Research Progress on Iron- and Steelmaking Iste Slag-Based Glass-Ceramics: Preparation and GHG Emission Reduction Potentials
Abstract
:1. Introduction
2. ISWS for the Preparation of Glass-Ceramics
2.1. Blast-Furnace-Slag-Based Glass-Ceramics
2.2. Steel-Slag-Based Glass-Ceramics
2.3. Ferroalloy-Slag-Based Glass-Ceramics
2.3.1. Mn-Fe-Slag-Based Glass-Ceramics
2.3.2. Si-Mn-Slag-Based Glass-Ceramics
2.3.3. Fe-Cr-Slag-Based Glass-Ceramics
2.3.4. Ni-Fe-Slag-Based Glass-Ceramics
3. Characteristics of ISWS for Preparing Glass-Ceramics
3.1. Melt Viscosity
3.2. Crystallization
3.3. Crystallization Temperature and other Properties
4. Corrosion Resistance Analysis of ISWS-Based Glass-Ceramics
5. Sensible Heat Utilization in ISWS-Based Glass-Ceramics
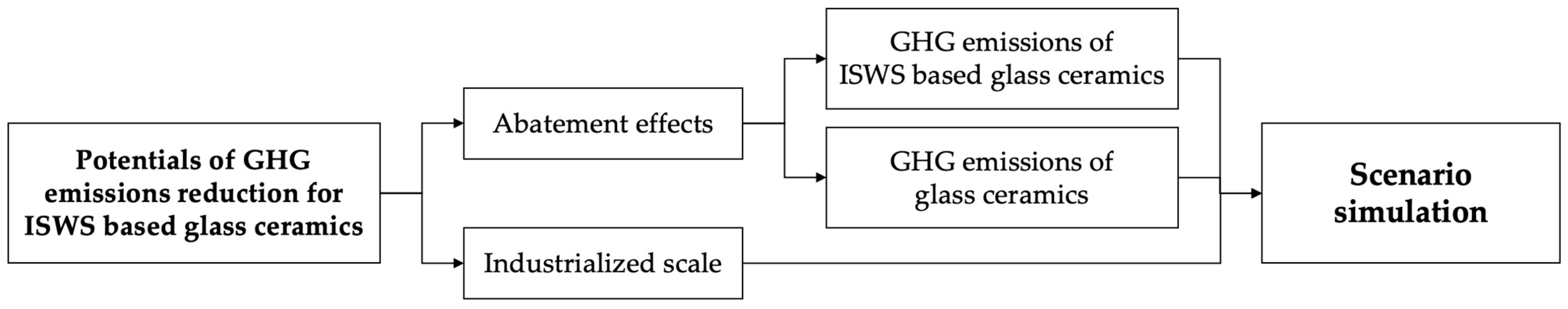
6. Potentials of GHG Emission Reduction for ISWS-Based Glass-Ceramics
- (1)
- Abatement effects
- (2)
- Industrialized Scale
6.1. GHG Emissions from Glass-Ceramic Production
- (1)
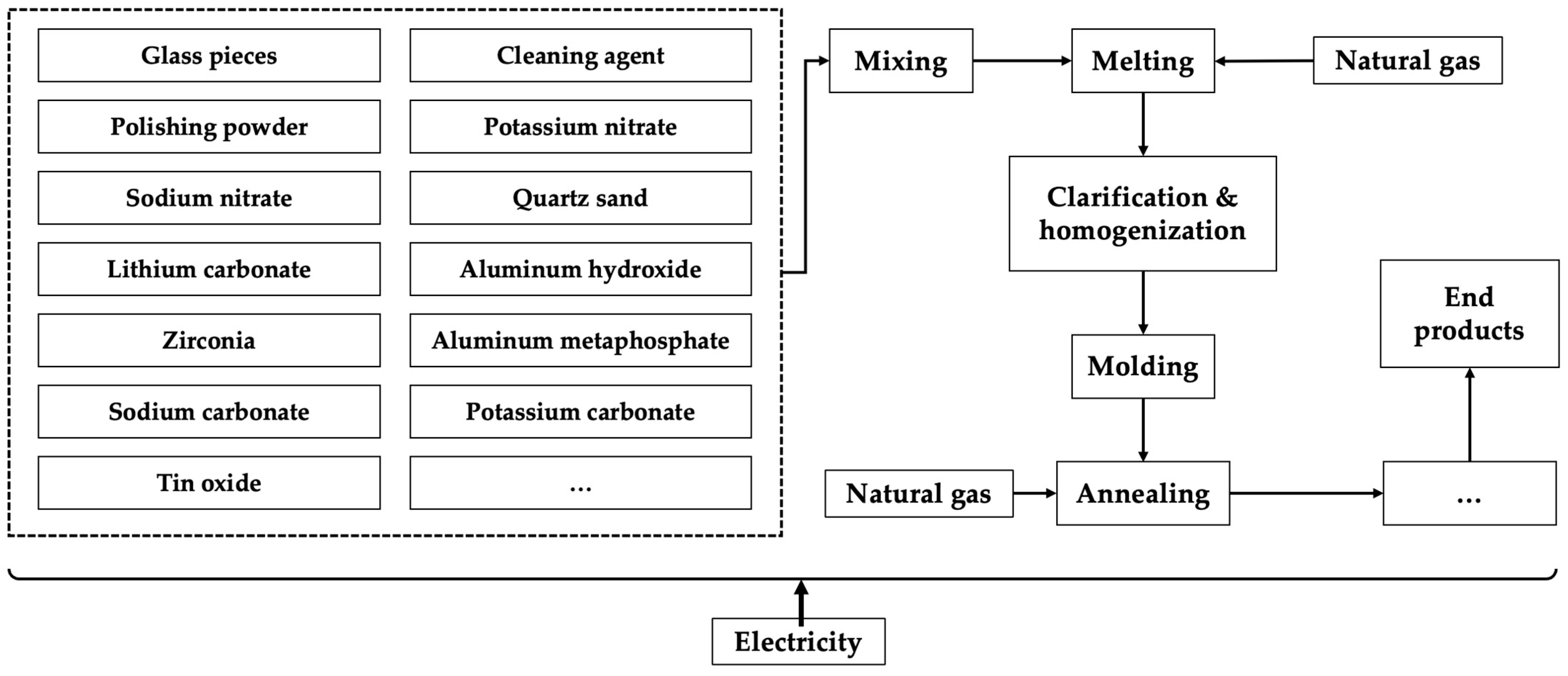
- Primary resource route
- (2)
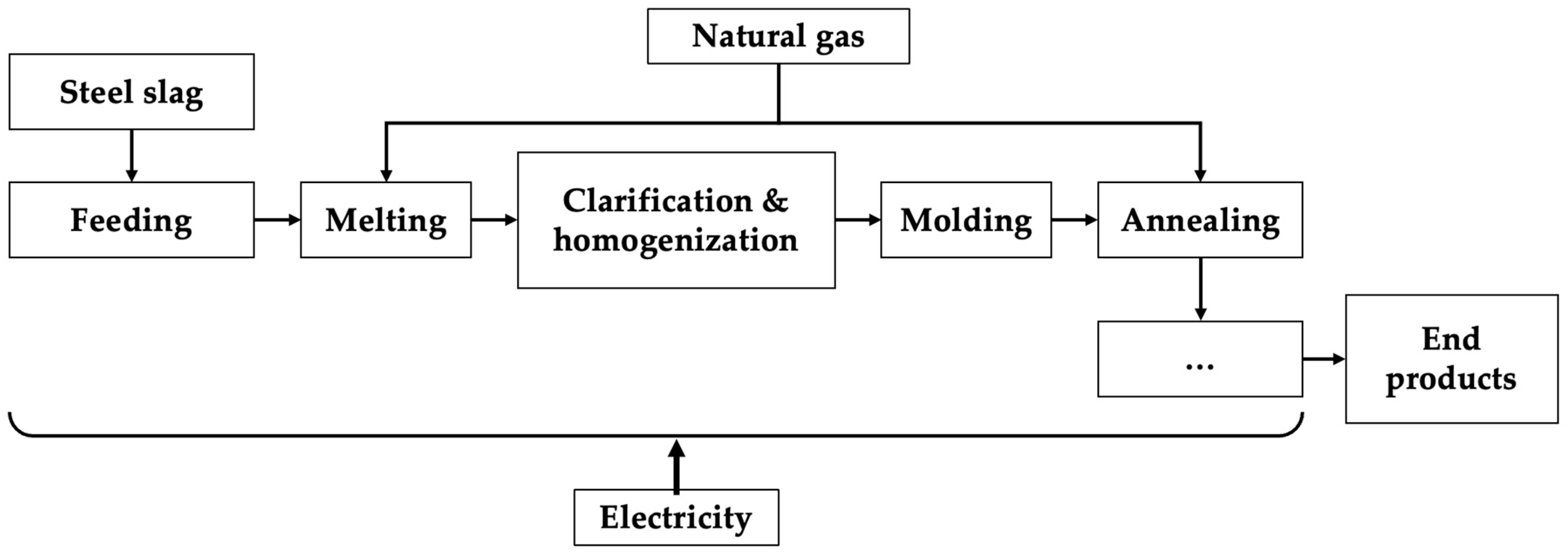
