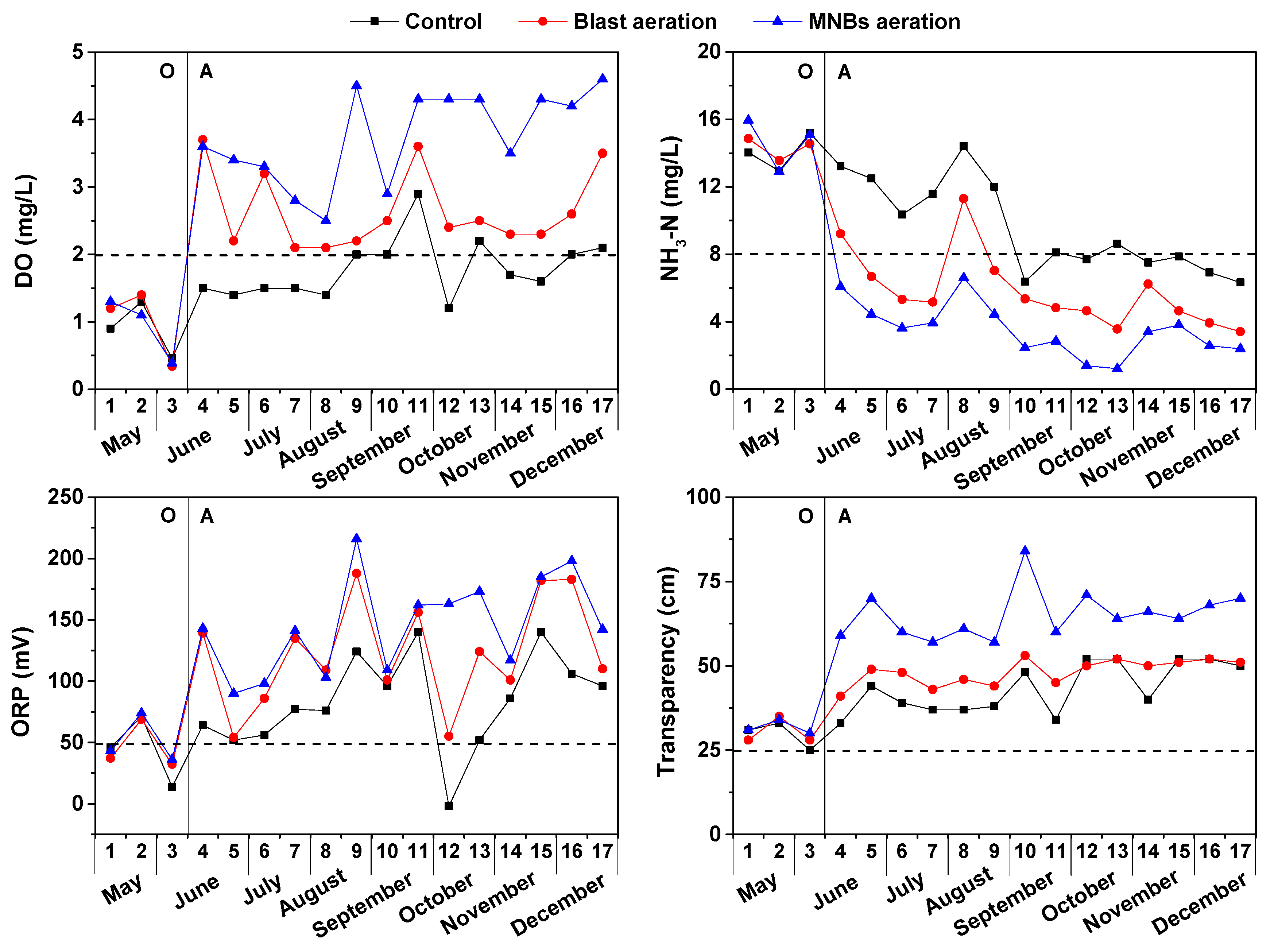
3.1. Physicochemical Characteristics of the Urban Black-Odor River
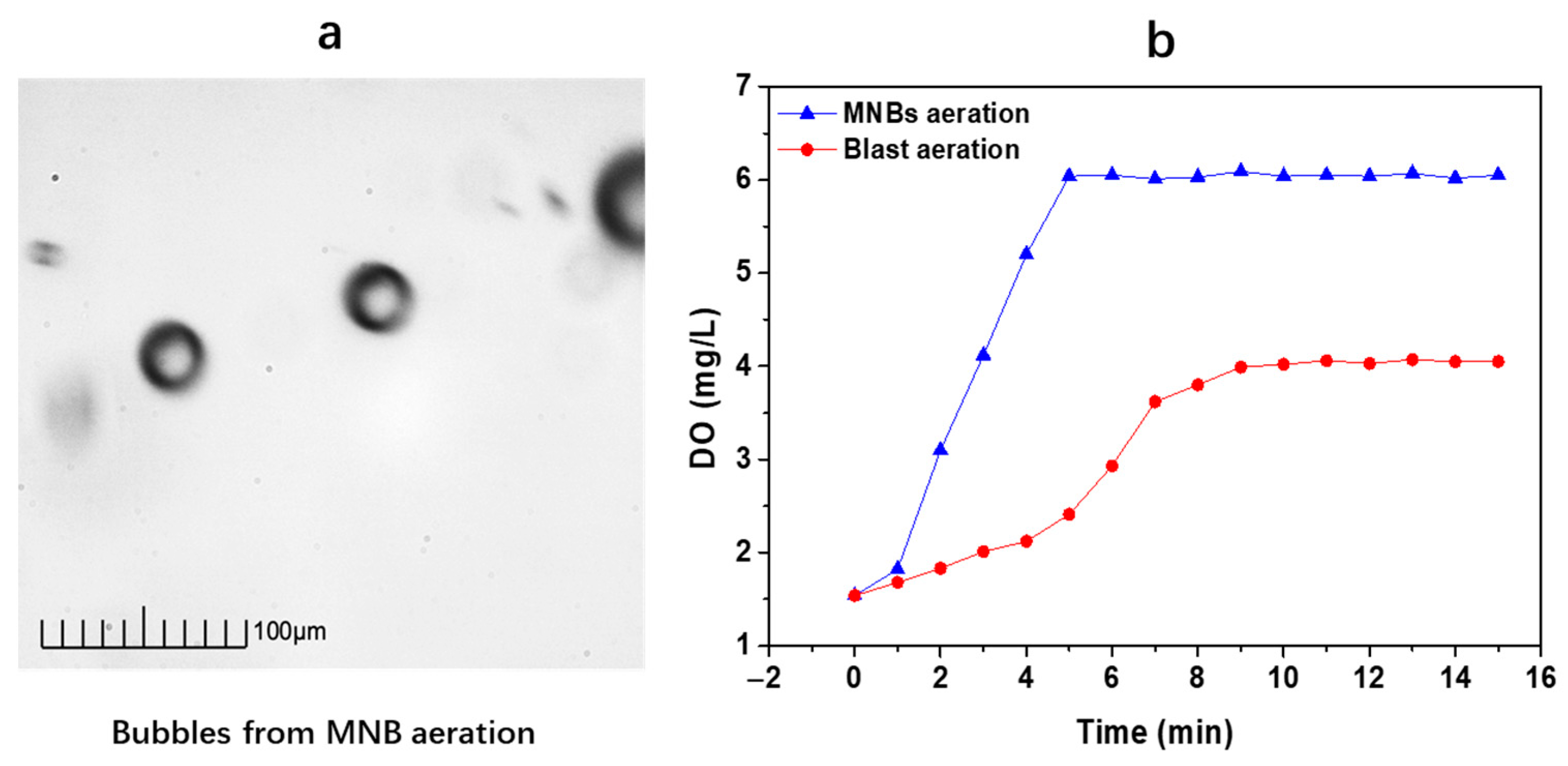
3.2. Performance of Water and Sediment after MNBs Aeration
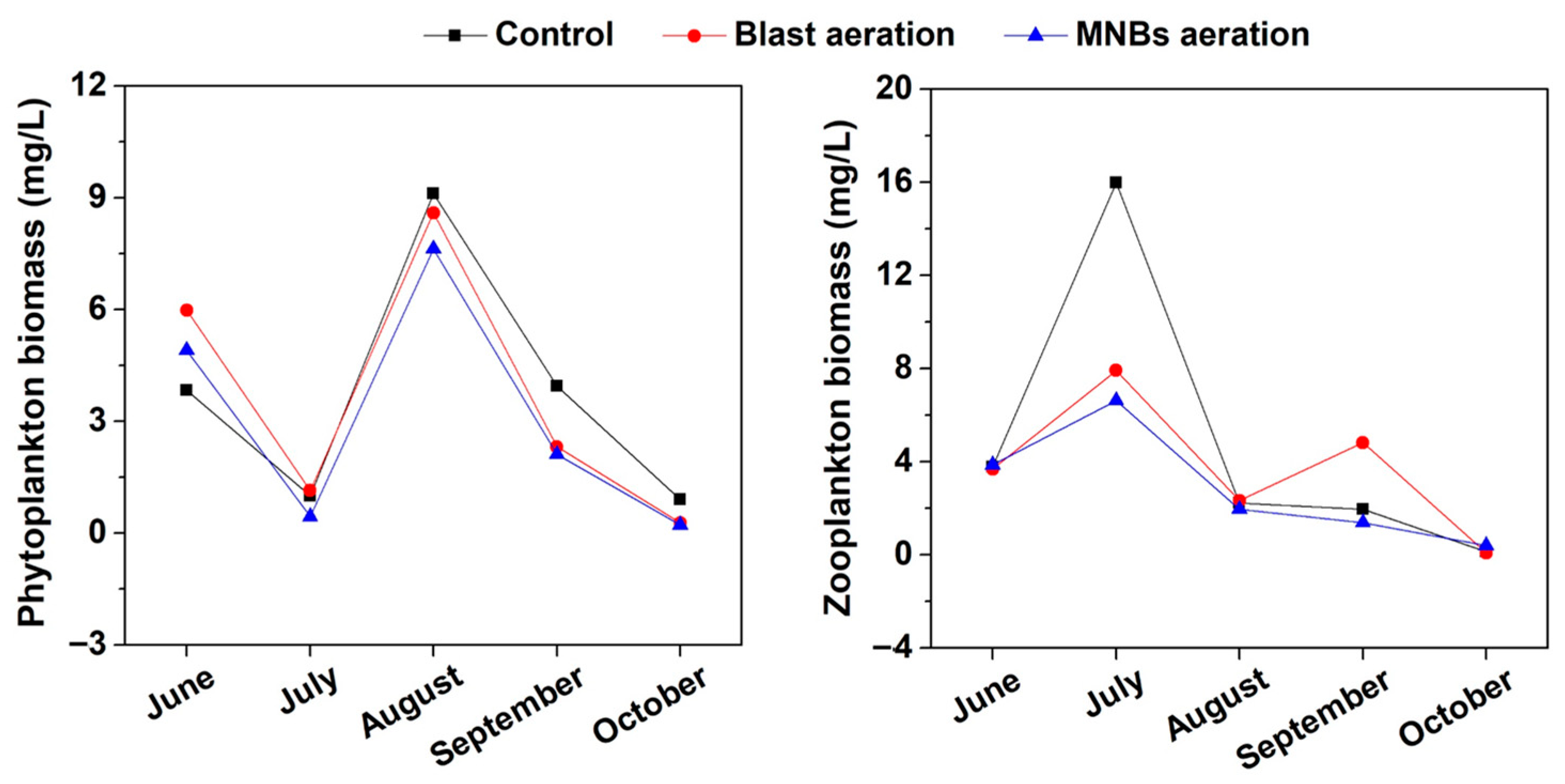
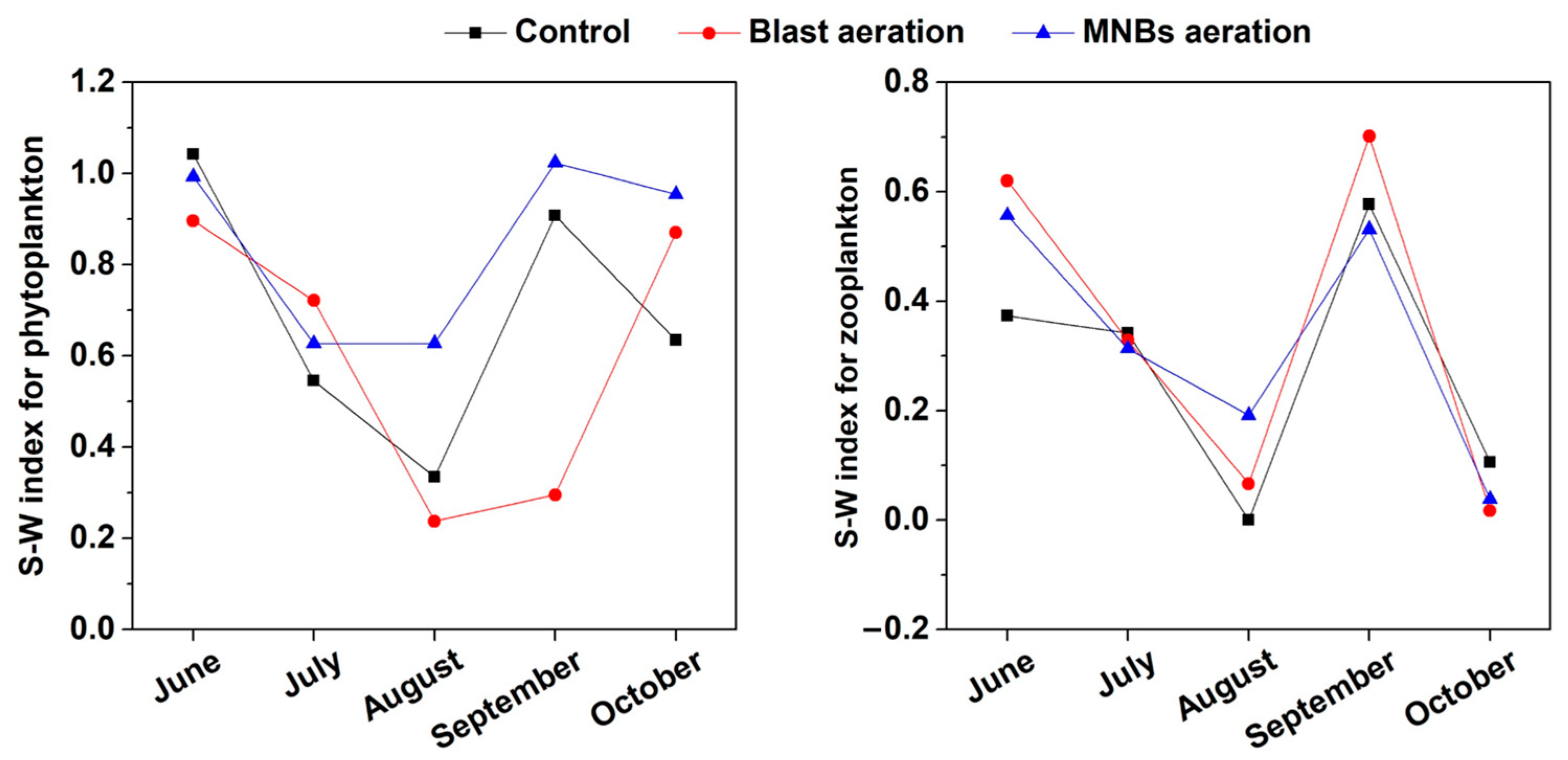
3.3. Response of Plankton Composition in Water to MNBs Aeration
3.4. Impacts of MNBs on Microbial Community in Sediment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Wu, H.; Huang, C.; Lin, H.; Li, W. Microbial compositions, ecological networks, and metabolomics in sediments of black-odour water in Dongguan, China. Environ. Res. 2022, 210, 112918. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, X.; Luo, H.; Zeng, D.; Huang, S. Linking microbial community and biological functions to redox potential during black-odor river sediment remediation. Environ. Sci. Pollut. Res. 2020, 27, 40392–40404. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Zhao, Z.; Dong, W.; Wu, Z.; Zhang, S.; Li, W.; Wu, X. Simultaneous elimination of black-odor and stabilization of heavy metals in contaminated sediment using calcium peroxide/hydroxyapatite: Microbial responses and ecotoxicological effects. J. Hazard. Mater. 2022, 429, 128298. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, S.; Zhang, N.; Liang, H.; Sun, L.; Zhao, X.; Guo, J.; Lu, H. Micro and nano bubbles promoted biofilm formation with strengthen of COD and TN removal synchronously in a blackened and odorous water. Sci. Total Environ. 