The Occurrence and Fate of Microplastics in Wastewater Treatment Plants in South Africa and the Degradation of Microplastics in Aquatic Environments—A Critical Review
Abstract
:1. Introduction
1.1. Nature of MPs Found in the Aquatic Environment
1.1.1. Primary MPs
1.1.2. Secondary MPs
1.2. Degradation of Polymers in the Environment
1.2.1. Physicochemical Degradation
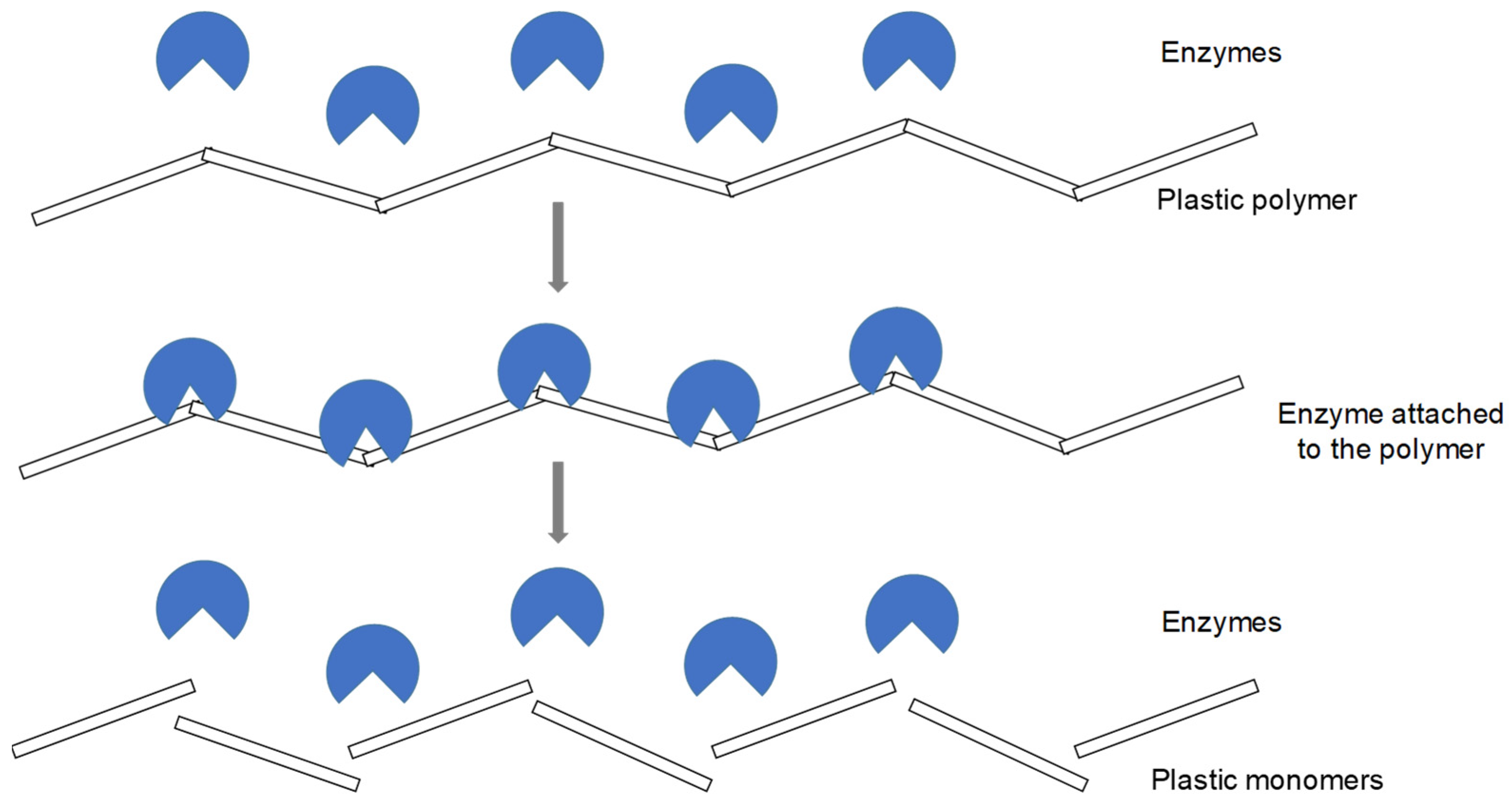
1.2.2. Biodegradation
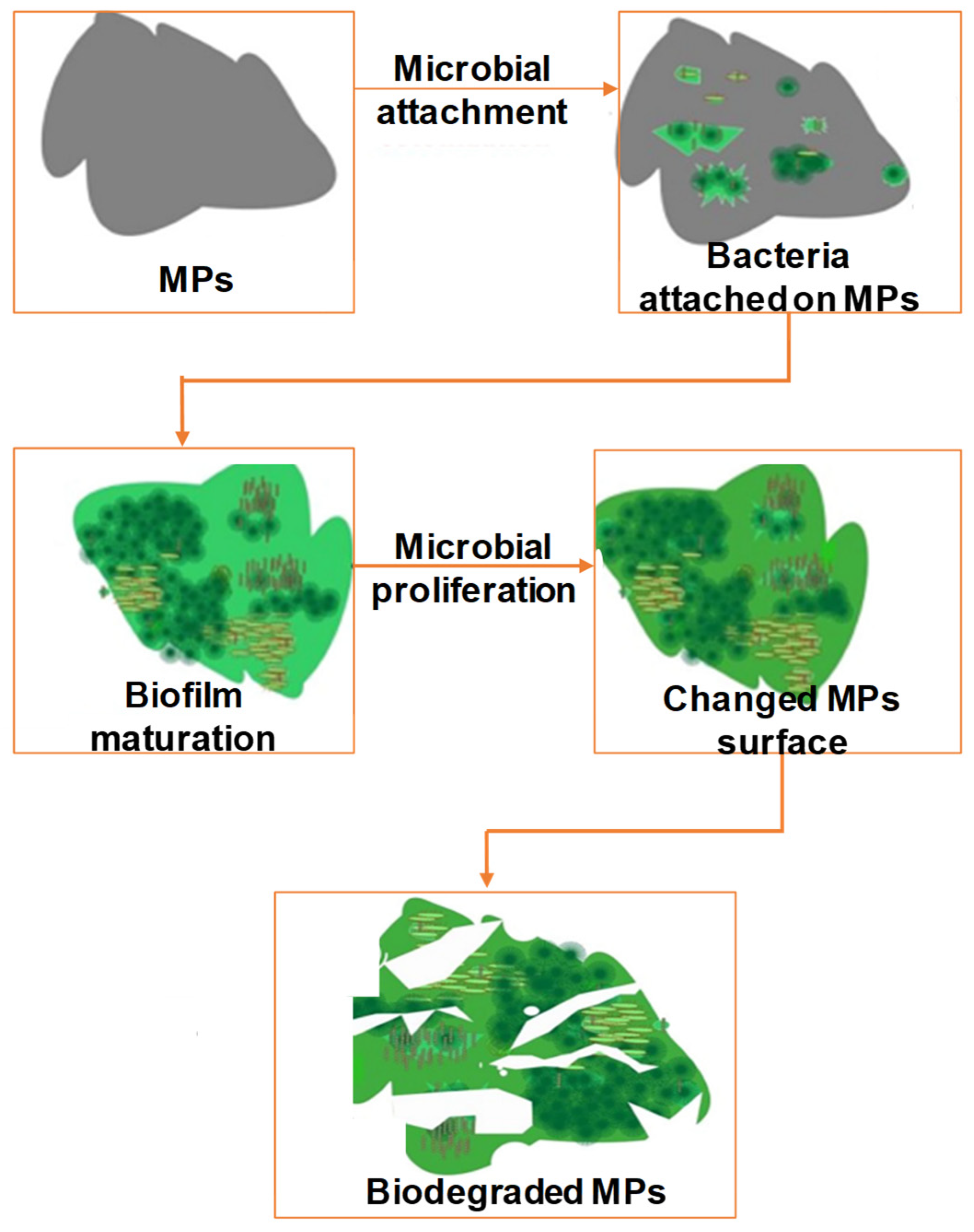
Effects of Biofilms on MP Degradation
1.3. Recent Trends of Plastic Pollution in South Africa
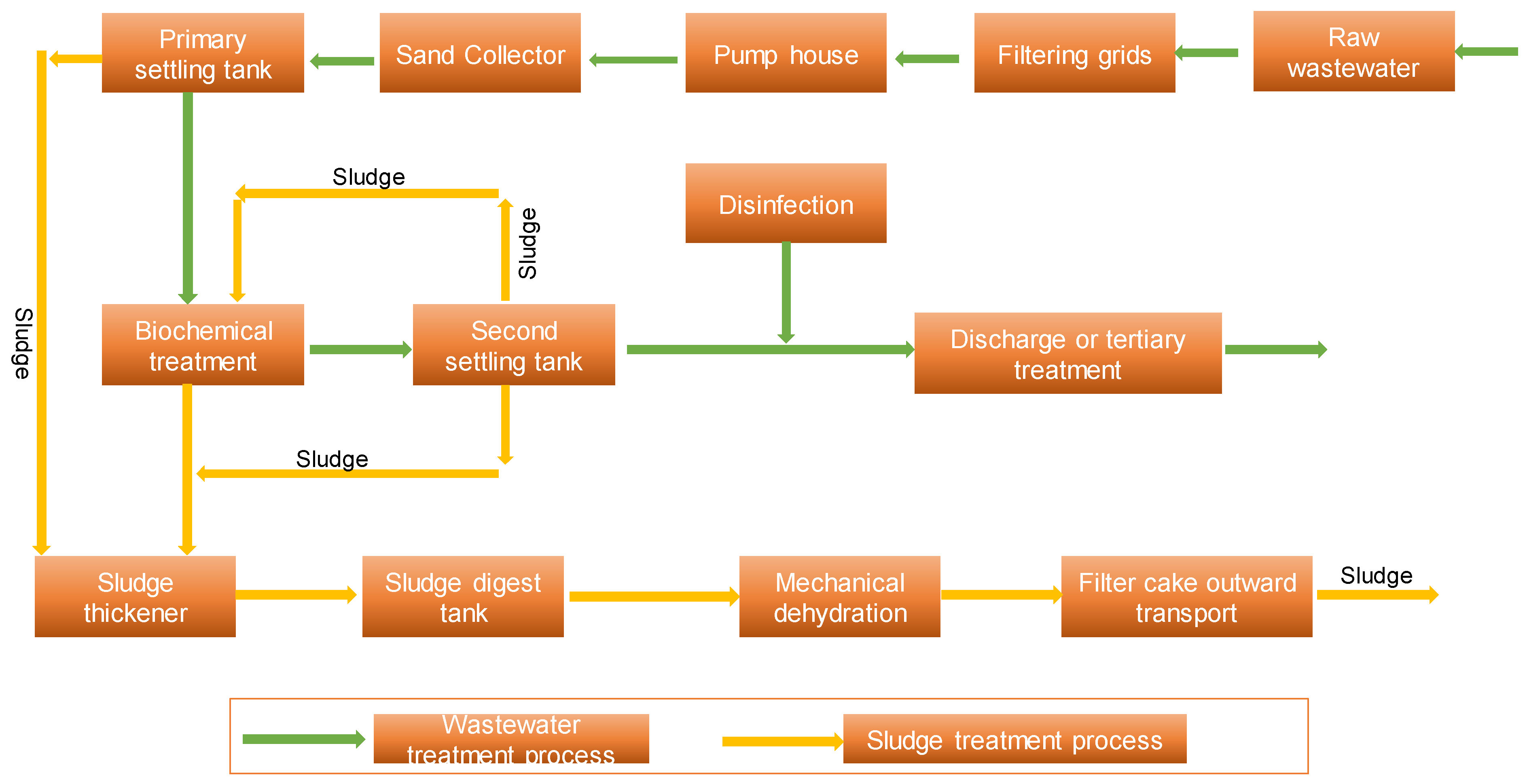
1.4. The Status of WWTPs in South Africa and Their Capabilities for the Removal of MPs
Effects of WWTPs on MPs
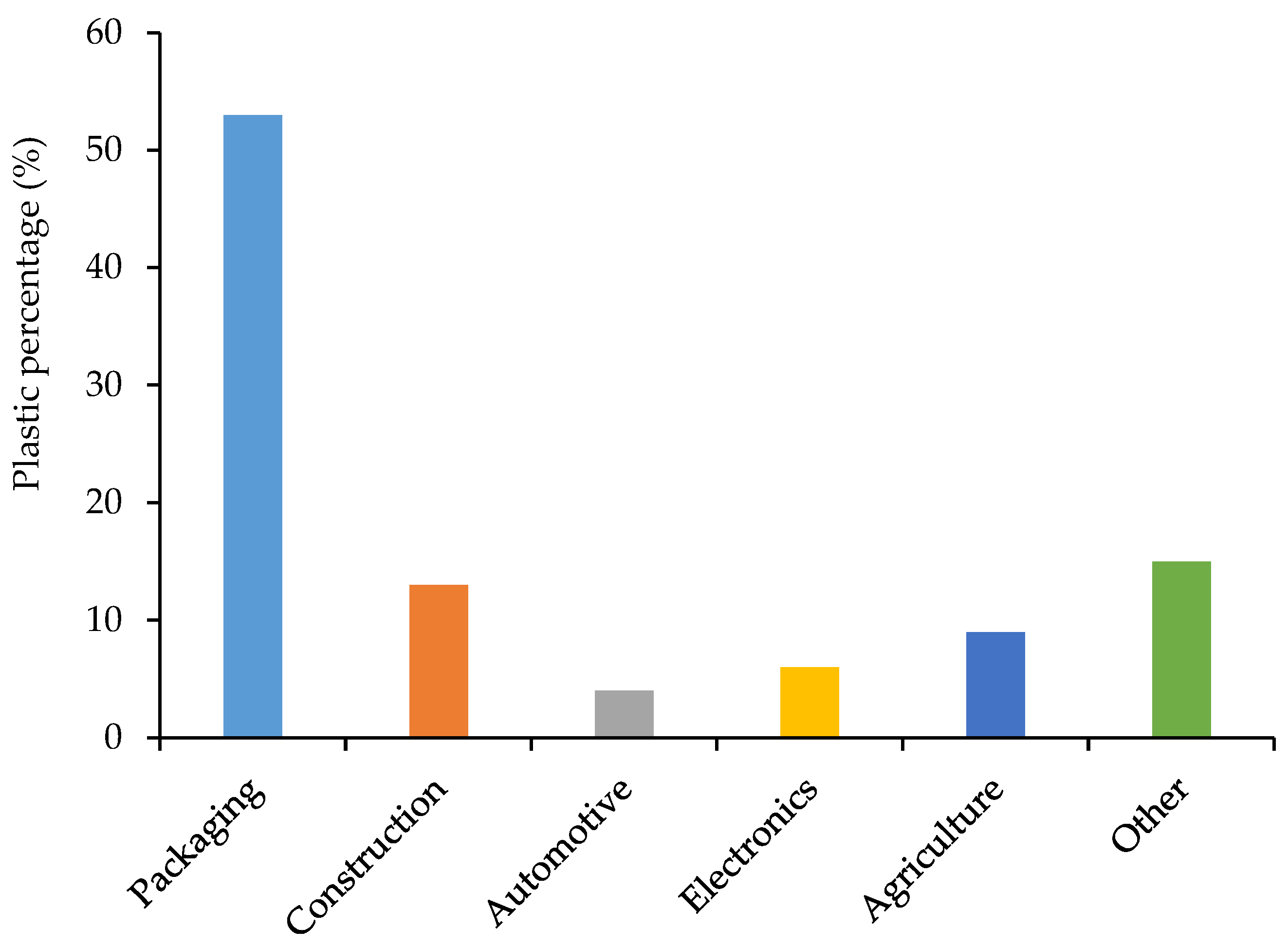
1.5. Different Polymers Used in Plastic Production in South Africa
1.6. Contamination of MPs in Groundwater and Drinking Water
Remediation of MPs in Freshwater
