Coagulation Enhanced with Adsorption and Ozonation Processes in Surface Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Research Course and Methodology
3. Results
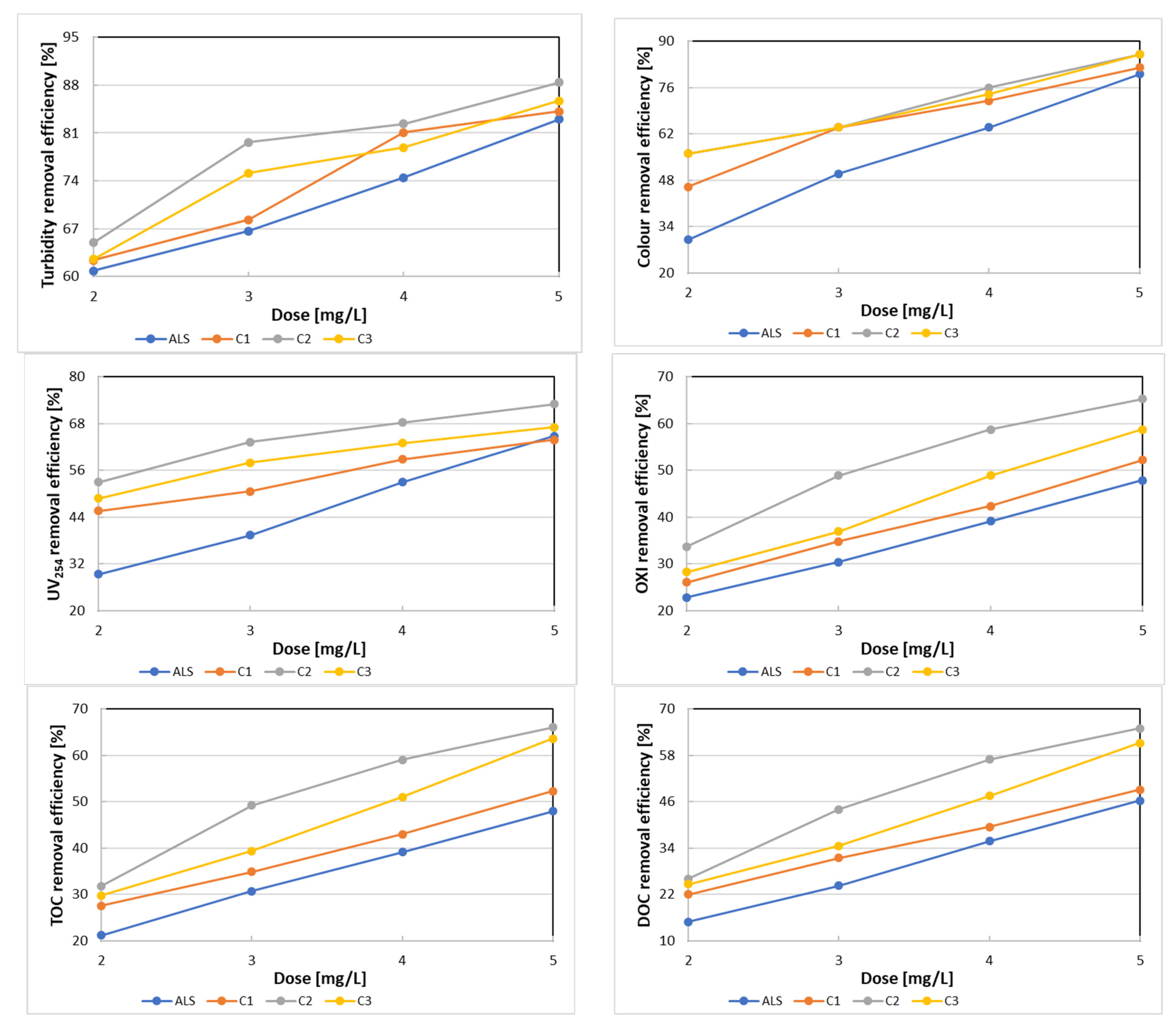
3.1. Coagulant Selection
3.2. Ozonation Process
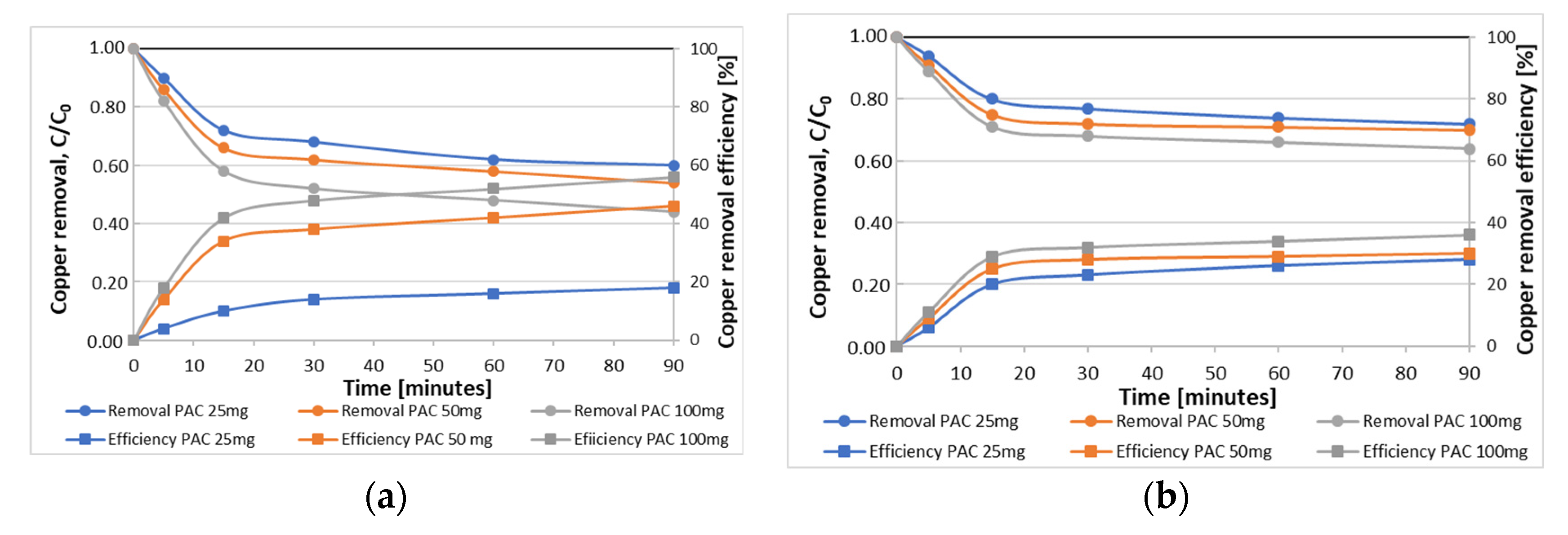
3.3. Optimisation of Adsorption Process
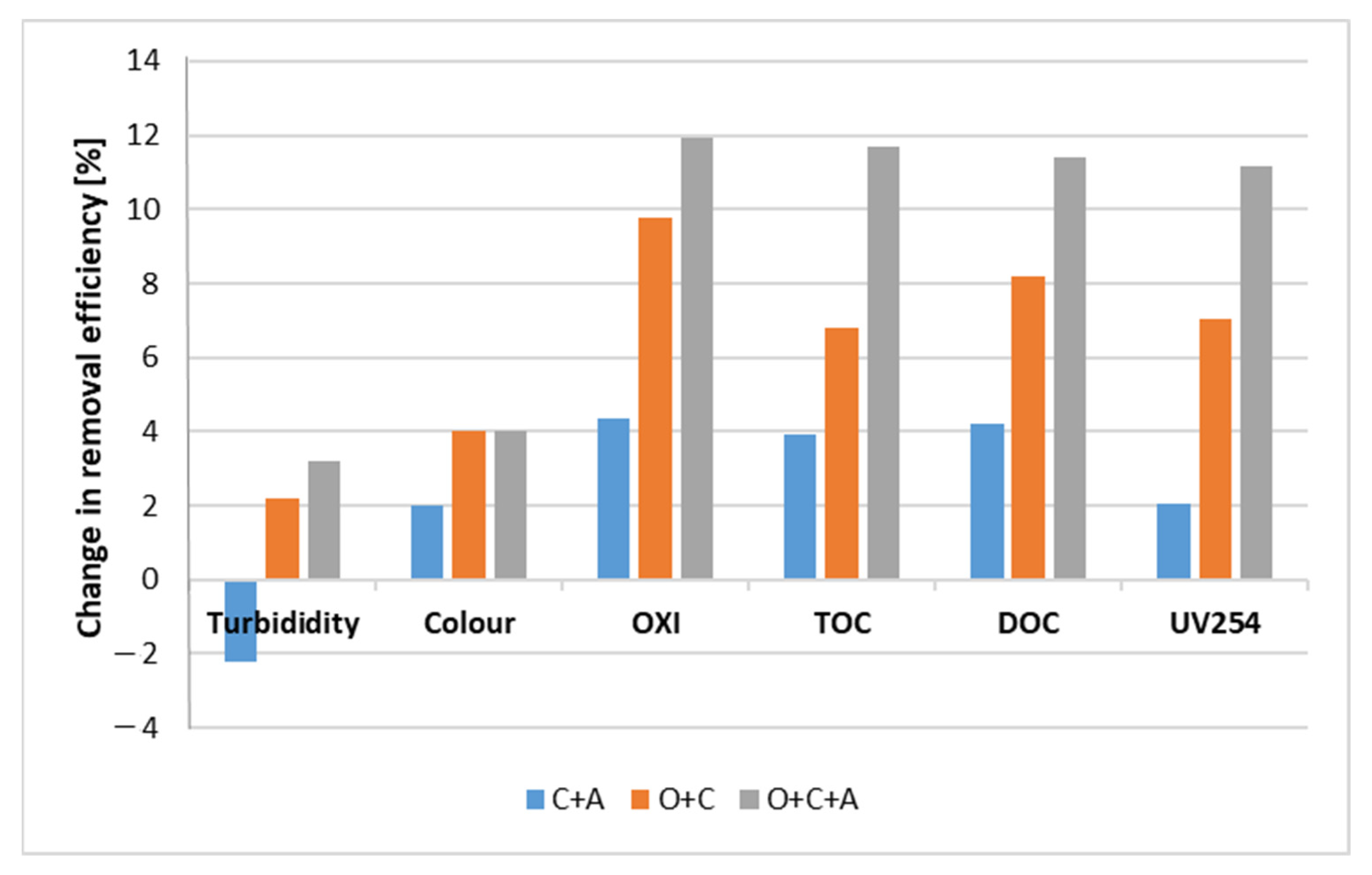
3.4. Connected Processes of Coagulation, Adsorption and Ozonation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.A.; Muhammad, M.H.; Ismail, N.I. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2023, 33, 101035. [Google Scholar] [CrossRef]
- He, H.; Li, T.; He, C.; Chen, J.; Chu, H.; Dong, B. Removal of natural organic matter in full-scale conventional and advanced water treatment plants: Assimilable organic carbon and its precursors. Chem. Eng. J. Adv. 2021, 8, 100183. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Huang, X.; Yu, Z.; Chen, P.; Cao, X. Application progress of enhanced coagulation in water treatment. RSC Adv. 2020, 10, 20231–20244. [Google Scholar] [CrossRef]
- Slavik, I.; Kostrowski, D.; Uhl, W. Effect of solar radiation on natural organic matter composition in surface waters and resulting impacts on drinking water treatment. Environ. Technol. 2023, 44, 1549–1565. [Google Scholar] [CrossRef]
- Joseph, L.; Flora, J.R.V.; Park, Y.-G.; Badawy, M.; Saleh, H.; Yoon, Y. Removal of natural organic matter from potential drinking water sources by combined coagulation and adsorption using carbon nanomaterials. Sep. Purif. Technol. 2012, 95, 64–72. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–541. [Google Scholar] [CrossRef]