2022, 837, 155578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, H.; Lan, S.; Wang, C.; Xie, Y. Evolution of urban black and odorous water: The characteristics of microbial community and driving-factors. J. Environ. Sci. 2022, 112, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ye, J.; Zhang, W.; Hu, F.; Guo, Q.; Xu, Z. The blackening process of black-odor water: Substance types determination and crucial roles analysis. J. Hazard. Mater. 2023, 443, 130295. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Liu, Y.; Du, P.P.; Zeng, L.J.; Mo, C.H.; Li, Y.W.; Lu, H.; Cai, Q.Y. Occurrence and distribution of antibiotics and antibiotic resistant genes in water and sediments of urban rivers with black-odor water in Guangzhou, South China. Sci. Total Environ. 2019, 670, 170–180. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment. Action Plan for Water Pollution Prevention and Control; Ministry of Ecology and Environment: Beijing, China, 2015. Available online: https://www.mee.gov.cn/zcwj/gwywj/201811/t20181129_676575.shtml (accessed on 15 September 2023).
- Liu, M.; Li, T.; Wang, Z.; Radu, T.; Jiang, H.; Wang, L. Effect of aeration on water quality and sediment humus in rural black-odorous water. J. Environ. Manag. 2022, 320, 115867. [Google Scholar] [CrossRef]
- Lyu, T.; Wu, Y.; Zhang, Y.; Fan, W.; Wu, S.; Mortimer, J.G.R.; Pan, G. Nanobubble aeration enhanced wastewater treatment and bioenergy generation in constructed wetlands coupled with microbial fuel cells. Sci. Total Environ. 2023, 895, 165131. [Google Scholar] [CrossRef]
- Zhou, S.; Nazari, S.; Hassanzadeh, A.; Bu, X.; Ni, C.; Peng, Y.; Xie, G.; He, Y. The effect of preparation time and aeration rate on the properties of bulk micro-nanobubble water using hydrodynamic cavitation. Ultrason. Sonochem. 2022, 84, 105965. [Google Scholar] [CrossRef]