2. Conclusions
3. Limitations and Perspectives
4. Recommendations and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heller, M.C.; Mazor, M.H.; Keoleian, G.A. Plastics in the US: Toward a material flow characterization of production, markets and end of life. Environ. Res. Lett. 2020, 15, 094034. [Google Scholar] [CrossRef]
- Amir, A.; Farhad, S.M.; Haque, N.; Kumar, B. Chemosphere Understanding the fate of nano-plastics in wastewater treatment plants and their removal using membrane processes. Chemosphere 2021, 284, 131430. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Kalčíková, G. The Response of Duckweed Lemna minor to Microplastics and Its Potential Use as a Bioindicator of Microplastic Pollution. Plants 2022, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; Duarte, A.C.; Rocha-santos, T.A.P. Analytica Chimica Acta Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S. Environmental Technology & Innovation Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Raza, M.; Lee, J.; Cha, J. Microplastics in soil and freshwater: Understanding sources, distribution, potential impacts, and regulations for management. Sci. Prog. 2022, 105, 368504221126676. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persisstence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]
- Du, S.; Zhu, R.; Cai, Y.; Xu, N.; Yap, P.; Zhang, Y. RSC Advances Environmental fate and impacts of microplastics in aquatic ecosystems: A review. RSC Adv. 2021, 11, 15762–15784. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Xiao, S.; Brahney, J.; Mahowald, N. Long-Distance Atmospheric Transport of Microplastic Bers Depends on Their Shapes. 2023. Available online: https://assets.researchsquare.com/files/rs-2416912/v1_covered_594e9af3-390e-44f1-bf05-8b7ae08ce9b9.pdf?c=1696004377 (accessed on 16 November 2023).