- Jin, X.; Jin, P.; Hou, R.; Yang, L.; Wang, X.C. Enhanced WWTP effluent organic matter removal in hybrid ozonation-coagulation (HOC) process catalyzed by Al-based coagulant. J. Hazard. Mater. 2017, 327, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Sadrnourmohamadi, M.; Gorczyca, B. Effects of ozone as a stand-alone and coagulation-aid treatment on the reduction of trihalomethanes precursors from high DOC and hardness water. Water Res. 2015, 73, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, B.; Yue, Q.; Bu, F.; Shen, X. Effects of ozonation, powdered activated carbon adsorption, and coagulation on the removal of disinfection by-product precursors in reservoir water. Environ. Sci. Pollut. Res. 2017, 24, 17945–17954. [Google Scholar] [CrossRef] [PubMed]
- Kristiana, I.; Joll, C.; Heitz, A. Powdered activated carbon coupled with enhanced coagulation for natural organic matter removal and disinfection by-product control: Application in a Western Australian water treatment plant. Chemosphere 2011, 83, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, Y.; Zhang, C.; Rong, H.; Zeng, G. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6509–6518. [Google Scholar] [CrossRef] [PubMed]
- Keochaiyom, B.; Wan, J.; Zeng, G.; Huang, D.; Xue, W.; Hu, L.; Huang, C.; Zhang, C.; Cheng, M. Synthesis and application of magnetic chlorapatite nanoparticles for zinc (II), cadmium (II) and lead (II) removal from water solutions. J. Colloid Interface Sci. 2017, 505, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanagiotou, C.; Christou, A.; Zissimos, A.M.; Chatzitheodoridis, E.; Varnavas, S.P. Contamination of stream waters, sediments, and agricultural soil in the surroundings of an abandoned copper mine by potentially toxic elements and associated environmental and potential human health–derived risks: A case study from Agrokipia, Cyprus. Environ. Sci. Pollut. Res. 2020, 27, 41279–41298. [Google Scholar] [CrossRef]
- Gupta, H.; Gogate, P.R. Intensified removal of copper from waste water using activated watermelon based biosorbent in the presence of ultrasound. Ultrason. Sonochem. 2016, 30, 113–122. [Google Scholar] [CrossRef]
- El-Agawany, N.I.; Kaamoush, M.I.A. Role of zinc as an essential microelement for algal growth and concerns about its potential environmental risks. Environ. Sci. Pollut. Res. 2023, 30, 71900–71911. [Google Scholar] [CrossRef]
- Mathimani, T.; Rene, E.R.; Devanesan, S.; AlSalhi, M.S. Removal of zinc by Selenastrum sp. and simultaneous biodiesel production from metal-resistant biomass for energy and environmental sustainability. Algal Res. 2023, 73, 103157. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Jiang, Y.; Tsang, D.C.W.; Zhang, C.; Liu, J.; Zhou, Y.; Yin, M.; Wang, J.; Shen, N.; et al. Health risks of metal(loid)s in maize (Zea mays L.) in an artisanal zinc smelting zone and source fingerprinting by lead isotope. Sci. Total Environ. 