- Soyluoglu, M.; Kim, D.; Zaker, Y.; Karanfil, T. Stability of Oxygen Nanobubbles under Freshwater Conditions. Water Res. 2021, 206, 117749. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Wu, S.; Mortimer, J.G.R.; Pan, G. Nanobubble technology in environmental engineering: Revolutionization potential and challenges. Environ. Sci. Technol. 2019, 53, 7175–7176. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zhang, Y.; Marhaba, T.; Zhang, W. Aeration and dissolution behavior of oxygen nanobubbles in water. J. Colloid Interface Sci. 2022, 609, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tian, W.; Zhang, Y.; Fan, W.; Liu, F.; Zhao, J.; Wang, M.; Liu, Y.; Lyu, T. Nanobubble technology enhanced ozonation process for ammonia removal. Water 2022, 14, 1865. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.; Zhang, J.; Lyu, T.; Cooper, M.; Pan, G. Reducing arsenic toxicity using the interfacial oxygen nanobubble technology for sediment remediation. Water Res. 2021, 205, 117657. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Li, P. Effect of micro-nano bubble aeration on aerobic microbial community in small water body. China Water Wastewater 2020, 36, 29–34. [Google Scholar]
- Zhou, Z.; Chai, X. Research on the rapid removal of odorous compounds in black and odorous water by micro-nano aeration. Shandong Chem. Ind. 2021, 2, 253–257. [Google Scholar]
- Ma, J.; Lin, X.; Yu, Z.; Wang, X.; Chen, J.; He, Q. Characterization, risk assessment and resource potential of sediments in the black-odor water in Hunan, China. Environ. Monit. Assess. 2022, 194, 1–11. [Google Scholar] [CrossRef]
