Interface Optimization of Cu2S Nanoparticles by Loading N-Doped Carbon for Efficient Sodium-Ion Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. General Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
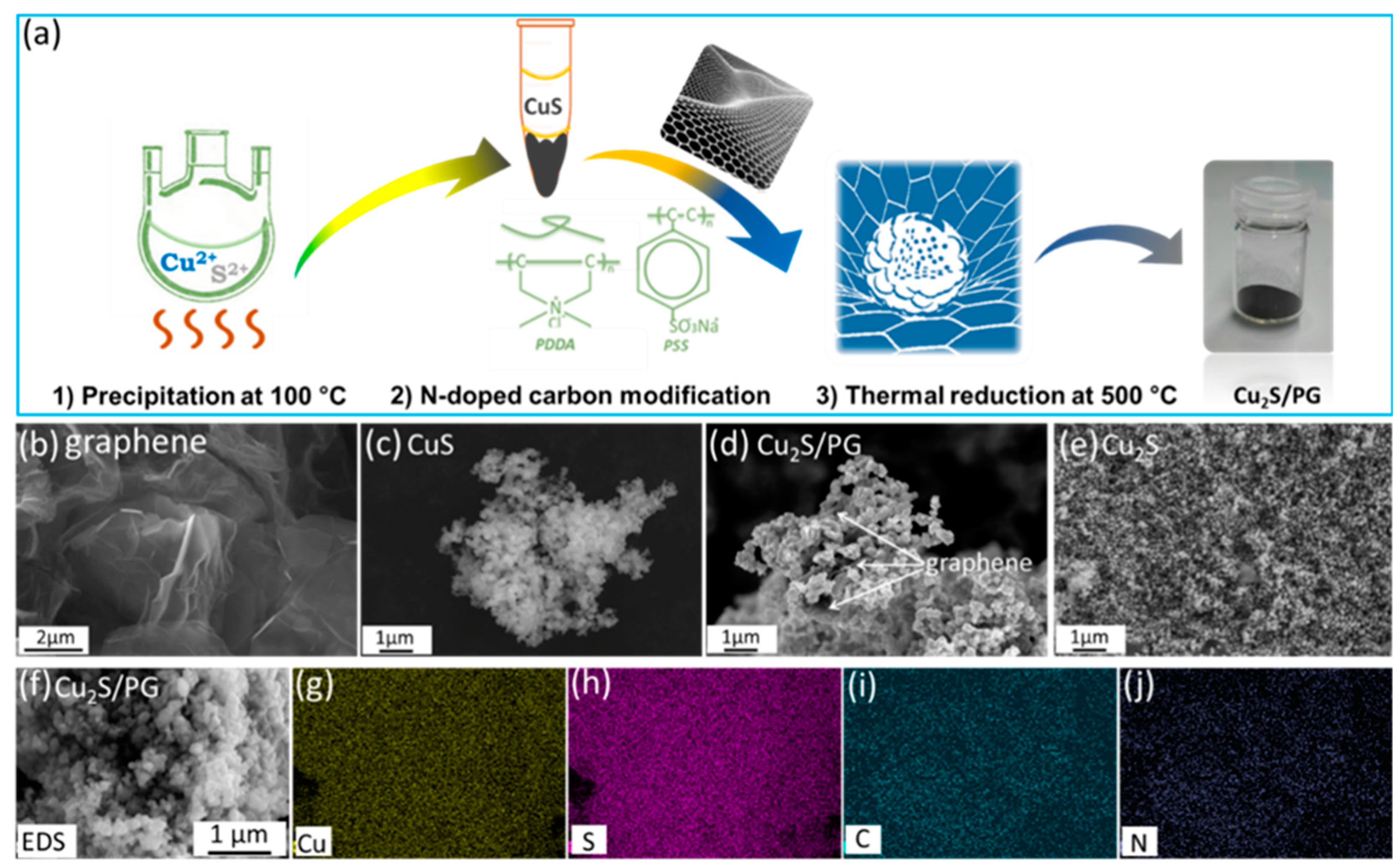
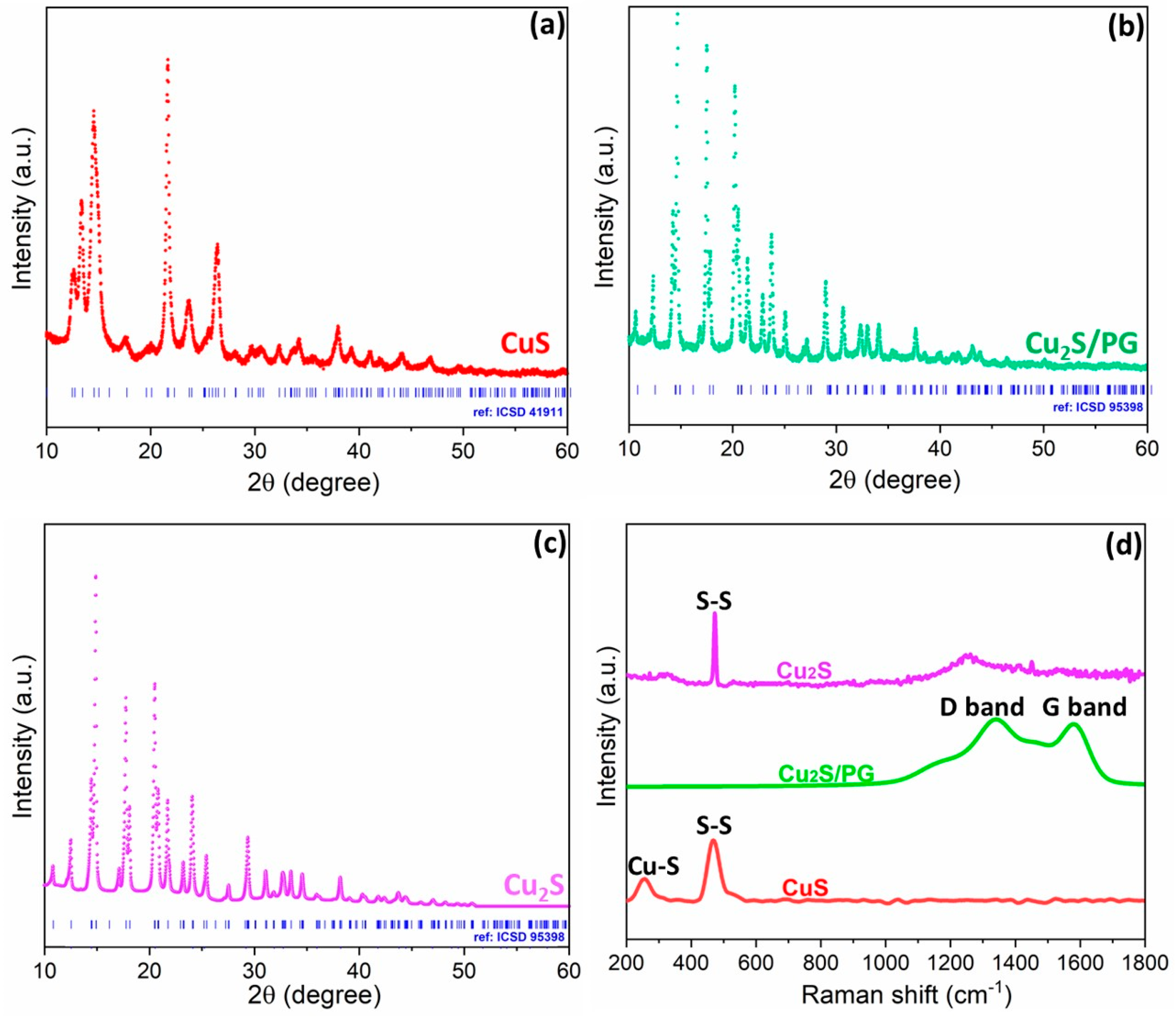
3.1. Synthesis and Characterization of Copper Sulfide Composites
3.2. Electrochemical Test
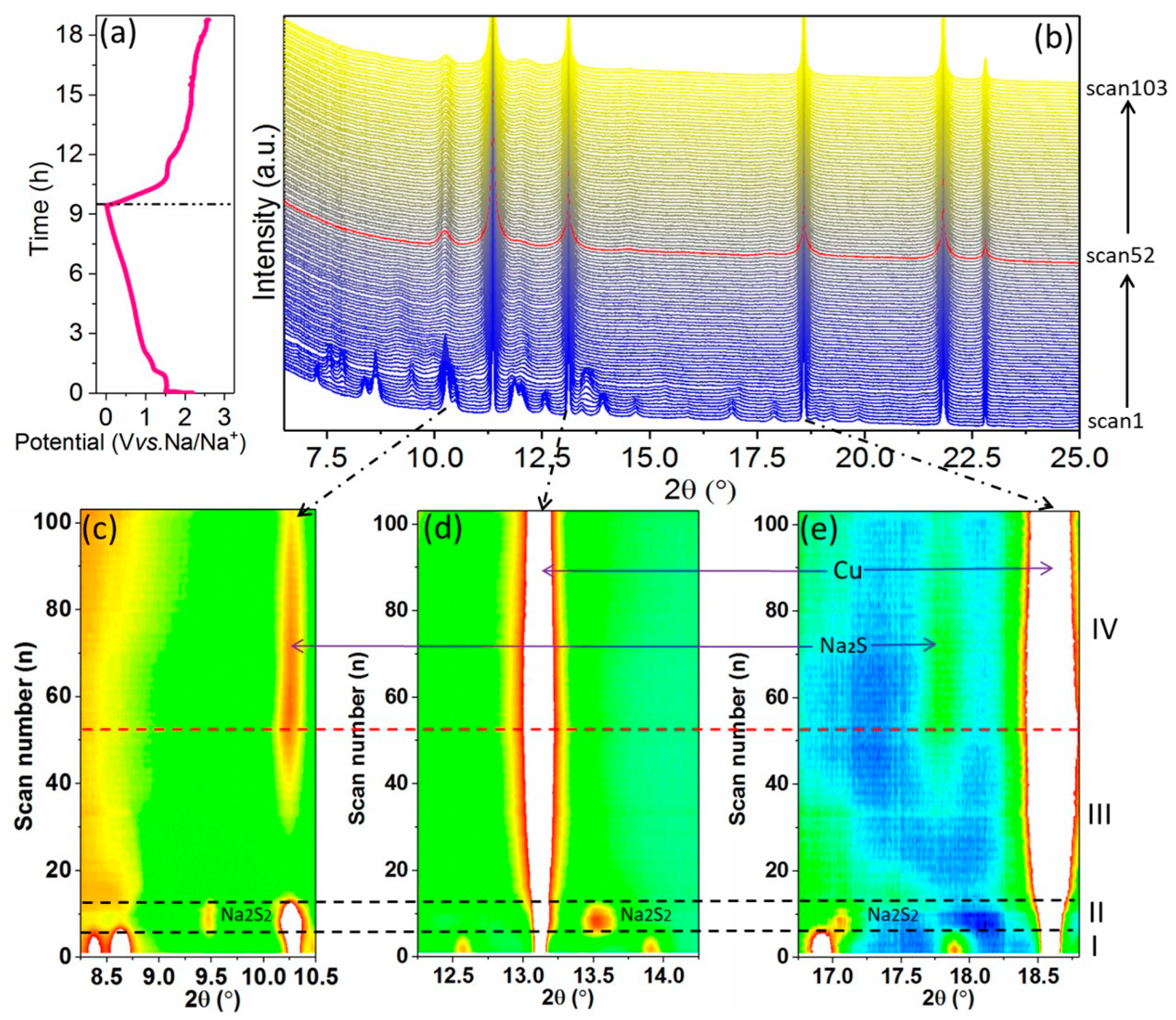
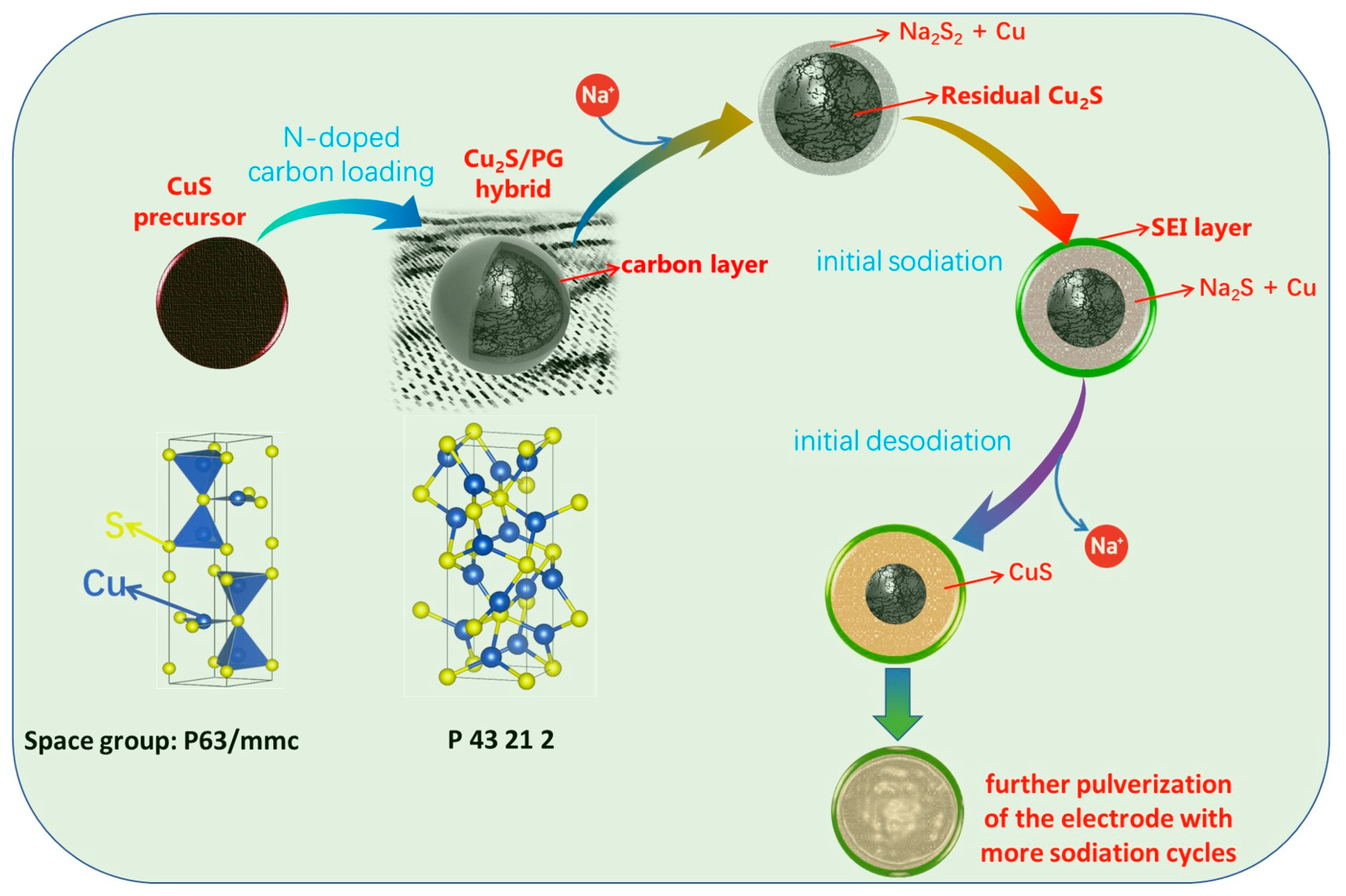
3.3. Mechanism Study for Initial Na+ Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Narins, T.P. The battery business: Lithium availability and the growth of the global electric car industry. Extr. Ind. Soc. 2017, 4, 321–328. [Google Scholar] [CrossRef]
- Deng, W.; Liu, W.; Zhu, H.; Chen, L.; Liao, H.; Chen, H. Click-chemistry and ionic cross-linking induced double cross-linking ionogel electrolyte for flexible lithium-ion batteries. J. Energy Storage 2023, 72, 108509. [Google Scholar] [CrossRef]
- Hannan, M.A.; Lipu, M.S.H.; Hussain, A.; Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations. Renew. Sustain. Energy Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, Y.; Xu, D.; Zhou, W.; Xiang, K.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Hasa, I.; Hassoun, J.; Passerini, S. Nanostructured Na-ion and Li-ion anodes for battery application: A comparative overview. Nano Res. 2017, 10, 3942–3969. [Google Scholar] [CrossRef]
- Cai, J.; Reinhart, B.; Eng, P.; Liu, Y.; Sun, C.-J.; Zhou, H.; Ren, Y.; Meng, X. Nitrogen-doped graphene-wrapped Cu2S as a superior anode in sodium-ion batteries. Carbon N. Y. 2020, 170, 430–438. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. Sodium-Ion Battery Materials and Electrochemical Properties Reviewed. Adv. Energy Mater. 2018, 8, 1800079. [Google Scholar] [CrossRef]