- Huang, Y.; He, T.; Yan, M.; Yang, L.; Gong, H.; Wang, W. Atmospheric transport and deposition of microplastics in a subtropical urban environment. J. Hazard. Mater. 2021, 416, 126168. [Google Scholar] [CrossRef] [PubMed]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and accumulation of nano/microplastics in plants: A critical review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. Handb. Environ. Chem. 2020, 95, 143–159. [Google Scholar] [CrossRef]
- Khoaele, K.K.; Gbadeyan, O.J.; Chunilall, V.; Sithole, B. The Devastation of Waste Plastic on the Environment and Remediation Processes: A Critical Review. Sustainability 2023, 15, 5233. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, M.; Jin, F.; Chen, M.; Liu, Z.; He, S.; Li, F.; Yang, L.; Fang, C.; Mu, J. Coupled effects of urbanization level and dam on microplastics in surface waters in a coastal watershed of Southeast China. Mar. Pollut. Bull. 2020, 154, 111089. [Google Scholar] [CrossRef]
- Strokal, M.; Bai, Z.; Franssen, W.; Hofstra, N.; Koelmans, A.A.; Ludwig, F.; Ma, L.; van Puijenbroek, P.; Spanier, J.E.; Vermeulen, L.C.; et al. Urbanization: An increasing source of multiple pollutants to rivers in the 21st century. NPJ Urban Sustain. 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Eom, J.; Yu, S.; Asrar, G.R. Projecting Global Urban Area Growth Through 2100 Based on Historical Time Series Data and Future Shared Socioeconomic Pathways. Earth’s Future 2019, 7, 351–362. [Google Scholar] [CrossRef]
- Flörke, M.; Schneider, C.; McDonald, R.I. Water competition between cities and agriculture driven by climate change and urban growth. Nat. Sustain. 2018, 1, 51–58. [Google Scholar] [CrossRef]
- Pal, P.; Thakura, R. Pharmaceutical waste treatment and disposal of concentrated rejects: A Review. Int. J. Eng. Technol. Sci. Res. 2017, 4, 130–155. [Google Scholar]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M. Integrated and Consolidated Review of Plastic Waste Management and Bio-Based Biodegradable Plastics: Challenges and Opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Jemec Kokalj, A.; Kuehnel, D.; Puntar, B.; Žgajnar Gotvajn, A.; Kalčikova, G. An exploratory ecotoxicity study of primary microplastics versus aged in natural waters and wastewaters. Environ. Pollut. 2019, 254, 112980. [Google Scholar] [CrossRef] [PubMed]
- van Wezel, A.; Caris, I.; Kools, S.A.E. Release of primary microplastics from consumer products to wastewater in the Netherlands. Environ. Toxicol. Chem. 2016, 35, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environmentA review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef]
- Dalziel, R.C.; de Klerk, N.; Bevan-Dye, A. Factors Influencing South African Female Generation Y Students’ Purchase Behaviour of Beauty Products. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2016. [Google Scholar]
- Gebashe, F.C.; Naidoo, D.; Amoo, S.O. Cosmeceuticals: A Newly Expanding Industry in South Africa. Cosmetics 2022, 9, 77. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Li, P.; Wang, Y.; Yang, J.; Zhang, W. Environmental Science Micro-nanobubble aeration promotes senescence of submerged macrophytes with low total antioxidant capacity in urban landscape water. Environ. Sci. Water Res. Technol. 2019, 6, 523–531. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Yurtsever, M. Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics. J. Agric. Environ. Ethics 2019, 32, 459–478. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Environmental Technology & Innovation Plastics and microplastics: A threat to environment. Environ. Technol. Innov. 2019, 14, 100352. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil. Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Efimova, I.; Bagaeva, M.; Bagaev, A.; Kileso, A.; Chubarenko, I.P. Secondary Microplastics Generation in the Sea Swash Zone With Coarse Bottom Sediments: Laboratory Experiments. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Unuabonah, I.E. The pathways of microplastics contamination in raw and drinking water. J. Water Process Eng. 2021, 41, 102073. [Google Scholar] [CrossRef]
- Nair, H.T.; Perumal, S. Trophic Transfer and Accumulation of Microplastics in Freshwater Ecosystem: Risk to Food Security and Human Health. Int. J. Ecol. 2022, 2022, 1234078. [Google Scholar] [CrossRef]
- Tiwari, N.; Santhiya, D.; Sharma, J.G. Microbial remediation of micro-nano plastics: Current knowledge and future trends. Environ. Pollut. 2020, 265, 115044. [Google Scholar] [CrossRef]
- Park, H.; Park, B. Review of Microplastic Distribution, Toxicity, Analysis Methods, and Removal Technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Kim, N.; Lee, J.; Yoon, Y. Potential of Adsorption of Diverse Environmental Contaminants onto Microplastics. Water 2022, 14, 4086. [Google Scholar] [CrossRef]
- Tseng, L.Y.; You, C.; Vu, C.; Chistolini, M.K.; Wang, C.Y.; Mast, K.; Luo, F.; Asvapathanagul, P.; Gedalanga, P.B.; Eusebi, A.L.; et al. Adsorption of Contaminants of Emerging Concern (CECs) with Potential Mechanisms. Water 2022, 14, 2581. [Google Scholar] [CrossRef]
- Mamitiana, R.; Ding, J.; Zhang, S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical Pollutants Sorbed to Ingested Microbeads from Personal Care Products Accumulate in Fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Ecotoxicology and Environmental Safety Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Predieri, B.; Iughetti, L. New insights on the effects of endocrine-disrupting chemicals on children. J. Pediatr. 2021, 98, S73–S85. [Google Scholar] [CrossRef]