- ISWS-based route
6.2. Future Scenario Settings of Ironmaking and Steelmaking
- (1)
- BF-BOF route
- (2)
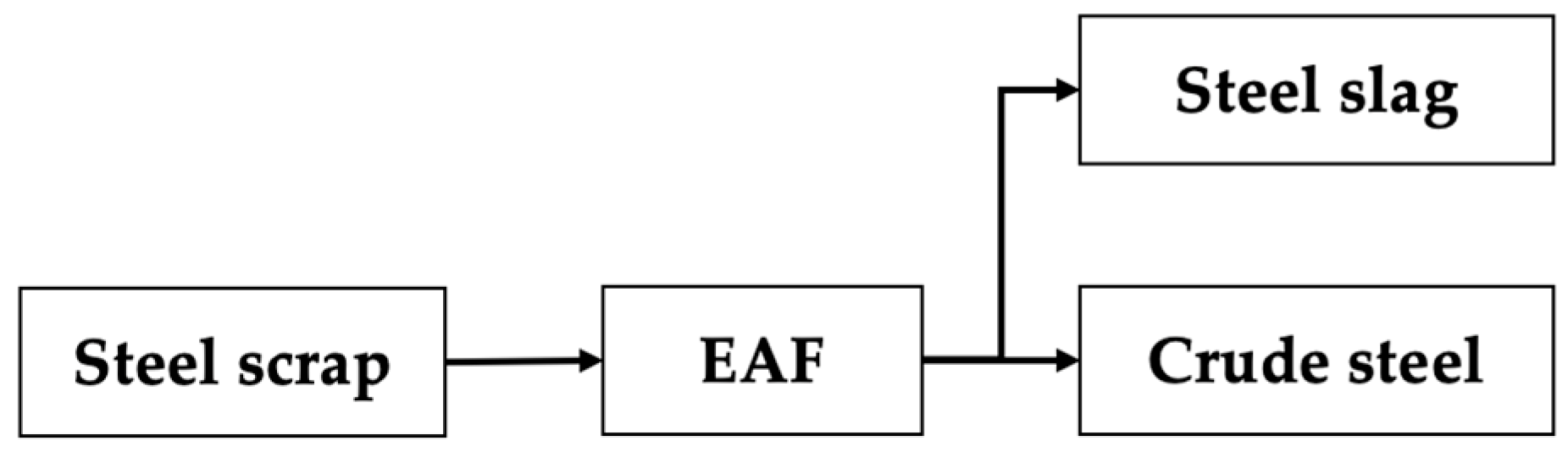
- EAF route
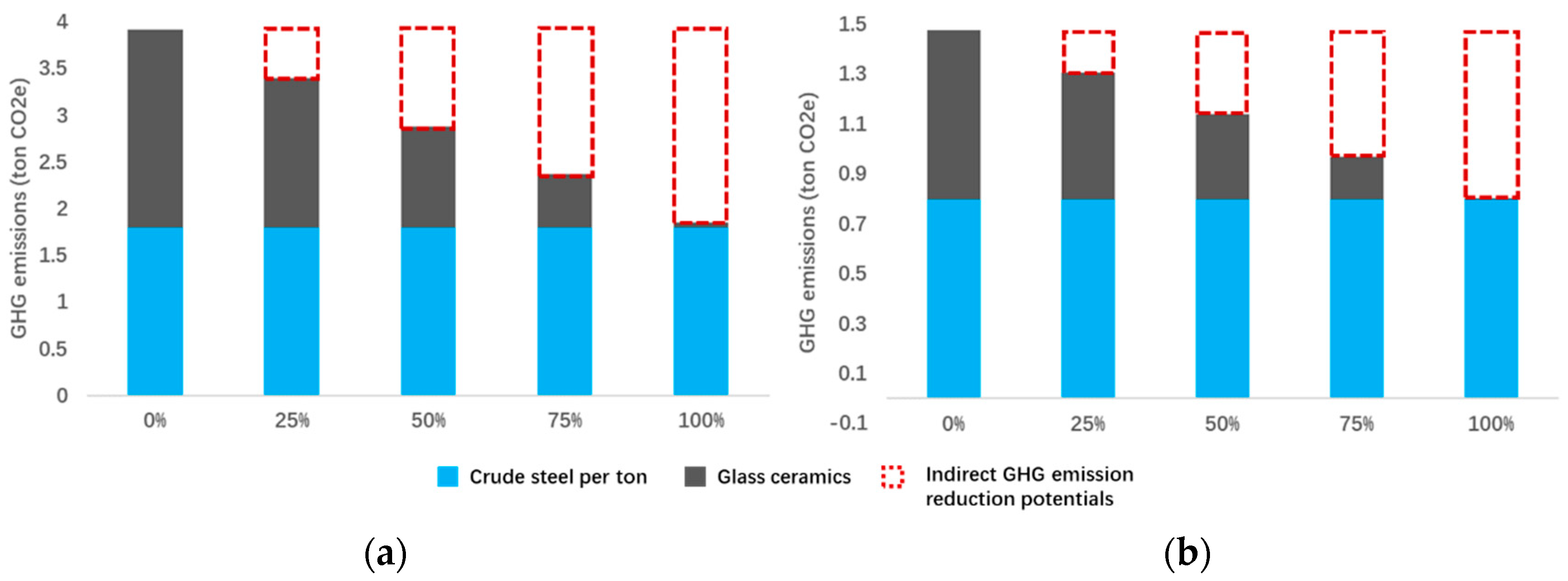
6.3. Potentials of GHG Emission Reduction for ISWS-Based Glass-Ceramics
6.4. Scenario Simulation
- (1)
- From the macro level, the glass-ceramics are assumed to have the same quality regardless of their production routes;
- (2)
- The differences between scenarios for each technology route are only attributed to the application scale of the technology.