- Fan, H.H.; Li, H.H.; Huang, K.C.; Fan, C.Y.; Zhang, X.Y.; Wu, X.L.; Zhang, J.P. Metastable Marcasite-FeS2 as a New Anode Material for Lithium Ion Batteries: CNFs-Improved Lithiation/Delithiation Reversibility and Li-Storage Properties. ACS Appl. Mater. Interfaces 2017, 9, 10708–10716. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Bresser, D.; Ji, Y.; Geiger, D.; Kaiser, U.; Streb, C.; Varzi, A.; Passerini, S. Cobalt Disulfide Nanoparticles Embedded in Porous Carbonaceous Micro-Polyhedrons Interlinked by Carbon Nanotubes for Superior Lithium and Sodium Storage. ACS Nano 2018, 12, 7220–7231. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Guo, F.; Ma, L.; Ren, Y.; Wu, T.; Zuo, P.; Yin, G.; Wang, J. Understanding the initial irreversibility of metal sulfides for sodium-ion batteries via operando techniques. Nano Energy 2018, 43, 184–191. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Jia, M.; Xu, J.; Pan, E. One-pot hydrothermal synthesis of ZnS quantum dots/graphene hybrids as a dual anode for sodium ion and lithium ion batteries. Appl. Surf. Sci. 2018, 437, 375–383. [Google Scholar] [CrossRef]
- Gao, S.; Chen, G.; Dall’Agnese, Y.; Wei, Y.; Gao, Z.; Gao, Y. Flexible MnS-Carbon Fiber Hybrids for Lithium-Ion and Sodium-Ion Energy Storage. Chem.-A Eur. J. 2018, 24, 13535–13539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zou, R.; Xia, W.; Ma, J.; Qiu, B.; Mahmood, A.; Zhao, R.; Yang, Y.; Xia, D.; Xu, Q. Facile Synthesis of Ultrasmall CoS2 Nanoparticles within Thin N-Doped Porous Carbon Shell for High Performance Lithium-Ion Batteries. Small 2015, 11, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Ma, C.; Ji, G.; Xu, C.; Xu, J.; Meng, Y.S.; Wang, T.; Lee, J.Y. Layered SnS2-reduced graphene oxide composite–A high-capacity, high-rate, and long-cycle life sodium-ion battery anode material. Adv. Mater. 