- Lu, P.; Lin, Y.H.; Yang, Z.Q.; Xu, Y.P.; Tan, F. Effects of application of corn straw on soil microbial community structure during the maize growing season. J. Basic Microbiol. 2014, 54, 1–11. [Google Scholar] [CrossRef]
- Wang, D.; Nie, L.; Li, J. Transfer characteristics of nutrient elements through hydrological process of Pinus tabulaeformis stad in Beijing Xishan area. Acta Ecol. Sin. 2006, 26, 2101–2107. [Google Scholar]
- Song, Q.; Li, Y.; Jiang, X. Spatial distribution of phosphorus fractions in the sediments of Meiliang Bay, Taihu Lake, China. Adv. Mater. Res. 2012, 610–613, 2766–2770. [Google Scholar] [CrossRef]
- Herath, I.K.; Wu, S.; Ma, M.; Ping, H. Heavy metal toxicity, ecological risk assessment, and pollution sources in a hydropower reservoir. Environ. Sci. Pollut. Res. 2022, 29, 32929–32946. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Feng, J.; Song, Y.; Ni, M.; Dietrich, A.M.; Chen, C.; Li, Q.; Gao, N. Release behavior of odor contaminants derived from Microcystis aeruginosa in rivers and a non-strict anaerobic aqueous system. AQUA 2015, 64, 812–823. [Google Scholar]
- Weng, B.; Yang, Y.; Yan, D.; Wang, J.; Dorjsuren, B. Shift in plankton diversity and structure: Influence of runoff composition in the Nagqu River on the Qinghai-Tibet Plateau. Ecol. Indic. 2020, 109, 105818. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. Msystems 2016, 1, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiang, Y.J.; He, W.; Liu, W.X.; Kong, X.Z.; Jørgensen, S.E.; Xu, F.L. The tempo-spatial variations of phytoplankton diversities and their correlation with trophic state levels in a large eutrophic Chinese lake. Ecol. Indic. 2016, 66, 153–162. [Google Scholar] [CrossRef]