7. Conclusions
- Based on a comprehensive summary of the types, different ISWS types are discussed from the perspective of glass-ceramic preparation, including blast furnace slag, steel slag, and ferroalloy slag;
- the inherent characteristics of these slags are analyzed focusing on their impacts on the preparing process of the glass-ceramics, the influence of the alkalinity due to the contents of ISWS on the melt viscosity, crystallization, crystallization temperature, and other properties. The performance of the ISWS-based glass-ceramics is tested according to existing literature;
- the GHG emission reduction of the ISWS-based glass-ceramics is estimated and calculated, and it is found that the ISWS-based glass-ceramics can avoid 0.87~0.91 tons of CO2 emissions compared to the primary resource routes. The scenario simulation suggests if the technology could be fully applied in association with the ironmaking and steelmaking industries, 2.07 and 0.67 tons of indirect CO2 reductions can be achieved for each ton of crude steel production from BF-BOF and EAF routes, respectively.
8. Limitations of This Paper
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, L.B.; Yun, F.; Jia, R.D.; Li, H.; Jia, X.L.; Shi, Y.; Zhang, X.F. Effect of SiO2/MgO ratio on the crystallization behavior, structure, and properties of wollastonite-augite glass-ceramics derived from stainless steel slag. Mater. Chem. Phys. 2020, 239, 122039. [Google Scholar] [CrossRef]
- Jiao, M.; Wu, H.; Li, Z.; Lai, F.; Li, J. Effect of Cr2O3 on the crystallization, structure, and properties of Ti-bearing blast furnace slag-based glass ceramics. J. Asian Ceram. Soc. 2021, 9, 1320–1330. [Google Scholar] [CrossRef]
- Li, Y.; Yi, Y.; Chen, K.; Meng, X. Optimization of performance and composition for glass ceramics prepared from mixing molten slags. Chin. J. Eng. 2019, 41, 1288–1297. [Google Scholar]
- Deng, L.; Jia, R.; Yun, F.; Zhang, X.; Li, H.; Zhang, M.; Jia, X.; Ren, D.; Li, B. Influence of Cr2O3 on the viscosity and crystallization behavior of glass ceramics based on blast furnace slag. Mater. Chem. Phys. 2020, 240, 122212. [Google Scholar] [CrossRef]
- Luo, Z.H.; He, F.; Zhang, W.T.; Xiao, Y.L.; Xie, J.L.; Sun, R.J.; Xie, M.Q. Effects of fluoride content on structure and properties of steel slag glass-ceramics. Mater. Chem. Phys. 2020, 242, 122531. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Liu, S.L.; Ou Yang, S.L.; Zhang, X.F.; Zhao, Z.W.; Jia, X.L.; Du, Y.S.; Deng, L.B.; Li, B.W. Transformation of unstable heavy metals in solid iste into stable state by the preparation of glass-ceramics. Mater. Chem. Phys. 2020, 252, 123061. [Google Scholar] [CrossRef]
- Chen, L.S.; Ge, X.X.; Long, Y.T.; Zhou, M.K.; Wang, H.D.; Chen, X. Crystallization and properties of high calcium glass-ceramics synthesized from ferromanganese slag. J. Non-Cryst. Solids 2020, 532, 119864. [Google Scholar] [CrossRef]
- Chen, L.; Long, Y.; Zhou, M.; Wang, H. Structure and Crystallization of High-Calcium, CMAS Glass Ceramics Synthesized with a High Content of Slag. Materials 2022, 15, 657. [Google Scholar] [CrossRef]
- Miao, X.; Bai, Z.; Qiu, G.; Tang, S.; Guo, M.; Cheng, F.; Zhang, M. Preparation of transparent Mn-doped CaF2 glass-ceramics from silicon-manganese slag: Dependence of colour-controllable change on slag addition and crystallization behavior. J. Eur. Ceram. Soc. 2020, 40, 3249–3261. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ma, C.; Han, C.T. Glass-ceramics prepared from C-Cr and Si-Mn alloy slag. Glass Enamel 2001, 29, 16–21. [Google Scholar]