2014, 26, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.; Geaney, H.; Bree, G.; Stokes, K.; Connolly, S.; Zaworotko, M.J.; Ryan, K.M. Copper Sulfide (CuxS) Nanowire-in-Carbon Composites Formed from Direct Sulfurization of the Metal-Organic Framework HKUST-1 and Their Use as Li-Ion Battery Cathodes. Adv. Funct. Mater. 2018, 28, 1800587. [Google Scholar] [CrossRef]
- Park, H.; Kwon, J.; Choi, H.; Shin, D.; Song, T.; Lou, X.W.D. Unusual Na+ Ion Intercalation/Deintercalation in Metal-Rich Cu1.8S for Na-Ion Batteries. ACS Nano 2018, 12, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Su, D.; Wang, X.; Wu, S.; Zhou, L.; Shi, Y.; Fang, S.; Cheng, H.-M.; Li, F. CuS Microspheres with Tunable Interlayer Space and Micropore as a High-Rate and Long-Life Anode for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1800930. [Google Scholar] [CrossRef]
- Evans, H.T., Jr. Djurleite (Cu1.94S) and Low Chalcocite (Cu2S): New Crystal Structure Studies. Science 1979, 203, 356–358. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, M.; Xu, H.; Liu, W.; Hei, J.; Su, L.; Wang, L. Cu2S@N,S Dual-Doped Carbon Matrix Hybrid as Superior Anode Materials for Lithium/Sodium ion Batteries. ChemElectroChem 2018, 5, 2135–2141. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, B.; Zhu, Y.F.; Ma, X.Z.; Lang, X.Y. Synthesis of Cu2S/carbon composites with improved lithium storage performance. Int. J. Hydrogen Energy 2015, 40, 670–674. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Lu, T.; Qin, W.; Yao, Y.; Pan, L. Significantly Improved Sodium-Ion Storage Performance of CuS Nanosheets Anchored into Reduced Graphene Oxide with Ether-Based Electrolyte. ACS Appl. Mater. Interfaces 2017, 9, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, C.; Xie, J.; Zhang, B.; Miao, L.; Cai, J.; Huang, Y.; Zhang, L. A Hierarchical N/S-Codoped Carbon Anode Fabricated Facilely from Cellulose/Polyaniline Microspheres for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501929. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Geiger, D.; Kaiser, U.; Zhang, H.; Kim, G.T.; Diemant, T.; Behm, R.J.; Varzi, A.; Passerini, S. ZnO/ZnFe2O4/N-doped C micro-polyhedrons with hierarchical hollow structure as high-performance anodes for lithium-ion batteries. Nano Energy 2017, 42, 341–352. [Google Scholar] [CrossRef]
- Tian, G.; Huang, C.; Luo, X.; Zhao, Z.; Peng, Y.; Gao, Y.; Tang, N.; Dsoke, S. Study of the Lithium Storage Mechanism of N-Doped Carbon-Modified Cu2S Electrodes for Lithium-Ion Batteries. Chem.-A Eur. J. 2021, 27, 13774–13782. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Scheiba, F.; Pfaffmann, L.; Fiedler, A.; Chakravadhanula, V.S.K.; Balachandran, G.; Zhao, Z.; Ehrenberg, H. Electrostatic self-assembly of LiFePO4 cathodes on a three-dimensional substrate for lithium ion batteries. Electrochim. Acta 2018, 283, 1375–1383. [Google Scholar] [CrossRef]
- Mousavi-kamazani, M.; Salavati-niasari, M.; Ramezani, M. Preparation and Characterization of Cu2S Nanoparticles Via Ultrasonic Method. J. Clust. Sci. 2013, 24, 927–934. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T. Sun, Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Hurma, T.; Kose, S. XRD Raman analysis and optical properties of CuS nanostructured film. Optik 2016, 127, 6000–6006. [Google Scholar] [CrossRef]