- Ministry of Housing and Urban Rual Development. The Work Guide of Urban Black and Odorous Water Body Remediation; Ministry of Housing and Urban Rual Development: Beijing, China, 2015; pp. 1–34. [Google Scholar]
- Qin, B.; Zhou, J.; Elser, J.J.; Gardner, W.S.; Deng, J.; Brookes, J.D. Water Depth Underpins the Relative Roles and Fates of Nitrogen and Phosphorus in Lakes. Environ. Sci. Technol. 2020, 54, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Dkmen, F. Temporal Variation of Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), and pH Values in Surface Waters of Gölcük-Kocaeli, Turkey. In Plants, Pollutants and Remediation; Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M.S.A., Hakeem, K.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 341–347. [Google Scholar]
- Duval, B.; Ludlam, D.S. The Black Water Chemocline of Meromictic Lower Mystic Lake, Massachusetts, USA. Int. Rev. Hydrobiol. 2001, 86, 165–181. [Google Scholar] [CrossRef]
- Dong, S.; Li, Z.; Chen, Q.; Zhiqiao, W. Total organic carbon and its environmental significance for the surface sediments in groundwater recharged lakes from the Badain Jaran Desert, Northwest China. J. Limnol. 2018, 77, 121–129. [Google Scholar] [CrossRef]
- Stockenberg, A.; Johnstone, R.W. Benthic Denitrification in the Gulf of Bothnia. Estuar. Coast. Shelf Sci. 1997, 45, 835–843. [Google Scholar] [CrossRef]
- León, J.G.; Pedrozo, F.L.; Temporetti, P.F. Phosphorus fractions and sorption dynamics in the sediments of two Ca-SO4 water reservoirs in the central Argentine Andes. Int. J. Sediment Res. 2017, 32, 442–451. [Google Scholar] [CrossRef]
