Optimizing Operational Conditions of Pilot-Scale Membrane Capacitive Deionization System
Abstract
:1. Introduction
2. Materials and Methods
2.1. MCDI Modules
2.2. Experimental Setup
2.3. TDS Removal Efficiency Test according to Different MCDI Operational Conditions
3. Results and Discussion
3.1. Performance of MCDI with Different Module Arrangements
3.2. Determination of Optimum Adsorption/Desorption Time and Flow Rate in the Operational-Scale MCDI Operation
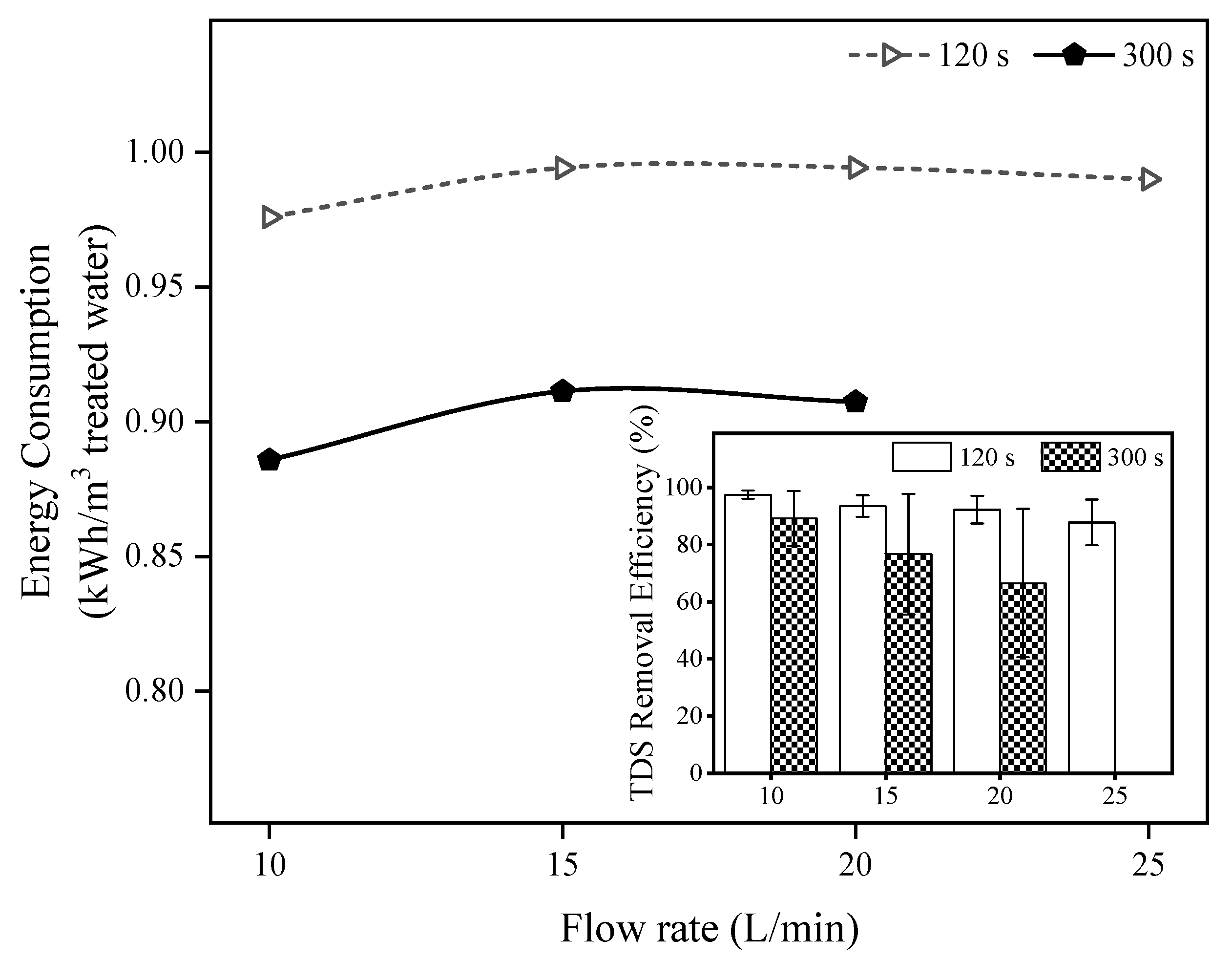
3.3. Energy Consumption Analysis of the Tested MCDI Operational Conditions
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tortajada, C.; Biswas, A.K. Achieving universal access to clean water and sanitation in an era of water scarcity: Strengthening contributions from academia. Curr. Opin. Environ. Sustain. 2018, 34, 21–25. [Google Scholar] [CrossRef]