- Bai, Z.; Qiu, G.; Peng, B.; Yue, C.; Zhang, M.; Guo, M. Regulating research on high-carbon ferrochrome slag-based glass-ceramics. Environ. Eng. 2019, 37, 158–163. [Google Scholar]
- Bo, X.; Jia, M.; Xue, X.; Tang, L.; Mi, Z.; Wang, S.; Cui, W.; Chang, X.; Ruan, J.; Dong, G.; et al. Effect of strengthened standards on Chinese ironmaking and steelmaking emissions. Nat. Sustain. 2021, 4, 811–820. [Google Scholar] [CrossRef]
- Wang, Y.-c.; Xin, W.-b.; Huo, X.-g.; Luo, G.-p.; Zhang, F. Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3. High. Temp. Mat. Pr. 2019, 38, 726–732. [Google Scholar] [CrossRef]
- Du, Y.S.; Ma, J.; Shi, Y.; Zhang, X.F.; Zhang, H.X.; Chen, H.; Ouyang, S.L.; Li, B.W. Crystallization characteristics and corrosion properties of slag glass-ceramic prepared from blast furnace slag containing rare earth. J. Non-Cryst. Solids 2020, 532, 119880. [Google Scholar]
- Luo, Y.; Wang, F.; Zhu, H.; Liao, Q.; Xu, Y.; Liu, L. Preparation and characterization of glass-ceramics with granite tailings and titanium-bearing blast furnace slags. J. Non-Cryst. Solids 2022, 582, 121463. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Zhang, Z.; Li, Z.; Jia, R.; Yun, F.; Li, H.; Ma, Y.; Wang, W. The viscosity and conductivity of the molten glass and crystallization behavior of the glass ceramics derived from stainless steel slag. Mater. Chem. Phys. 2020, 251, 123159. [Google Scholar] [CrossRef]
- Ma, J.; Shi, Y.; Zhang, H.; Ouyang, S.; Deng, L.; Chen, H.; Zhao, M.; Du, Y. Crystallization of CaO-MgO-Al2O3-SiO2 glass ceramic derived from blast furnace slag via one-step method. Mater. Chem. Phys. 2021, 261, 124123. [Google Scholar] [CrossRef]
- Montoya-Quesada, E.; Villaquiran-Caiced, M.A.; Mejia de Gutierrez, R.; Munoz-Saldana, J. Effect of ZnO content on the physical, mechanical and chemical properties of glass-ceramics in the CaO-SiO2-Al2O3 system. Ceram. Int. 2020, 46, 4322–4328. [Google Scholar] [CrossRef]
- Jia, R.D.; Deng, L.B.; Yun, F.; Li, H.; Zhang, X.F.; Jia, X.L. Effects of SiO2/CaO ratio on viscosity, structure, and mechanical properties of blast furnace slag glass ceramics. Mater. Chem. Phys. 2019, 233, 155–162. [Google Scholar] [CrossRef]
- Zhang, W.T.; He, F.; Xiao, Y.L.; Xie, M.Q.; Xie, J.L.; Sun, R.J.; Yang, H.; Luo, Z.H. Effects of Al/Na and heat treatment on the structure and properties of glass ceramics from molten blast furnace slag. Ceram. Int. 2019, 45, 13692–13700. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Qu, Z. Physicochemical property and chromium leaching behavior in different environments of glass ceramics prepared from AOD stainless steel slag. J. Alloys Compd. 2019, 805, 1106–1116. [Google Scholar] [CrossRef]
- OuYang, S.L.; Zhang, Y.X.; Chen, Y.X.; Zhao, Z.W.; Wen, M.; Li, B.W.; Shi, Y.; Zhang, M.Z.; Liu, S.L. Preparation of Glass-ceramics Using Chromium-containing Stainless Steel Slag: Crystal Structure and Solidification of Heavy Metal Chromium. Sci. Rep. 2019, 9, 1964. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Leng, M.; Li, J.; Liu, Q. The Crystallization Behaviors of SiO2-Al2O3-CaO-MgO-TiO2 Glass-Ceramic Systems. Crystals 2020, 10, 794. [Google Scholar] [CrossRef]
- Reben, M.; Kosmal, M.; Ziabka, M.; Pichniarczyk, P.; Grelowska, I. The influence of TiO2 and ZrO2 on microstructure and crystallization behavior of CRT glass. J. Non-Cryst. Solids 2015, 425, 118–123. [Google Scholar] [CrossRef]