- Kim, L.H.; Choi, E.; Stenstrom, M.K. Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere 2003, 50, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Y.; Wu, Y.; Zeng, C.; Zheng, W. Applicationof black-odorriverof aerobic-oxygen-enrichedaerationbiological treatment in-situremediation. Chin. J. Environ. Eng. 2017, 11, 2780–2784. [Google Scholar]
- Zhou, S.; Liu, M.; Chen, B.; Sun, L.; Lu, H. Microbubble- and nanobubble-aeration for upgrading conventional activated sludge process: A review. Bioresour. Technol. 2022, 362, 127826. [Google Scholar] [CrossRef] [PubMed]
- Gin, Y.H.; Gopalakrishnan, A.P. Sediment Oxygen Demand and Nutrient Fluxes for a Tropical Reservoir in Singapore. J. Environ. Eng. 2010, 136, 78–85. [Google Scholar] [CrossRef]
- Goñi, M.A.; Thomas, K.A. Sources and transformations of organic matter in surface soils and sediments from a tidal estuary (North Inlet, South Carolina, USA). Estuaries 2000, 23, 548–564. [Google Scholar] [CrossRef]
- Roden, E.E.; Wetzel, R.G. Competition between Fe(III)-reducing and methanogenic bacteria for acetate in iron-rich freshwater sediments. Microb. Ecol. 2003, 45, 252–258. [Google Scholar] [CrossRef]
- Li, P.; Song, Y.; Yu, S. Removal of Microcystis aeruginosa using hydrodynamic cavitation: Performance and mechanisms. Water Res. 2014, 62, 241–248. [Google Scholar] [CrossRef]
- Xu, C.; Yang, J.; Ma, M.; Hu, X.; You, W. Characteristics of Phytoplankton Community Changes in Dianshan Lake During Peak Period of Algal Blooms. Environ. Sci. 2012, 33, 1136–1145. [Google Scholar]
- Zhao, L.; Liu, Y.; Wang, Y.; Zhao, R.; Ren, W.; Xu, M. Bacterial community structure and diversity of sediments in a typical plateau lakeshore. Microbiol. China 2020, 47, 401–410. [Google Scholar]