- Ishii, M.; Shibata, K.; Nozaki, H. Anion distributions and phase transitions in CuS1−xSex(x = 0–1) studied by raman spectroscopy. J. Solid State Chem. 1993, 105, 504–511. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Bai, J.; Jiang, X. A facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar] [CrossRef] [PubMed]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon N. Y. 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.; Zhao, C.; Wang, Z.; Chen, L.; Yang, Y. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Yu, D.; Li, M.; Yu, T.; Wang, C.; Zeng, Y.; Hu, X.; Chen, G.; Yang, G.; Du, F. Nanotube-assembled pine-needle-like CuS as an effective energy booster for sodium-ion storage. J. Mater. Chem. A 2019, 7, 10619–10628. [Google Scholar] [CrossRef]
- Zhao, D.; Yin, M.; Feng, C.; Zhan, K.; Jiao, Q.; Li, H.; Zhao, Y. Rational Design of N-Doped CuS@C Nanowires toward High-Performance Half/Full Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 11317–11327. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Lu, X.; He, X.; Jiang, N.; Huo, Y.; Xu, C.; Lin, D. Metal-organic framework derived in-situ nitrogen-doped carbon-encapsulated CuS nanoparticles as high-rate and long-life anode for sodium ion batteries. J. Alloys Compd. 2021, 854, 157132. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Passerini, S. Nanocrystalline TiO2 (B) as Anode Material for Sodium-Ion Batteries. J. Electrochem. Soc. 2014, 162, A3052–A3058. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, G.; Sarapulova, A.; Trouillet, V.; Fu, Q.; Geckle, U.; Ehrenberg, H.; Dsoke, S. Elucidating the energy storage mechanism of ZnMn2O4 as promising anode for Li-ion batteries. J. Mater. Chem. A 2018, 6, 19381–19392. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, G.; Sarapulova, A.; Melinte, G.; Gómez-Urbano, J.L.; Li, C.; Liu, S.; Welter, E.; Etter, M.; Dsoke, S. Mechanism Study of Carbon Coating Effects on Conversion-Type Anode Materials in Lithium-Ion Batteries: Case Study of ZnMn2O4 and ZnO-MnO Composites. ACS Appl. Mater. Interfaces 2019, 11, 29888–29900. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Li, Y.; Zhang, L.; Yang, X. Hollow heterostructured Cu1.96S/NiS microspheres coupled with nitrogen/sulfur dual-doped carbon realizing superior reaction kinetics and sodium storage. New J. Chem. 2023, 47, 14746–14757. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Kohl, M.; Althues, H.; Kaskel, S. Shuttle suppression in room temperature sodium–sulfur batteries using ion selective polymer membranes. Chem. Commun. 2014, 50, 3208. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhao, Z.; Sarapulova, A.; Das, C.; Zhu, L.; Liu, S.; Missiul, A.; Welter, E.; Maibach, J.; Dsoke, S. Understanding the Li-ion storage mechanism in a carbon composited zinc sulfide electrode. J. Mater. Chem. A 2019, 7, 15640–15653. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, Z.; Yang, D.; Song, K.; Mi, L.; Zhai, Y.; Guan, X.; Chen, W. Enhanced interfacial compatibility of FeS@N,S-C anode with ester-based electrolyte enables stable sodium-ion full cells. J. Energy Chem. 2022, 68, 27–34. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, M.; Zhang, L.; Li, Y.; Li, Y.; Tan, C.; Zheng, F.; Huang, Y.; Wang, H.; Li, Q. FeSe2@C Microrods as a Superior Long-Life and High-Rate Anode for Sodium Ion Batteries. ACS Nano 2020, 14, 17683–17692. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. In situ growth of N/O-codoped carbon nanotubes in wood-derived thick carbon scaffold to boost the capacitive performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131018. [Google Scholar] [CrossRef]