- Ding, L.; Ning, W.; Wang, Q.; Shi, D.; Luo, L. Preparation and characterization of glass–ceramic foams from blast furnace slag and iste glass. Mater. Lett. 2015, 141, 327–329. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Yan, B.; Hu, S.; Guo, H.; Chen, D. Recycling of Blast Furnace Slag and Fluorite Tailings into Diopside-Based Glass-Ceramics with Various Nucleating Agents’ Addition. Sustainability 2021, 13, 11144. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, C.; Wang, G.; Cai, Y.; Zhao, C.J.M.R.T. Stabilization of free CaO in molten BOF slag by addition of silica at high temperature. Metall. Res. Technol. 2018, 115, 414. [Google Scholar] [CrossRef]
- Lu, X.; Huang, X.L.; Wei, R.F.; Chen, W.; Cang, D.Q.; Yang, F.H.; Pu, C.L. Novel method for improving iron recovery from electric arc furnace slag: On-site hot modification. J. Iron. Steel. Res. Int. 2022, 29, 1224–1235. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Wang, M.; Zhao, Z.; Gao, L.; Chu, M. New understanding on reduction mechanism and alloying process of rich manganese slag: Phase formation and morphological evolution. Powd. Technol. 2021, 380, 229–245. [Google Scholar] [CrossRef]
- Fan, W.-D.; Yang, Q.-W.; Guo, B.; Liu, B.; Zhang, S.-G. Crystallization mechanism of glass-ceramics prepared from stainless steel slag. Rare Metals 2018, 37, 413–420. [Google Scholar] [CrossRef]
- Tong, Z.; Sun, J.; Wang, J.; Tan, Z.; Liu, S. Iron reduction and diopside-based glass ceramic preparation based on mineral carbonation of steel slag. Environ. Sci. Pollut. R. 2021, 28, 796–804. [Google Scholar] [CrossRef]
- He, F.; Fang, Y.; Xie, J.; Xie, J. Fabrication and characterization of glass-ceramics materials developed from steel slag iste. Mater. Des. 2012, 42, 198–203. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Liu, W.; Yang, J. Preparation of glass-ceramics from molten steel slag using liquid-liquid mixing method. Chemosphere 2011, 85, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Z.; Li, Y.; Dai, W.B.; Cang, D.Q. Crystallization mechanism and properties of high basicity steel slag-derived glass-ceramics. J. Ceram. Soc. Jpn. 2016, 124, 247–250. [Google Scholar] [CrossRef]
- Yang, Z.H.; Lin, Q.; Lu, S.C.; He, Y.; Liao, G.D.; Ke, Y. Effect of CaO/SiO2 ratio on the preparation and crystallization of glass-ceramics from copper slag. Ceram. Int. 2014, 40, 7297–7305. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, Y.X.; Ouyang, S.L.; Li, H.R.; Li, X.; Li, B.W. Microstructural transformation of stainless steel slag-based CAMS glass ceramics prepared by SPS. Ceram. Int. 2021, 47, 1284–1293. [Google Scholar] [CrossRef]
- Song, Y.X.; Lan, S.D.; Di, J.H.; Jiang, B.Q. Present situation and development trend of comprehensive utilization of ferroalloy slag in China. China Metall 2017, 27, 73–77. [Google Scholar]
- Doweidar, H. Density of CaO–Al2O3–SiO2 glasses with (CaO/Al2O3) ≥ 1; the hidden factors. J. Non-Cryst. Solids 2017, 471, 344–348. [Google Scholar] [CrossRef]
- Xia, F.; Liu, Y.; Wang, Y.; Chen, G. Expanded and Enhanced Deep-UV Excitations of Mn2+-Doped Phosphate Glasses Sensitized by Gd3+ Ions. J. Am. Ceram. Soc. 2015, 98, 2720–2723. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, X.; Wang, J.; Du, Y.; Wang, P.; Chen, D. Effect of Al3+ on the photoluminescence and radioluminescence properties of Mn2+-Doped high silica glass. Ceram. Int. 2022, 48, 20010–20019. [Google Scholar] [CrossRef]
- Wen, L.; Guo, H.W.; Li, P.; Liang, Z.; Huang, X.F.; Yan, B.J. Preparation of Glass Ceramics Based on Medium Ti-bearing Blast Furnace Slag and Ferrochrome Slag. Metal World 2020, 68–72. [Google Scholar]
- Zhang, W.; Li, Y.; Li, H.; Cang, D. Research of Preparing CMSA Glass-ceramics with the Nickel Iron Slag and Fly Ash. B. Chin. Ceram. Soc. 2014, 33, 3359–3365. [Google Scholar]
- Park, H.S.; Park, J.H. Vitrification of red mud with mine istes through melting and granulation process—Preparation of glass ball. J. Non-Cryst. Solids 2017, 475, 129–135. [Google Scholar] [CrossRef]