- Shen, X.; Xu, M.; Li, M.; Zhao, Y.; Shao, X. Response of sediment bacterial communities to the drainage of wastewater from aquaculture ponds in different seasons. Sci. Total Environ. 2020, 717, 137180. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; He, Q.; Fu, Y.; Liu, Y. Construction of the comprehensive evaluation system of waterbody pollution degree and the response of sedimentary microbial community. Environ. Pollut. 2023, 317, 120837. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhang, L.; Qin, C.; Lu, P.; Yuan, S. Temporal changes of microbial community structure and nitrogen cycling processes during the aerobic degradation of phenanthrene. Chemosphere 2021, 1, 131709. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Sun, J.; Pang, Y.; Zhang, S.; Xu, R. Effects of different habitats on the bacterial community composition in the water and sediments of Lake Taihu, China. Environ. Sci. Pollut. Res. 2020, 27, 44983–44994. [Google Scholar] [CrossRef]
- Liu, P. Studies on the microorganisms of three domains associated with seagrass (Zostera japonica) meadows: Distributions, ecological functions, and environmental drivers. In Marine Biology 2022; University of Chinese Academy of Sciences: Yantai, China, 2022; p. 193. [Google Scholar]
- Zhang, L.; Zhao, T.; Shen, T.; Gao, G. Seasonal and spatial variation in the sediment bacterial community and diversity of Lake Bosten, China. J. Basic Microbiol. 2018, 59, 224–233. [Google Scholar] [CrossRef]
- He, Y.; Long, L.; Tian, X. Recent advances in the class Acidimicrobiia. Microbiol. China 2020, 47, 1945–1957. [Google Scholar]