- Balali-mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Y.; Jiang, H.; Wang, H. Environmental Technology & Innovation Adsorption of rhodamine B on polyvinyl chloride, polystyrene, and polyethylene terephthalate microplastics in aqueous environments. Environ. Technol. Innov. 2022, 27, 102495. [Google Scholar] [CrossRef]
- Barrick, A.; Champeau, O.; Chatel, A.; Manier, N.; Northcott, G.; Tremblay, L.A. Plastic additives: Challenges in ecotox hazard assessment. PeerJ 2021, 9, 1–26. [Google Scholar] [CrossRef]
- Prata, J.C.; Costa, J.P.; Lopes, I.; Duarte, A.C. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2019, 702, 134455. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Science of the Total Environment Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, Y.; Shi, Z.; Li, Z.; Liang, X. Comparative Biochemistry and Physiology—Part D Ecotoxicoproteomic assessment of microplastics and plastic additives in aquatic organisms: A review. Comp. Biochem. Physiol. Part D 2020, 36, 100713. [Google Scholar] [CrossRef]
- Zhang, K.; Hossein, A.; Tubi, A.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef]
- Bond, T.; Ferrandiz-mas, V.; Felipe-sotelo, M.; Van Sebille, E.; Bond, T.; Ferrandiz-mas, V.; Felipe-sotelo, M.; Van, E. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: A review The occurrence and degradation of aquatic plastic litter. Crit. Rev. Environ. Sci. Technol. 2018, 48, 685–722. [Google Scholar] [CrossRef]
- Binda, G.; Zanetti, G.; Bellasi, A.; Spanu, D.; Boldrocchi, G.; Bettinetti, R.; Pozzi, A.; Nizzetto, L. Physicochemical and biological ageing processes of (micro) plastics in the environment: A multi-tiered study on polyethylene. Environ. Sci. Pollut. Res. 2023, 30, 6298–6312. [Google Scholar] [CrossRef]
- Ouyang, Z.; Yang, Y.; Zhang, C.; Zhu, S.; Qin, L.; Wang, W.; He, D.; Zhou, Y.; Luo, H.; Qin, F. plastics and plastic-derived chemicals. J. Mater. Chem. A 2021, 9, 13402–13441. [Google Scholar] [CrossRef]
- Luo, L.; Chen, T.; Li, Z.; Zhang, Z.; Zhao, W.; Fan, M. Heteroatom self-doped activated biocarbons from fir bark and their excellent performance for carbon dioxide adsorption. J. CO2 Util. 2018, 25, 89–98. [Google Scholar] [CrossRef]
- Pischedda, A.; Tosin, M.; Degli-innocenti, F. Biodegradation of plastics in soil: The effect of temperature. Polym. Degrad. Stab. 2019, 170, 109017. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics Bio-plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Dolma, J.; Jyot, J.; Sehgal, S. Materials Today: Proceedings Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2020, 39, 4–7. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Science of the Total Environment Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Huo, Y.; Yang, Y. Microbial Degradation and Valorization of Plastic Wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Shilpa; Basak, N.; Meena, S.S. Microbial biodegradation of plastics: Challenges, opportunities, and a critical perspective. Front. Environ. Sci. Eng. 2022, 16, 1–22. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, S.; Ray, S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. 2013, 20, 4339–4355. [Google Scholar] [CrossRef]
- Muthukumar, A.; Veerappapillai, S. Biodegradation of Plastics—A Brief Review. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 204–209. [Google Scholar]
- Razi, A.; Hassimi, O.; Hasan, A.; Hafizuddin, M.; Nur, M.; Ismail, I. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; de Almeida, M.P.; Neves, C.V.; Neto, J.A.B.; da Fonseca, E.M. The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems. Micro 2023, 3, 320–337. [Google Scholar] [CrossRef]
- Sun, X.; Xiang, H.; Xiong, H.; Fang, Y.; Wang, Y. Science of the Total Environment Bioremediation of microplastics in freshwater environments: A systematic review of bio fi lm culture, degradation mechanisms, and analytical methods. Sci. Total Environ. 2023, 863, 160953. [Google Scholar] [CrossRef] [PubMed]
- Plakunov, V.K.; Gannesen, A.V.; Mart, S.V.; Zhurina, M.V. Biocorrosion of Synthetic Plastics: Degradation Mechanisms and Methods of Protection. Microbiology 2020, 89, 647–659. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Science of the Total Environment Distinct community structure and microbial functions of bio fi lms colonizing microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, T.; Oi, T.; Aiso, H.; Suzuki, T.; Okura, T.; Sato, S. Biofilm formation and degradation of commercially available biodegradable plastic films by bacterial consortiums in freshwater environments. Microbes Environ. 2018, 33, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Delacuvellerie, A.; Benali, S.; Cyriaque, V.; Moins, S.; Raquez, J.M.; Gobert, S.; Wattiez, R. Microbial biofilm composition and polymer degradation of compostable and non-compostable plastics immersed in the marine environment. J. Hazard. Mater. 2021, 419, 126526. [Google Scholar] [CrossRef]
- Sangeetha Devi, R.; Rajesh Kannan, V.; Nivas, D.; Kannan, K.; Chandru, S.; Robert Antony, A. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef]
- Bhavsar, P.; Bhave, M.; Webb, H.K. Solving the plastic dilemma: The fungal and bacterial biodegradability of polyurethanes. World J. Microbiol. Biotechnol. 2023, 39, 122. [Google Scholar] [CrossRef]
- Lear, G.; Kingsbury, J.M.; Franchini, S.; Gambarini, V.; Maday, S.D.M.; Wallbank, J.A.; Weaver, L.; Pantos, O. Plastics and the microbiome: Impacts and solutions. Environ. Microbiomes 2021, 16, 1–19. [Google Scholar] [CrossRef]
- Statistics South Africa Mid-Year Population Estimates. 2022; pp. 1–50. Available online: https://www.statssa.gov.za/publications/P0302/MidYear2022.pdf (accessed on 13 February 2023).