- Kang, J.; Chen, Z.; Zhu, X.; Zhou, S.; Zhou, L.; Wang, Z.; Wang, J.; Khater, G.A.; Yue, Y. Effect of replacement of Na2O by Fe2O3 on the crystallization behavior and acid resistance of MgOAl2O3SiO2 glass-ceramics. J. Non-Cryst. Solids 2019, 503–504, 1–6. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, J.; Li, X.; Shao, G. Effect of MgO addition on sinterability, crystallization kinetics, and flexural strength of glass–ceramics from iste materials. Ceram. Int. 2016, 42 Pt B, 3452–3459. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, G.H.; Chou, K.C.; Fan, D.Q. Effects of CaO/SiO2 ratio and heat treatment parameters on the crystallization behavior, microstructure and properties of SiO2-CaO-Al2O3-Na2O glass ceramics. J. Non-Cryst. Solids 2020, 538, 120023. [Google Scholar] [CrossRef]
- Fu, Y.; Li, P.Z.; Tao, H.J.; Zhang, L.M.; Xin, M.; Chang, Y.; Xia, Y.S.; Zhou, H.Q. The effects of Ca/Si ratio and B2O3 content on the dielectric properties of the CaO-B2O3-SiO2 glass-ceramics. J. Mater. Sci.-Mater. Electron. 2019, 30, 14053–14060. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Dai, W.; Cang, D. Effect of composition and sintering process on mechanical properties of glass ceramics from solid iste. Adv. Appl. Ceram. 2016, 115, 13–20. [Google Scholar] [CrossRef]
- Ljatifi, E.; Kamusheva, A.; Grozdanov, A.; Paunovic, P.; Karamanov, A. Optimal thermal cycle for production of glass-ceramic based on istes from ferronickel manufacture. Ceram. Int. 2015, 41, 11379–11386. [Google Scholar] [CrossRef]
- Topateş, G.; Tarhan, B.; Tarhan, M. Chemical durability of zircon containing glass-ceramic glazes. Ceram. Int. 2017, 43, 12333–12337. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.; Yang, X.; Zhang, X.; Zhao, M.; Chen, H.; Ouyang, S.; Li, B. Influence of rare earth ions on metal ions distribution and corrosion behavior of tailing-derived glass-ceramics. J. Non-Cryst. Solids 2018, 482, 105–115. [Google Scholar] [CrossRef]
- Dai, W.B.; Li, Y.; Cang, D.Q.; Lu, X.; Zhao, G.Z.; Guo, J.X. Research on a novel modifying furnace for converting hot slag directly into glass-ceramics. J. Clean. Prod. 2018, 172, 169–177. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, X.; Qiu, Y.J.; Li, K.; Chen, R.; Liao, Q. A review of iste heat recovery technologies towards molten slag in steel industry. Appl. Energy 2013, 112, 956–966. [Google Scholar] [CrossRef]
- Ghenai, C.; Inayat, A.; Shanableh, A.; Al-Sarairah, E.; Janajreh, I. Combustion and emissions analysis of Spent Pot lining (SPL) as alternative fuel in cement industry. Sci. Total Environ. 2019, 684, 519–526. [Google Scholar] [CrossRef]
- Min, Y.; Liu, C.J.; Shi, P.Y.; Qin, C.D.; Feng, Y.T.; Liu, B.C. Effects of the addition of municipal solid iste incineration fly ash on the behavior of polychlorinated dibenzo-p-dioxins and furans in the iron ore sintering process. Iste Manag. 2018, 77, 287–293. [Google Scholar]


| / | SiO2 | Al2O3 | CaO | MgO | TFe | MnO2 | TiO2 | K2O | Na2O | Cr2O3 | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 25.59–38.21 | 11.38–15.88 | 36.79–50.74 | 6.13–9.96 | 0.34–1.48 | 0.13–0.9 | 0.84–6.71 | 0.48–1.75 | 0.23–0.74 | 0.02 | [1,2,3] |
| Steel slag | 11.4~27.81 | 2.7–7.00 | 34.93–49.73 | 6.42–12.51 | 0.43–30.8 | 3.5–3.77 | 0.02–0.8 | 0–0.56 | 0–0.35 | 0.2–4.53 | [4,5,6] |
| Mn-Fe slag | 40.77–40.87 | 4.46–4.49 | 41.72–41.78 | 6.87–6.91 | 0.26 | 3.99 | / | / | / | / | [7,8] |
| Si-Mn slag | 41.5–45 | 3.5–9.0 | 22.0–43.7 | 6.5–22.0 | 0.0–0.75 | 4.5–12.0 | 0–0.22 | / | 0–0.12 | / | [9] |