- Xu, X. The research of in-situ remediation black-ordorous river by aeration enhancing functional strains. In Environmental Science and Engineering 2017; Chengdu University of Technology: Chengdu, China, 2017; p. 60. [Google Scholar]




| Water | Sediment | ||
|---|---|---|---|
| Parameters (Units) | Mean (Range) | Parameters (Units) | Mean (Range) |
| pH | 7.57 (7.57–7.58) | TOC (g/kg) | 6.90 (6.80–7.00) |
| Transparency (cm) | 27.7 (25.0–30.0) | NH3-N (mg/kg) | 61.4 (59.6–62.9) |
| DO (mg/L) | 0.40 (0.34–0.46) | TN (mg/kg) | 162 (131–197) |
| ORP (mV) | 27.3 (13.8–36.1) | NOx-N (mg/kg) | 3.01 (2.57–3.40) |
| TN (mg/L) | 16.7 (16.2–17.1) | TP (mg/kg) | 582 (528–628) |
| NH3-N (mg/L) | 15.0 (14.6–15.2) | Fe (g/kg) | 20.9 (19.6–23.0) |
| Nitrate (mg/L) | 0.21 (0.10–0.41) | Fe/Al-P (mg/kg) | 186 (168–196) |
| TP (mg/L) | 1.38 (1.32–1.45) | ||
| COD (mg/L) | 24.0 (19.0–28.0) | ||
| BOD5 (mg/L) | 8.87 (8.00–9.70) | ||
| SO42− (mg/L) | 35.8 (33.6–38.1) | ||
| S2− (mg/L) | 0.02 (0.02–0.03) | ||
| Fe2+ (mg/L) | 0.34 (0.29–0.38) | ||
| Mn2+ (mg/L) | 0.06 (0.06–0.07) | ||
| DMTS (ng/L) | 2824 (2081–3419) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Zhou, Z.; Chai, X. Micro- and Nano-Bubbles Enhanced the Treatment of an Urban Black-Odor River. Sustainability 2023, 15, 16695. https://doi.org/10.3390/su152416695
Xu Q, Zhou Z, Chai X. Micro- and Nano-Bubbles Enhanced the Treatment of an Urban Black-Odor River. Sustainability. 2023; 15(24):16695. https://doi.org/10.3390/su152416695
Chicago/Turabian StyleXu, Qinqin, Zheng Zhou, and Xiaoli Chai. 2023. "Micro- and Nano-Bubbles Enhanced the Treatment of an Urban Black-Odor River" Sustainability 15, no. 24: 16695. https://doi.org/10.3390/su152416695