2020, 742, 140321. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Wei, C.; Luo, K. Hydrogeochemical characteristics, source identification and health risks of surface water and groundwater in mining and non-mining areas of Handan, China. Environ. Earth Sci. 2019, 78, 402. [Google Scholar] [CrossRef]
- Çiner, F.; Sunkari, E.D.; Şenbaş, B.A. Geochemical and multivariate statistical evaluation of trace elements in groundwater of Niğde municipality, south-central Turkey: Implications for arsenic contamination and human health risks assessment. Arch. Environ. Contam. Toxicol. 2021, 80, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, Q.; Nadeem, R.; Iqbal, M.; Saeed, R.; Ansari, T.M. Organic acids pretreatment effect on Rosa bourbonia phyto-biomass for removal of Pb(II) and Cu (II) from aqueous media. Bioresour. Technol. 2013, 132, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Meng, F.; Luo, L. Naked-eye detection and sustainable removal of zinc ions in water by a conjugated polymer with ratiometric absorption. Org. Electron. 2021, 99, 106347. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 (accessed on 7 October 2023).
- Regulation of the Minister of Health of 7 December 2017 on the Quality of Water Intended for Human Consumption) Dz.U.2017 poz. 2294. (In Polish). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 7 October 2023).
- WHO. Guidelines for Drinking-Water Quality—4th Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022.