- Statistics South Africa Mid-Year Population Estimates. 2018. Available online: http://www.statssa.gov.za/publications/P0302/Media_Presentation.pdf (accessed on 13 February 2018).
- Annual Report. 2022. Available online: https://www.saplasticspact.org.za/2022/12/29/sa-plastics-pact-2021-annual-report-published/ (accessed on 13 February 2023).
- Department of Forestry, Fisheries and the Environment (DFFE). Available online: https://www.dffe.gov.za/plasticpollution (accessed on 1 April 2023).
- Herbig, F.J.W. Talking dirty-effluent and sewage irreverence in South Africa: A conservation crime perspective. Cogent Soc. Sci. 2019, 5, 1701359. [Google Scholar] [CrossRef]
- Smale, Z. Waste Khoro Presentation: Gauteng. 2013. Available online: https://documents.pub/document/gauteng-waste-management-report-waste-khoro-894kb.html?page=1 (accessed on 11 March 2023).
- Government Gazette. 2012, pp. 1–41. Available online: https://cer.org.za/wp-content/uploads/2012/06/municipalwaste_sectorplan.pdf (accessed on 25 September 2023).
- le Grange, L. The Government Role of Local Government in the Recycling of Domestic Waste; North-West University: Potchefstroom, South Africa, 2022. [Google Scholar]
- Aoyi, O.; Onyango, M.; Majozi, T.; Seid, E.; Leswifi, T.; Rwanga, S.; Kesi, J. Water and Wastewater Management in Local Government: Skills Needs and Development Final Report Part II to the Local Government Sector Education and Training (LGSETA). 2015. Available online: https://cdn.lgseta.co.za/resources/performance_monitoring_and_reporting_documents/Water%20%20Wastewater%20Management%20Research%20Report%20II.pdf (accessed on 30 October 2023).
- Edokpayi, J.N.; Enitan-folami, A.M.; Adeeyo, A.O. Chapter 9—Recent Trends and National Policies for Water Provision and Wastewater Treatment in South Africa; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128183397. [Google Scholar]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Iloms, E.; Ololade, O.O.; Ogola, H.J.O. Investigating Industrial Effluent Impact on Municipal Wastewater Treatment Plant in Vaal, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M.; Yang, S.J.; Wang, Q.; Wang, X.C.; Ao, D. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhao, Y.; Lyczko, N.; Nzihou, A. Current Status and Outlook of Odor Removal Technologies in Wastewater Treatment Plant. Waste Biomass Valorization 2019, 10, 1443–1458. [Google Scholar] [CrossRef]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in eastern cape province, south africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Bahamon, D.; Carro, L.; Guri, S.; Vega, L.F. Computational study of ibuprofen removal from water by adsorption in realistic activated carbons. J. Colloid Interface Sci. 2017, 498, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Dalu, T.; Banda, T.; Mutshekwa, T.; Munyai, L.F.; Cuthbert, R.N. Effects of urbanisation and a wastewater treatment plant on microplastic densities along a subtropical river system. Environ. Sci. Pollut. Res. 2021, 28, 36102–36111. [Google Scholar] [CrossRef]
- Lehutso, R.F.; Daso, A.P.; Okonkwo, J.O. Occurrence and environmental levels of triclosan and triclocarban in selected wastewater treatment plants in Gauteng Province, South Africa. Emerg. Contam. 2017, 3, 107–114. [Google Scholar] [CrossRef]
- Cristaldi, A.; Fiore, M.; Zuccarello, P.; Conti, G.O.; Grasso, A.; Nicolosi, I.; Copat, C.; Ferrante, M. Efficiency of wastewater treatment plants (Wwtps) for microplastic removal: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 8014. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, X.; Ye, Z. Removal and generation of microplastics in wastewater treatment plants: A review. J. Clean. Prod. 2021, 291, 125982. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Vilakati, B.; Sivasankar, V.; Nyoni, H.; Mamba, B.B.; Omine, K.; Msagati, T.A.M. The Py—GC-TOF-MS analysis and characterization of microplastics (MPs) in a wastewater treatment plant in Gauteng Province, South Africa. Ecotoxicol. Environ. Saf. 2021, 222, 112478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhao, H.; Sun, H.; Zhao, J.; Sun, Y. Abundance, morphology, and removal efficiency of microplastics in two wastewater treatment plants in Nanjing, China. Environ. Sci. Pollut. Res. 2021, 28, 9327–9337. [Google Scholar] [CrossRef] [PubMed]
- Vardar, S.; Onay, T.T.; Demirel, B.; Kideys, A.E. Evaluation of microplastics removal efficiency at a wastewater treatment plant discharging to the Sea of Marmara. Environ. Pollut. 2021, 289, 117862. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; SCB, S.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hidayaturrahman, H.; Peera, S.G.; Lee, T.G. Elimination of Microplastics at Different Stages in Wastewater Treatment Plants. Water 2022, 14, 2404. [Google Scholar] [CrossRef]
- Vetrimurugan, E.; Jonathan, M.P.; Sarkar, S.K.; Rodríguez-gonzález, F.; Roy, P.D.; Velumani, S.; Sakthi, J.S. Science of the Total Environment Occurrence, distribution and provenance of micro plastics: A large scale quantitative analysis of beach sediments from southeastern coast of South Africa. Sci. Total Environ. 2020, 746, 141103. [Google Scholar] [CrossRef]
- Sparks, C.; Awe, A.; Maneveld, J. Abundance and characteristics of microplastics in retail mussels from Cape. Mar. Pollut. Bull. 2021, 166, 112186. [Google Scholar] [CrossRef]
- Naidoo, T.; Thompson, R.C.; Rajkaran, A. Quantification and characterisation of microplastics ingested by selected juvenile fish species associated with mangroves in KwaZulu-Natal, South Africa. Environ. Pollut. 2019, 257, 113635. [Google Scholar] [CrossRef] [PubMed]