| Cr-Fe slag | 28.24–63.94 | 3.18–30.6 | 1.66–3.3 | 7.51–21.8 | 2.19–4.4 | 0–0.18 | / | 0–4.05 | 0–4.31 | 5.91–8.92 | [10] |
| Raw Materials | System | Method | Heat Treatment Conditions | Main Phases | Bulk Density (g/cm3) | Flexural Strength (MPa) | Vickers Microhardness (GPa) | Refs. |
|---|---|---|---|---|---|---|---|---|
| BFS, SiO2, Al2O3, MgO, Na2CO3, Cr2O3 | CMAS | Casting method | 1450 °C 3 h, 600 °C 2 h | Diopside, spinel | / | 123 MPa | 7.76 | [14] |
| BFS, granite tailings | CMAS | Casting method | 1550 °C 2 h, 600 °C 0.5 h | Diopside, wollastonite | 2.92–3.48 | / | 8.6 | [15] |
| BFS, SiO2, borax, MgO, Al2O3, Cr2O3 | CAMS | Casting method | 1500 °C 2 h, 600 °C 3 h | Diopside, spinel | 2.97 | 196.69 | / | [16] |
| BFS, SiO2, Cr2O3 | / | Casting method | 1500 °C 3 h, 600 °C 3 h | Anorthite, diopside, akermanite | / | 94.37 | 0.97 | [2] |
| BFS, SiO2 MgO, Al2O3, Na2CO3, Cr2O3 | CMAS | Casting method | 1450 °C 18 h, 600 °C 5 h | Diopside, spinel | / | / | / | [17] |
| BFS, fly ash, glass cullet | CAS | Sintering method | 1450 °C 2 h, 957 °C 2 h | Anorthite | 2.66–2.85 | / | 0.63–0.65 | [18] |
| BFS, SiO2, CaO, MgO, Al2O3, Cr2O3 | CMAS | Melting method | 1500 °C 1 h, 800 °C 2 h | Diopside, augite, gehlenite | 2.78 | 182.86 | 7.34 | [19] |
| BFS, chemically pure reagents | CAS | Casting method | 1500 °C 1 h, 780 °C 2 h, 880 °C 3 h | Akermanite, gehlenite, nepheline | / | 81.31 | / | [20] |
| Raw Materials | System | Method | Heat Treatment Conditions | Main Phases | Bulk Density (g/cm3) | Flexural Strength (MPa) | Vickers Microhardness (GPa) | Refs. |
|---|---|---|---|---|---|---|---|---|
| SS, Na2CO3, Na2B4O7, etc. | CAMS | Spark plasma sintering | 1450 °C 3 h, 700–850 ℃ 3 min | Diopside | 2.79–2.83 | 67–134 | 9.7–16.3 | [29] |
| SS, carbonate, Na2SiF6 | / | Casting method | 1400 °C 3 h, 550 ℃ 1 h | Nepheline, cuspidine | / | 177.76 MPa | / | [5] |
| SS, fly ash | CMAS | Casting method | 1450 °C 2 h, 922 °C 1 h | / | / | / | / | [30] |
| SS, MgO, SiO2, CaO, Na2CO3 | CMAS | Melting method | 1500 °C 2 h, 600 °C 3 h | Diopside Anorthite | 2.9 | 222.9 | 7.15 | [22] |
| SS, SiO2, powdered coal | CMSA | Melting method | 1500 °C 1 h, 800 °C 1 h, 970 °C 1 h | Melilite, diopside | / | / | / | [31] |
| SS, chemical reagent | CMAS | Casting method | 1500 °C 3 h, annealing crystallization | Augite, anorthite, wollastonite | / | / | / | [1] |
| SS, oxides, carbonates | CMAS | Casting method | 1000 °C 1 h, 1500 °C 3 h, 600 °C 0.5 h | Wollastonite | / | 145.6 | / | [32] |
| SS, SiO2, emery powder, CaO, MgO, TiO2 | / | Casting method | 1350 °C 1 h, 550 °C 2 h | Diopside | / | / | / | [33] |
| Routes | Kg CO2 Emissions Per Ton of Glass-Ceramics | Kg CO2 Emissions Consumed Per Ton of ISWS |
|---|---|---|
| Primary resource route | 907.70 | - |
| BFS-based route | 33.51 | 50.26 |
| Steel-slag-based route | 2.67 | 6.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Liu, X.; Hu, G.; Xue, K.; Wu, Y. Research Progress on Iron- and Steelmaking Iste Slag-Based Glass-Ceramics: Preparation and GHG Emission Reduction Potentials. Sustainability 2023, 15, 16925. https://doi.org/10.3390/su152416925
Wei Z, Liu X, Hu G, Xue K, Wu Y. Research Progress on Iron- and Steelmaking Iste Slag-Based Glass-Ceramics: Preparation and GHG Emission Reduction Potentials. Sustainability. 2023; 15(24):16925. https://doi.org/10.3390/su152416925
Chicago/Turabian StyleWei, Zichao, Xiaomin Liu, Guangwen Hu, Kai Xue, and Yufeng Wu. 2023. "Research Progress on Iron- and Steelmaking Iste Slag-Based Glass-Ceramics: Preparation and GHG Emission Reduction Potentials" Sustainability 15, no. 24: 16925. https://doi.org/10.3390/su152416925