- Wu, H.P.; Lai, C.; Zeng, G.M.; Liang, J.; Chen, J.; Xu, J.J.; Dai, J.; Li, X.D.; Liu, J.F.; Chen, M.; et al. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: A review. Crit. Rev. Biotechnol. 2016, 37, 754–764. [Google Scholar] [CrossRef]
- Kanrar, S.; Ghosh, A.; Ghosh, A.; Chowdhury, S.; Sadhukhan, M.; Ghosh, U.C.; Sasikumar, P. Tailored hybrid Ce-Zr-La hydrous oxide material: Preparation, characterization and application towards removal of fluoride and copper(II) from their contaminated water. Inorg. Chem. Commun. 2023, 158, 111381. [Google Scholar] [CrossRef]
- Khan, N.A.; Ibrahim, S.; Subramaniam, P. Elimination of heavy metals from wastewater using agricultural wastes as adsorbents. Malays. J. Sci. 2004, 23, 43–51. [Google Scholar]
- Park, D.; Yun, Y.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Karwowska, B.; Sperczyńska, E. Organic matter and heavy metal ions removal from surface water in processes of oxidation with ozone, UV irradiation, coagulation and adsorption. Water 2022, 14, 3763. [Google Scholar] [CrossRef]
- APHA. AWWA Standard Methods for Examination of Water and Wastewater, 20th ed.; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; APHA Publishing House: Washington, DC, USA, 1998. [Google Scholar]
- Mustereț, C.P.; Morosanu, I.; Ciobanu, R.; Plavan, O.; Gherghel, A.; Al-Refai, M.; Roman, I.; Teodosiu, C. Assessment of coagulation–flocculation process efficiency for the natural organic matter removal in drinking water treatment. Water 2021, 13, 3073. [Google Scholar] [CrossRef]
- Rucka, K.; Solipiwko-Pieścik, A.; Wolska, M. Effectiveness of humic substance removal during the coagulation process. SN Appl. Sci. 2019, 1, 535. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Chang, E.-E.; Chang, P.-C.; Huang, C.-P. Effects of pre-ozonation on the removal of THM precursors by coagulation. Sci. Total Environ. 2009, 407, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Tubić, A.; Agbaba, J.; Jazić, J.M.; Watson, M.; Dalmacija, B.; Tubi, A.; Jazi, J.M. Pilot scale investigation of coagulation combined with ozonation and pH adjustment in treatment of NOM rich water. Water Supply 2016, 16, 837–844. [Google Scholar] [CrossRef]