- Nel, H.A.; Froneman, P.W. A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Mar. Pollut. Bull. 2015, 101, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Van Der Lingen, C.D.; Preston-whyte, F.; Bali, A.; Geja, Y.; Harmer, R.; Maes, T.; Turner, A. Microplastics in Commercially Important Small Pelagic Fish Species From South Africa. Front. Mar. Sci. 2020, 7, 574663. [Google Scholar] [CrossRef]
- Apetogbor, K.; Pereao, O.; Sparks, C.; Opeolu, B. Spatio-temporal distribution of microplastics in water and sediment samples of the Plankenburg river, Western Cape, South Africa. Environ. Pollut. 2023, 323, 121303. [Google Scholar] [CrossRef] [PubMed]
- Sparks, C.; Immelman, S. Microplastics in offshore fish from the Agulhas Bank, South Africa. Mar. Pollut. Bull. 2020, 156, 111216. [Google Scholar] [CrossRef] [PubMed]
- Govender, J.; Naidoo, T.; Rajkaran, A.; Cebekhulu, S.; Bhugeloo, A. Serchen Towards Characterising Microplastic Abundance. Water 2020, 12, 2802. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Mei, Q.; Niu, S.; Wang, X.; Xu, H.; Dong, B.; Dai, X.; Zhou, J.L. Science of the Total Environment Aging microplastics in wastewater pipeline networks and treatment processes: Physicochemical characteristics and Cd adsorption. Sci. Total Environ. 2021, 797, 148940. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, Z.; Yang, L.; Liu, X. Chemosphere Effects of particle size and solution chemistry on Triclosan sorption on polystyrene microplastic. Chemosphere 2019, 231, 308–314. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in freshwater sediment: A review on methods, occurrence, and sources. Sci. Total Environ. 2021, 754, 141948. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, Y.; Pan, S. Integration of Green Energy and Advanced Energy-Efficient Technologies for Municipal Wastewater Treatment Plants. Int. J. Environ. Res. Public Health 2019, 16, 1282. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.; Macleod, M. Pathways for degradation of plastics polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Samir, S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Gela, S.M.; Aragaw, T.A. Abundance and Characterization of Microplastics in Main Urban Ditches Across the Bahir Dar City, Ethiopia. Front. Environ. Sci. 2022, 10, 831417. [Google Scholar] [CrossRef]
- Mangala, S.; Norashikin, S.; Shaifuddin, M.; Akizuki, S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Amin, A.A.; Karimi, H.; Pirsaheb, M.; Kim, H.; Hossini, H. Occurrence of microplastic particles in the most popular Iranian bottled mineral water brands and an assessment of human exposure. J. Water Process Eng. 2021, 39, 101708. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C. Plastics in municipal drinking water and wastewater treatment plant effluents: Challenges and opportunities for South Africa—A review. Environ. Sci. Pollut. Res. 2020, 39, 12953–12966. [Google Scholar] [CrossRef]
- Latif, A.; Abbas, A.; Iqbal, J.; Azeem, M.; Asghar, W.; Ullah, R.; Chen, Z. Remediation of Environmental Contaminants Through Phytotechnology. Water Air Soil Pollut. 2023, 234, 1–22. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery from Various Environments—A Review. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 1–19. [Google Scholar] [CrossRef]
- Shehata, S.M.; Badawy, R.K.; Aboulsoud, Y.I.E. Phytoremediation of some heavy metals in contaminated soil. Bull. Natl. Res. Cent. 2019, 43, 189. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Kumar, M.; Hussain, A.; Wang, Q.; Li, R.; Zhang, Z. Ecotoxicology and Environmental Safety Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Heng, S.; Farooq, M.; Munis, H.; Fhad, S.; Yang, X. Phytoremediation of Heavy Metals Assisted by Plant Growth Promoting (PGP) Bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Chemosphere Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef]
- Tripathi, S.; Singh, V.K.; Srivastava, P.; Singh, R.; Devi, R.S.; Kumar, A.; Bhadouria, R. Pollutants: Current Status and Future Directions; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128180952. [Google Scholar]
- Tang, D.; Ho, K. Effects of Microplastics on Agriculture: A Mini-review Effects of Microplastics on Agriculture: A Mini-review. Asian J. Environ. Ecol. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Gong, X.; Shi, G.; Zou, D.; Wu, Z.; Qin, P.; Yang, Y.; Hu, X. Micro- and nano-plastics pollution and its potential remediation pathway by phytoremediation. Planta 2023, 257, 1–13. [Google Scholar] [CrossRef]
- Farraji, H.; Zaman, N.Q.; Tajuddin, R.M.; Faraji, H. Advantages and disadvantages of phytoremediation: A concise review. Environ. Sci. 2016, 2, 69–75. [Google Scholar]
- Ekta, P.; Modi, N.R. A review of phytoremediation. J. Pharmacogn. Phytochem. 2018, 7, 1485–1489. [Google Scholar]
- Lv, M.; Jiang, B.; Xing, Y.; Ya, H.; Zhang, T.; Wang, X. Recent advances in the breakdown of microplastics: Strategies and future prospectives. Environ. Sci. Pollut. Res. 2022, 29, 65887–65903. [Google Scholar] [CrossRef] [PubMed]
- Etim, E. Phytoremediation and Its Mechanisms: A Review. Int. J. Environ. Bioenergy 2012, 2, 120–136. [Google Scholar]
- Sivarajasekar, S.M.N.; Paramasivan, J.S.V.T.; Naushad, M. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Kibria, G.; Imtiaz, N.; Rafat, M.; Huy, S.; Nguyen, Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; Volume 17, ISBN 0123456789. [Google Scholar]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. Current Research in Food Science An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef]
- Department of Forestry, Fisheries and the Environment (DFFE). Available online: https://www.dffe.gov.za/SouthAfricawelcomestheadoptionoftheresolutiontoendplasticpollution (accessed on 26 September 2023).