- Bremere, I.; Kennedy, M.; Stikker, A.; Schippers, J. How water scarcity will effect the growth in the desalination market in the coming 25 years. Desalination 2001, 138, 7–15. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, H.-W.; Chung, J.W.; Kwak, S.-Y. Magnetic core-hydrophilic shell nanosphere as stability-enhanced draw solute for forward osmosis (FO) application. Desalination 2016, 397, 22–29. [Google Scholar] [CrossRef]
- Dhakal, N.; Salinas-Rodriguez, S.G.; Hamdani, J.; Abushaban, A.; Sawalha, H.; Schippers, J.C.; Kennedy, M.D. Is Desalination a Solution to Freshwater Scarcity in Developing Countries? Membranes 2022, 12, 381. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Liu, M.; He, M.; Han, J.; Sun, Y.; Jiang, H.; Li, Z.; Li, Y.; Zhang, H. Recent Advances in Capacitive Deionization: Research Progress and Application Prospects. Sustainability 2022, 14, 14429. [Google Scholar] [CrossRef]
- Tan, C.; He, C.; Tang, W.; Kovalsky, P.; Fletcher, J.; Waite, T.D. Integration of photovoltaic energy supply with membrane capacitive deionization (MCDI) for salt removal from brackish waters. Water Res. 2018, 147, 276–286. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of Capacitive Deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Tang, W.; Liang, J.; He, D.; Gong, J.; Tang, L.; Liu, Z.; Wang, D.; Zeng, G. Various cell architectures of capacitive deionization: Recent advances and future trends. Water Res. 2019, 150, 225–251. [Google Scholar] [CrossRef]
- Hassanvand, A.; Wei, K.; Talebi, S.; Chen, G.Q.; Kentish, S.E. The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation. Membranes 2017, 7, 54. [Google Scholar] [CrossRef]
- Bales, C.; Lian, B.; Fletcher, J.; Wang, Y.; Waite, T.D. Site specific assessment of the viability of membrane Capacitive Deionization (mCDI) in desalination of brackish groundwaters for selected crop watering. Desalination 2021, 502, 114913. [Google Scholar] [CrossRef]
- Feng, C.; Hou, C.-H.; Chen, S.; Yu, C.-P. A Microbial Fuel Cell Driven Capacitive Deionization Technology for Removal of Low-Level Dissolved Ions. Chemosphere 2013, 91, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Kovalsky, P.; Cao, B.; He, D.; Waite, T.D. Fluoride Removal from Brackish Groundwaters by Constant Current Capacitive Deionization (CDI). Environ. Sci. Technol. 2016, 50, 10570–10579. [Google Scholar] [CrossRef]
- Choi, J.-H. New operation method of a membrane capacitive deionization system with a dual-solution mode for improving the desorption rate. Desalination 2023, 549, 116364. [Google Scholar] [CrossRef]
- Mossad, M.; Zou, L. A Study of the Capacitive Deionisation Performance under Various Operational Conditions. J. Hazard. Mater. 2012, 213–214, 491–497. [Google Scholar] [CrossRef]
- Tang, W.; He, D.; Zhang, C.; Waite, T.D. Optimization of sulfate removal from brackish water by membrane capacitive deionization (MCDI). Water Res. 2017, 121, 302–310. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Ziaedini, A.; Rashedi, H.; Alaie, E.; Zeinali, M. Performance assessment of the stacked microbial desalination cells with internally parallel and series flow configurations. J. Environ. Chem. Eng. 2018, 6, 5079–5086. [Google Scholar] [CrossRef]
- Ramachandran, A.; Oyarzun, D.I.; Hawks, S.A.; Stadermann, M.; Santiago, J.G. High water recovery and improved thermodynamic efficiency for capacitive deionization using variable flowrate operation. Water Res. 2019, 155, 76–85. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, Y.; Wang, Y.; Miller, C.J.; Fletcher, J.; Lian, B.; Waite, T.D. Insufficient desorption of ions in constant-current membrane capacitive deionization (MCDI): Problems and solutions. Water Res. 2023, 242, 120273. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Yoon, N.; Park, S.; Son, M.; Lee, J.; Park, J.; Cho, K.H. Optimization of a nanofiltration and membrane capacitive deionization (NF-MCDI) hybrid system: Experimental and modeling studies. Desalination 2020, 493, 114658. [Google Scholar] [CrossRef]
- Zhao, R.; Porada, S.; Biesheuvel, P.M.; van der Wal, A. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. [Google Scholar] [CrossRef]
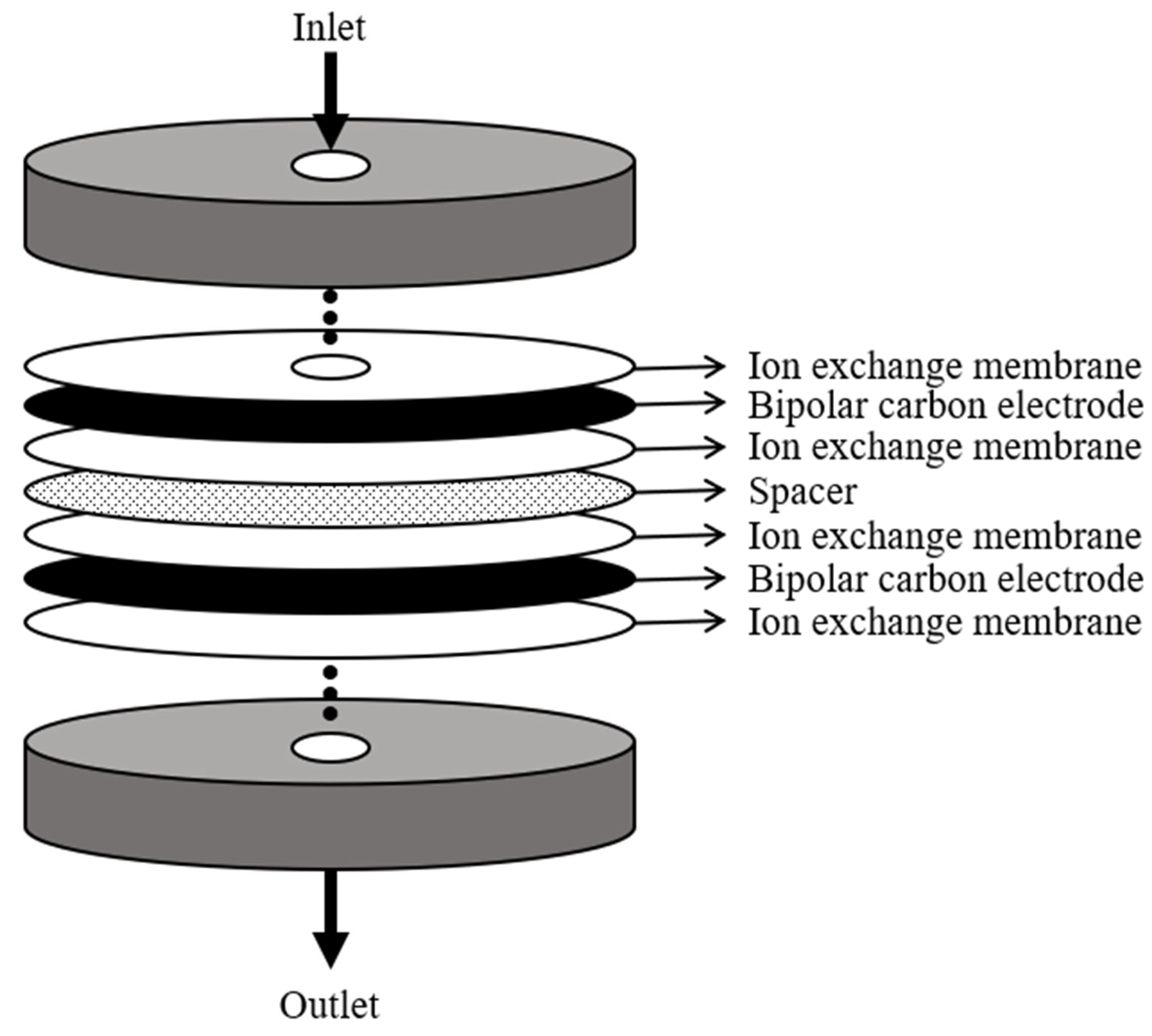
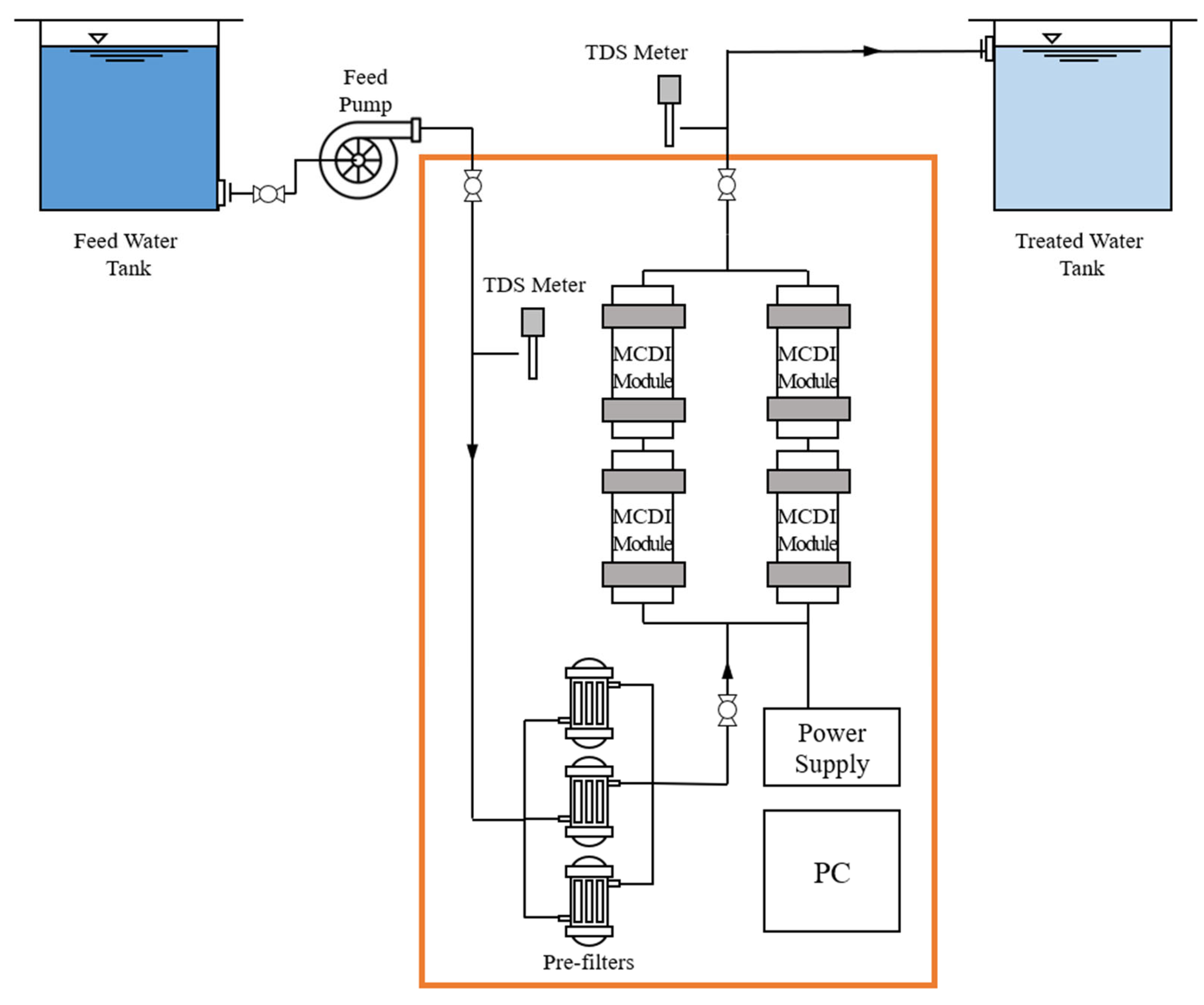
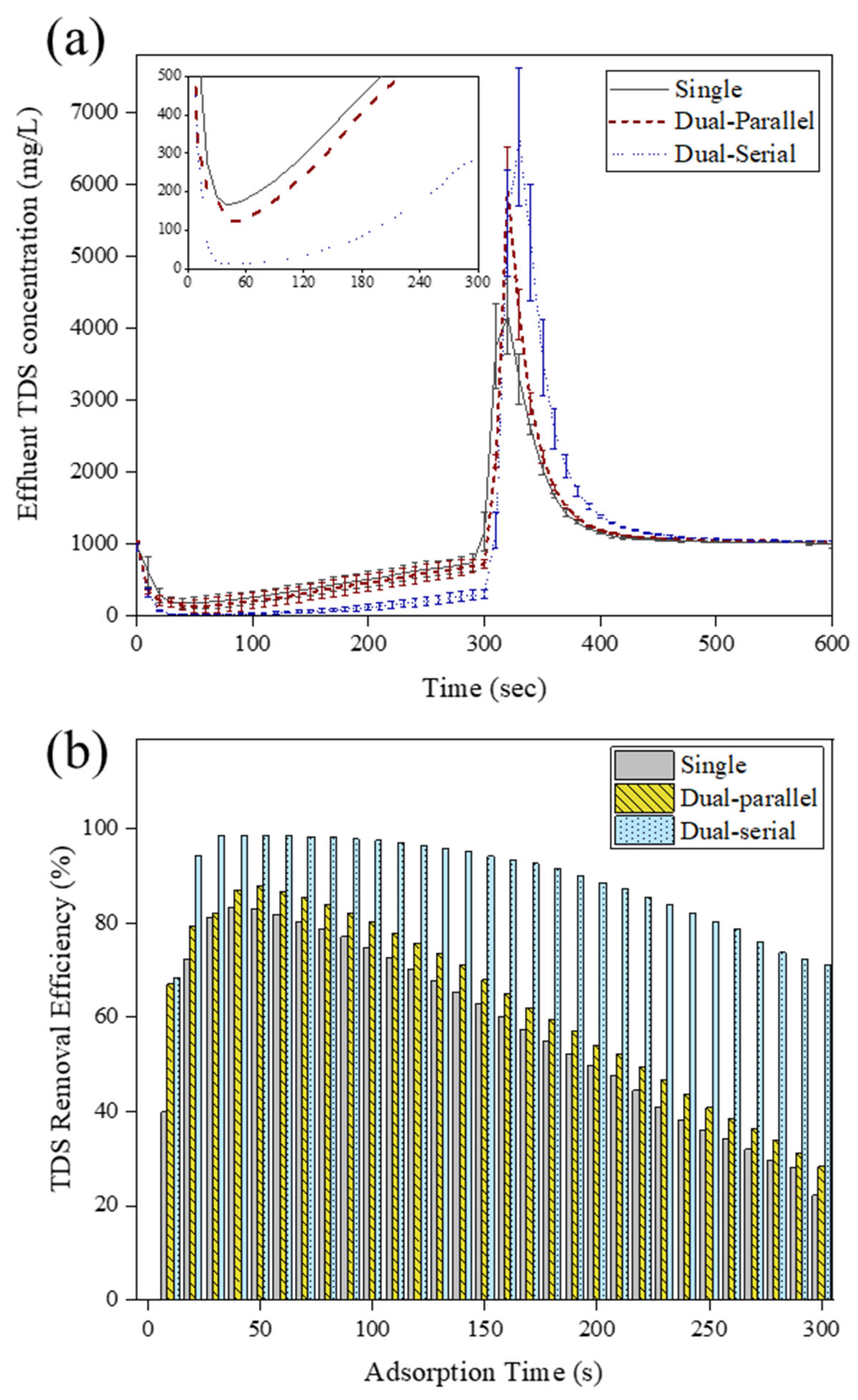
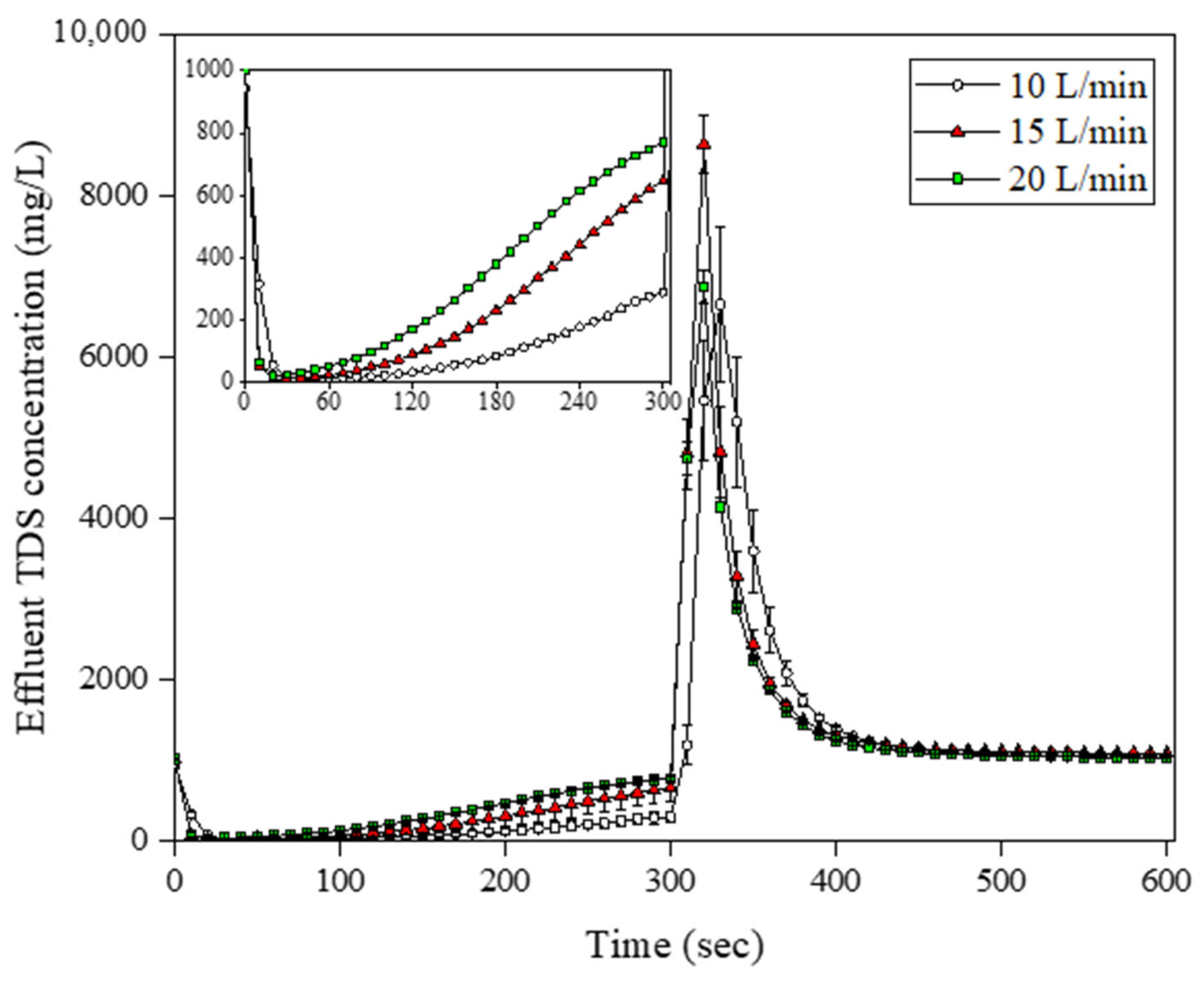
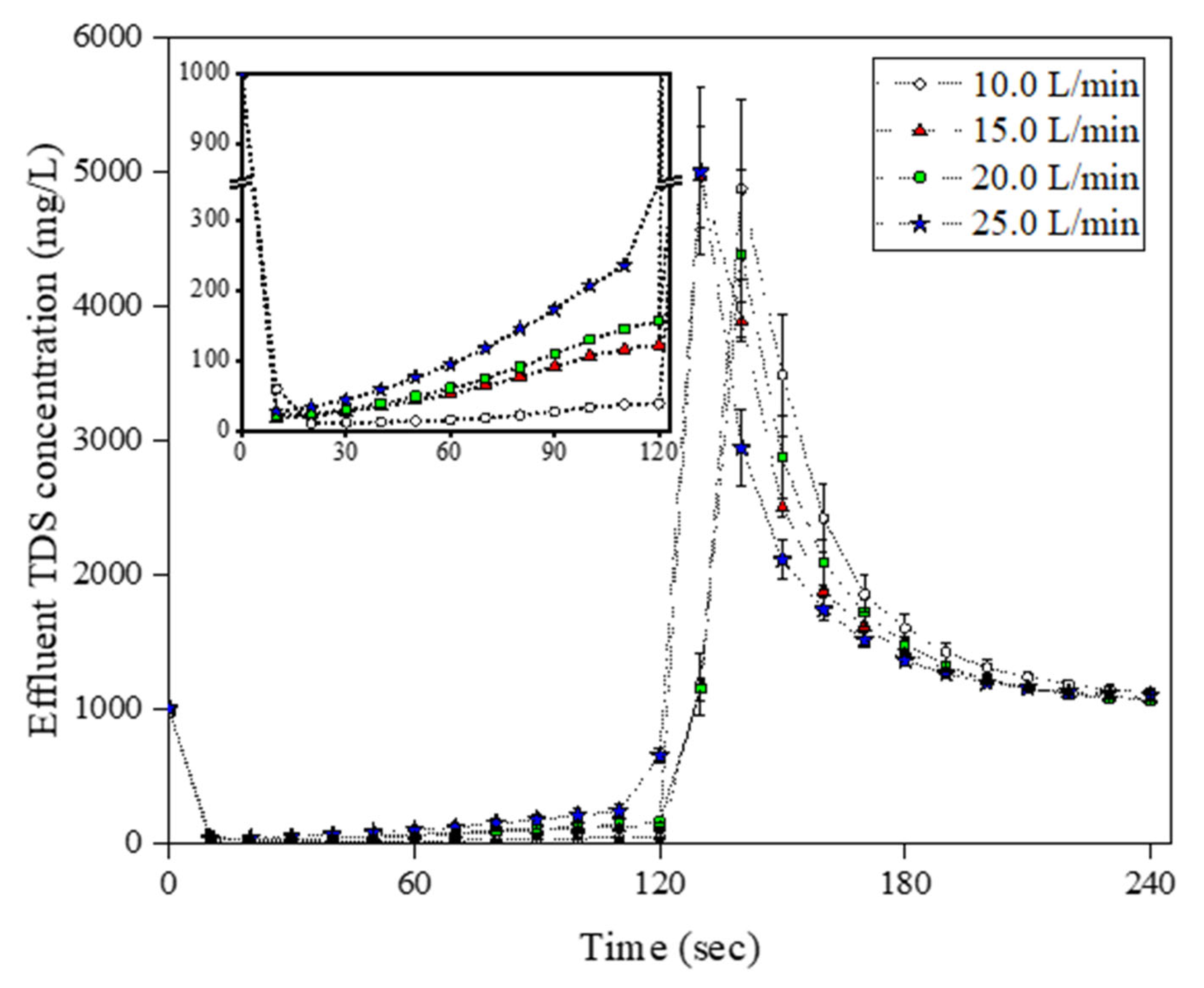
| No. | Flow Rate | Adsorption and Desorption Time (s) | Module Arrangement |
|---|---|---|---|
| 1 | 10 L/min | 300 | Single mode |
| 2 | 10 L/min | 300 | Dual (Series) |
| 3 | 20 L/min | 300 | Dual (Parallel) |
| 4 | 15 L/min | 300 | Dual (Series) |
| 5 | 20 L/min | 300 | Dual (Series) |
| 6 | 10 L/min | 120 | Dual (Series) |
| 7 | 15 L/min | 120 | Dual (Series) |
| 8 | 20 L/min | 120 | Dual (Series) |
| 9 | 25 L/min | 120 | Dual (Series) |
| Flow Rate | TDS Removal Efficiency (%) | Energy Consumption (kWh/m3) |
|---|---|---|
| 10 L/min | 97.4 | 0.976 |
| 15 L/min | 93.5 | 0.994 |
| 20 L/min | 92.2 | 0.994 |
| 25 L/min | 87.8 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Oh, C.; An, J.; Yeon, S.; Oh, H.J. Optimizing Operational Conditions of Pilot-Scale Membrane Capacitive Deionization System. Sustainability 2023, 15, 16809. https://doi.org/10.3390/su152416809
Lee B, Oh C, An J, Yeon S, Oh HJ. Optimizing Operational Conditions of Pilot-Scale Membrane Capacitive Deionization System. Sustainability. 2023; 15(24):16809. https://doi.org/10.3390/su152416809
Chicago/Turabian StyleLee, Bokjin, Changseog Oh, Jusuk An, Seungjae Yeon, and Hyun Je Oh. 2023. "Optimizing Operational Conditions of Pilot-Scale Membrane Capacitive Deionization System" Sustainability 15, no. 24: 16809. https://doi.org/10.3390/su152416809
APA StyleLee, B., Oh, C., An, J., Yeon, S., & Oh, H. J. (2023). Optimizing Operational Conditions of Pilot-Scale Membrane Capacitive Deionization System. Sustainability, 15(24), 16809. https://doi.org/10.3390/su152416809