- Bu, F.; Gao, B.; Shen, X.; Wang, W.; Yue, Q. The combination of coagulation and ozonation as a pre-treatment of ultrafiltration in water treatment. Chemosphere 2019, 231, 349–356. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2022, 278, 119510. [Google Scholar] [CrossRef]
- Jena, P.S.; Pradhan, A.; Nanda, S.P.; Aditya Kishore Dash, A.K.; Naik, B. Biosorption of heavy metals from wastewater using Saccharomyces cerevisiae as a biosorbent: A mini review. Mater. Today 2022, 67, 1140–1146. [Google Scholar] [CrossRef]
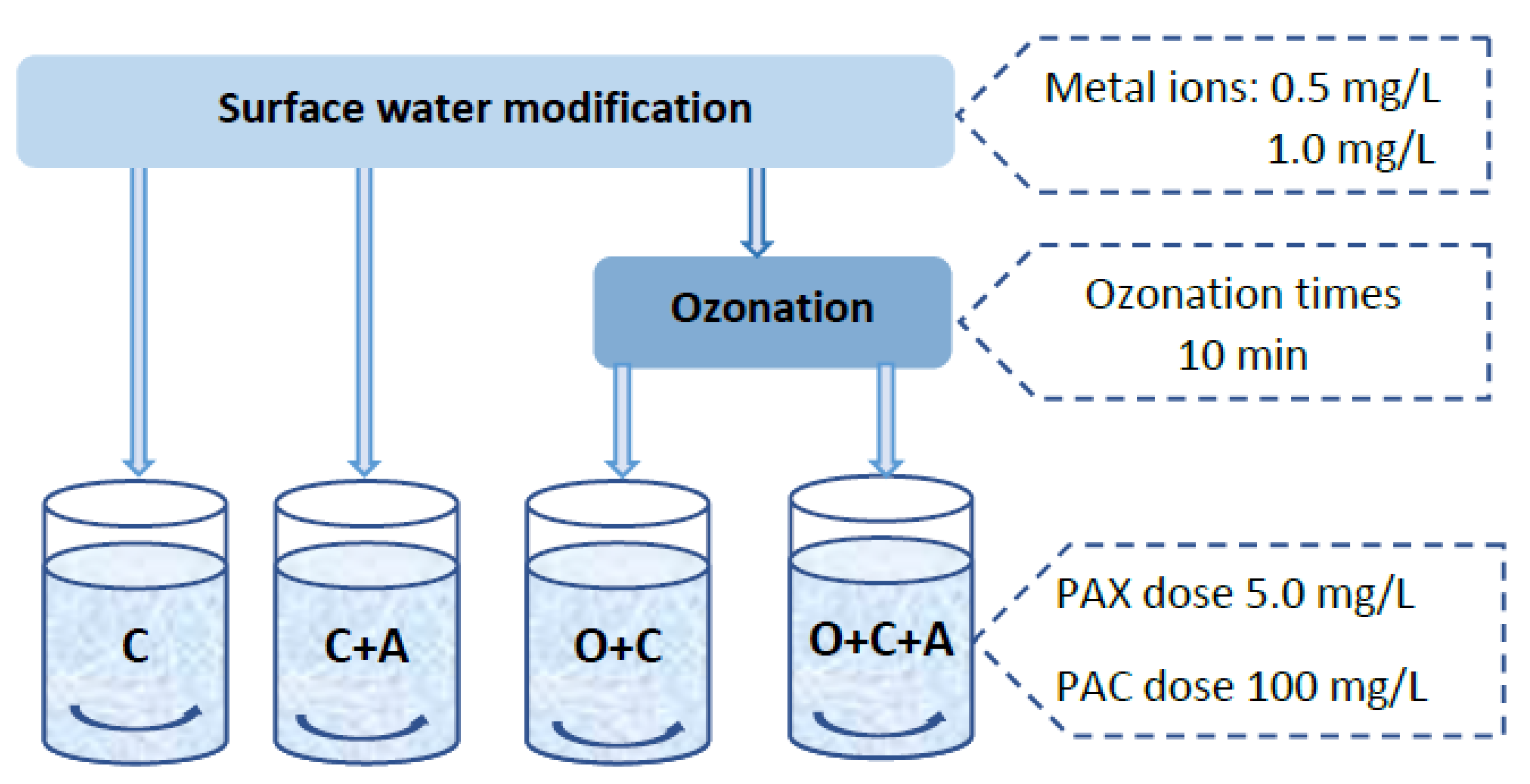


| Coagulant | Al, % | Cl, % | Basicity, % | pH |
|---|---|---|---|---|
| low-basicity C1 | 7.2 ± 0.3 | 21.0 ± 3.0 | 26 ± 6 | <1 |
| medium-basicity C2 | 5.3 ± 0.3 | 13.0 ± 2.0 | 65 ± 5 | 2.5 ± 0.5 |
| high-basicity C3 | 6.0 ± 0.5 | 5.0 ± 1.0 | 85 ± 5 | 3.6 ± 0.4 |
| Parameter | Unit | Value |
|---|---|---|
| specific surface area | m2/g | 960 |
| granulation < 0.06 mm | % | 93 |
| iodine number | mg/g | 1032 |
| methylene number | cm3 | 29 |
| Parameter | Modified Surface Water | ALS | C1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.0 mg/L | 3.0 mg/L | 4.0 mg/L | 5.0 mg/L | 2.0 mg/L | 3.0 mg/L | 4.0 mg/L | 5.0 mg/L | ||
| pH | 7.67 ± 0.01 | 7.34 ± 0.02 | 7.22 ± | 7.03 ± 0.03 | 6.81 ± 0.03 | 7.62 ± 0.01 | 7.59 ± 0.01 | 7.57 ± 0.03 | 7.56 ± 0.02 |
| Turbidity, NTU | 7.76 ± 0.02 | 3.04 ± 0.03 | 2.59 ± 0.03 | 1.98 ± 0.02 | 1.32 ± 0.03 | 2.92 ± 0.02 | 2.46 ± 0.03 | 1.47 ± 0.02 | 1.23 ± 0.03 |
| Colour mg Pt/L | 50 ± 3 | 35 ± 2 | 25 ± 2 | 18 ± 1 | 10 ± 1 | 27 ± 3 | 18 ± 3 | 14 ± 1 | 9 ± 1 |
| UV254 | 0.341 ± 0.011 | 0.240 ± 0.011 | 0.206 ± 0.009 | 0.160 ± 0.006 | 0.120 ± 0.006 | 0.185 ± 0.008 | 0.168 ± 0.006 | 0.140 ± 0.009 | 0.123 ± 0.002 |
| OXI, mg O2/L | 9.2 ± 0.2 | 7.1 ± 0.1 | 6.4 ± 0.1 | 5.6 ± 0.2 | 4.8 ± 0.1 | 6.8 ± 0.2 | 6.0 ± 0.2 | 5.3 ± 0.1 | 4.4 ± 0.1 |