- Seetal, A.; Mathye, M.; Mahlangu, W.; Godfrey, L. Enabling South Africa’s Water Security through a Circular Economy. 2023. Available online: https://www.circulareconomy.co.za/wp-content/uploads/2023/08/Water_Report-CE-Opportunities.pdf (accessed on 28 September 2023).






| Country | Technologies (Primary and Secondary Treatment) | Removal Efficiency (%) | References |
|---|---|---|---|
| Italy | Biological treatment, sedimentation, sand filter treatment, disinfection | 84 | [102] |
| China | Anaerobic anoxic oxic (A2O), membrane bio-rector (MBR), | 97.67 | [104] |
| cyclic activated sludge technology (CAST), fiber rotary filter (FRF) | 98.46 | ||
| Turkey | Biological and physical treatment | 93.0 | [105] |
| Germany | Gravity filters, membrane reactor | 97 | [106] |
| USA | Sedimentation, filtration, disinfection | 77.7–95.9 | [107] |
| Australia | Biological and UV treatment | 76.61 | [108] |
| South Korea | Physical and biological treatment, filtration | 74.76–91.04 | [109] |
| South Africa | Bio-filtration, disinfection | 79.35 | [103] |
| Province | Source of MPs | Extraction Method | Type of MPs | Type of Polymer | MPs Concentration | Reference |
|---|---|---|---|---|---|---|
| Kwa-Zulu Natal | Beach sediment | H2O2 followed by density separation | Fibers, films | PP, HDPE, LDPE, PES, PS, PET | 84 MPs/g | [110] |
| Western Cape | Mussels | 10% KOH; oven heated at 60 °C for 48 h | Filaments and fragments | PET, PVC, HDPE | 0.04 MPs/g | [111] |
| Kwa-Zulu Natal | Fish | Proteinase K; incubated at 39 °C overnight | Fibers and fragments | PES, PVC | 0.79 ± 1.00/fish | [112] |
| Western Cape, Eastern Cape | Beach sediment | Saline treatment | Fibers and fragments | Not provided | 688.9 ± 9 and 348.2 ± 1449 particles/m2 | [113] |
| Western Cape | Fish | 30% KOH; incubated at 40 °C for 24 h | Fibers and fragments | PP, PS, PE | 1.36 MPs/fish | [114] |
| Western Cape | River water | 10% KOH; incubated at 50 °C for 24 h | Fibers and Films | PE, PP, PET | 5.13 ± 6.62 MP/L | [115] |
| Western Cape | Fish | 10% KOH; incubated at 60 °C for 24 h | Fibers | PE | 2.8–4.6 MPs/fish | [116] |
| Kwa-Zulu Natal | Estuarine sediment and water | Not provided | Fibers, films, foams | PE, PP, PS, PUR | 18.5 ± 34.4/500 g and 11.9 ± 11.2/10,000 L | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malematja, K.C.; Melato, F.A.; Mokgalaka-Fleischmann, N.S. The Occurrence and Fate of Microplastics in Wastewater Treatment Plants in South Africa and the Degradation of Microplastics in Aquatic Environments—A Critical Review. Sustainability 2023, 15, 16865. https://doi.org/10.3390/su152416865
Malematja KC, Melato FA, Mokgalaka-Fleischmann NS. The Occurrence and Fate of Microplastics in Wastewater Treatment Plants in South Africa and the Degradation of Microplastics in Aquatic Environments—A Critical Review. Sustainability. 2023; 15(24):16865. https://doi.org/10.3390/su152416865
Chicago/Turabian StyleMalematja, Kholofelo Clifford, Funzani Asnath Melato, and Ntebogeng Sharon Mokgalaka-Fleischmann. 2023. "The Occurrence and Fate of Microplastics in Wastewater Treatment Plants in South Africa and the Degradation of Microplastics in Aquatic Environments—A Critical Review" Sustainability 15, no. 24: 16865. https://doi.org/10.3390/su152416865
APA StyleMalematja, K. C., Melato, F. A., & Mokgalaka-Fleischmann, N. S. (2023). The Occurrence and Fate of Microplastics in Wastewater Treatment Plants in South Africa and the Degradation of Microplastics in Aquatic Environments—A Critical Review. Sustainability, 15(24), 16865. https://doi.org/10.3390/su152416865