- Kim, N.R.; Choi, J.; Yoon, H.J.; Lee, M.E.; Son, S.U.; Jin, H.J.; Yun, Y.S. Conversion Reaction of Copper Sulfide Based Nanohybrids for Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2017, 5, 9802–9808. [Google Scholar] [CrossRef]
- Tao, H.; Tang, Y.; Zhou, M.; Wang, R.; Wang, K.; Li, H.; Jiang, K. Porous Copper Sulfide Microflowers Grown In Situ on Commercial Copper Foils as Advanced Binder-Free Electrodes with High Rate and Long Cycle Life for Sodium-Ion Batteries. ChemElectroChem 2021, 8, 157–163. [Google Scholar] [CrossRef]
- Kim, H.; Sadan, M.K.; Kim, C.; Choe, S.H.; Cho, K.K.; Kim, K.W.; Ahn, J.H.; Ahn, H.J. Simple and scalable synthesis of CuS as an ultrafast and long-cycling anode for sodium ion batteries. J. Mater. Chem. A 2019, 7, 16239–16248. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, X.; Wang, Y.; Tian, G.; Zhao, Z. Interface Optimization of Cu2S Nanoparticles by Loading N-Doped Carbon for Efficient Sodium-Ion Storage. Sustainability 2023, 15, 16846. https://doi.org/10.3390/su152416846
Wang J, Chen X, Wang Y, Tian G, Zhao Z. Interface Optimization of Cu2S Nanoparticles by Loading N-Doped Carbon for Efficient Sodium-Ion Storage. Sustainability. 2023; 15(24):16846. https://doi.org/10.3390/su152416846
Chicago/Turabian StyleWang, Jinhui, Xue Chen, Yang Wang, Guiying Tian, and Zijian Zhao. 2023. "Interface Optimization of Cu2S Nanoparticles by Loading N-Doped Carbon for Efficient Sodium-Ion Storage" Sustainability 15, no. 24: 16846. https://doi.org/10.3390/su152416846
APA StyleWang, J., Chen, X., Wang, Y., Tian, G., & Zhao, Z. (2023). Interface Optimization of Cu2S Nanoparticles by Loading N-Doped Carbon for Efficient Sodium-Ion Storage. Sustainability, 15(24), 16846. https://doi.org/10.3390/su152416846