| TOC, mg C/L | 8.66 ± 0.05 | 6.98 ± 0.04 | 6.14 ± 0.02 | 5.39 ± 0.04 | 4.61 ± 0.02 | 6.42 ± 0.03 | 5.77 ± 0.02 | 5.05 ± 0.02 | 4.23 ± 0.01 |
| DOC, mg C/L | 7.82 ± 0.04 | 6.65 ± 0.03 | 5.92 ± 0.01 | 5.02 ± 0.02 | 4.20 ± 0.02 | 6.10 ± 0.03 | 5.36 ± 0.05 | 4.73 ± 0.02 | 3.98 ± 0.02 |
| Aluminium, mg/L | <0.01 | 0.12 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.04 ± 0.01 | <0.01 |
| Parameter | Modified Surface Water | C2 | C3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.0 mg/L | 3.0 mg/L | 4.0 mg/L | 5.0 mg/L | 2.0 mg/L | 3.0 mg/L | 4.0 mg/L | 5.0 mg/L | ||
| pH | 7.67 ± 0.01 | 7.63 ± 0.01 | 7.60 ± 0.02 | 7.58 ± 0.02 | 7.57 ± 0.02 | 7.65 ± 0.02 | 7.63 ± 0.03 | 7.61 ± 0.01 | 7.59 ± 0.02 |
| Turbidity, NTU | 7.76 ± 0.02 | 2.72 ± 0.01 | 1.58 ± 0.02 | 1.37 ± 0.03 | 0.90 ± 0.02 | 2.91 ± 0.01 | 1.93 ± 0.02 | 1.64 ± 0.02 | 1.11 ± 0.03 |
| Colour mg Pt/L | 50 ± 3 | 22 ± 2 | 18 ± 1 | 12 ± 1 | 7 ± 2 | 22 ± 2 | 18 ± 2 | 13 ± 1 | 7 ± 1 |
| UV254, 1/cm | 0.341 ± 0.011 | 0.160 ± 0.011 | 0.125 ± 0.009 | 0.108 ± 0.006 | 0.092 ± 0.009 | 0.174 ± 0.005 | 0.143 ± 0.011 | 0.126 ± 0.008 | 0.112 ± 0.006 |
| OXI, mg O2/L | 9.2 ± 0.2 | 6.1 ± 0.2 | 4.7 ± 0.1 | 3.8 ± 0.2 | 3.2 ± 0.1 | 6.6 ± 0.2 | 5.8 ± 0.2 | 4.7 ± 0.2 | 3.8 ± 0.1 |
| TOC, mg C/L | 8.66 ± 0.05 | 6.04 ± 0.04 | 4.50 ± 0.02 | 3.63 ± 0.02 | 3.01 ± 0.04 | 6.22 ± 0.04 | 5.37 ± 0.01 | 4.34 ± 0.01 | 3.23 ± 0.02 |
| DOC, mg C/L | 7.82 ± 0.04 | 5.78 ± 0.04 | 4.38 ± 0.01 | 3.37 ± 0.01 | 2.74 ± 0.02 | 5.89 ± 0.04 | 5.12 ± 0.03 | 4.11 ± 0.01 | 3.04 ± 0.03 |
| Aluminium, mg/L | <0.01 | 0.05 ± 0.01 | <0.01 | <0.01 | <0.01 | 0.06 ± 0.01 | <0.01 | <0.01 | <0.01 |
| Parameter | Modified Surface Water | Ozonated Water |
|---|---|---|
| pH | 7.67 ± 0.01 | 7.86 ± 0.02 |
| Colour, mg Pt/L | 50 ± 3 | 30 ± 2 |
| UV254, 1/cm | 0.341 ± 0.011 | 0.114 ± 0.006 |
| Oxidisability (OXI), mg C/L | 9.2 ± 0.2 | 6.7 ± 0.1 |
| Total organic carbon (TOC), mg C/L | 8.66 ± 0.05 | 6.32 ± 0.03 |
| Dissolved organic carbon (DOC), mg C/L | 7.82 ± 0.04 | 6.12 ± 0.04 |
| Water Treatment Process | Zn2+ | Cu2+ | ||
|---|---|---|---|---|
| 0.5 mg/L | 1.0 mg/L | 0.5 mg/L | 1.0 mg/L | |
| ozonation | 0.49 ± 0.01 | 0.97 ± 0.02 | 0.48 ± 0.01 | 0.98 ± 0.01 |
| PAC Dose; mg/L | Zinc | |||||||||||
| C0 = 0.5 mg/L | C0 = 1.0 mg/L | |||||||||||
| Contact Time (min) | ||||||||||||
| 0 | 5 | 15 | 30 | 60 | 90 | 0 | 5 | 15 | 30 | 60 | 90 | |
| 25 | 0.50 ± 0.02 | 0.48 ± 0.02 | 0.45 ± 0.01 | 0.43 ± 0.02 | 0.42 ± 0.01 | 0.41 ± 0.01 | 1.00 ± 0.01 | 0.96 ± 0.02 | 0.88 ± 0.02 | 0.85 ± 0.02 | 0.82 ± 0.01 | 0.81 ± 0.01 |
| 50 | 0.50 ± 0.02 | 0.46 ± 0.01 | 0.39 ± 0.02 | 0.37 ± 0.01 | 0.36 ± 0.02 | 0.36 ± 0.02 | 1.00 ± 0.01 | 0.93 ± 0.02 | 0.81 ± 0.01 | 0.77 ± 0.01 | 0.76 ± 0.01 | 0.75 ± 0.01 |
| 100 | 0.50 ± 0.02 | 0.44 ± 0.02 | 0.36 ± 0.01 | 0.34 ± 0.01 | 0.33 ± 0.02 | 0.32 ± 0.01 | 1.00 ± 0.01 | 0.90 ± 0.02 | 0.75 ± 0.01 | 0.70 ± 0.01 | 0.69 ± 0.01 | 0.69 ± 0.01 |
| PAC Dose; mg/L | Copper | |||||||||||
| C0 = 0.5 mg/L | C0 = 1.0 mg/L | |||||||||||
| Contact Time (min) | ||||||||||||
| 0 | 5 | 15 | 30 | 60 | 90 | 0 | 5 | 15 | 30 | 60 | 90 | |
| 25 | 0.50 ± 0.02 | 0.45 ± 0.02 | 0.36 ± 0.01 | 0.34 ± 0.01 | 0.31 ± 0.02 | 0.30 ± 0.02 | 1.00 ± 0.01 | 0.94 ± 0.02 | 0.80 ± 0.02 | 0.77 ± 0.01 | 0.74 ± 0.02 | 0.72 ± 0.01 |
| 50 | 0.50 ± 0.02 | 0.43 ± 0.01 | 0.33 ± 0.01 | 0.31 ± 0.02 | 0.29 ± 0.02 | 0.27 ± 0.01 | 1.00 ± 0.01 | 0.91 ± 0.02 | 0.75 ± 0.01 | 0.72 ± 0.01 | 0.71 ± 0.02 | 0.70 ± 0.01 |
| 100 | 0.50 ± 0.02 | 0.41 ± 0.02 | 0.29 ± 0.01 | 0.26 ± 0.02 | 0.24 ± 0.01 | 0.22 ± 0.01 | 1.00 ± 0.01 | 0.89 ± 0.01 | 0.71 ± 0.01 | 0.68 ± 0.01 | 0.66 ± 0.02 | 0.64 ± 0.02 |
| Treatment Process | Parameter | ||||||
|---|---|---|---|---|---|---|---|
| Turbidity, NTU | Colour, mg Pt/L | OXI, mg O2/L | TOC, mg C/L | DOC, mg C/L | UV254 1/cm | Al3+, mg/L | |
| Raw water | 7.76 ± 0.02 | 50 ± 3 | 9.2 ± 0.2 | 8.66 ± 0.05 | 7.82 ± 0.04 | 0.341 ± 0.011 | <0.01 |
| C | 0.98 ± 0.02 | 7 ± 2 | 3.2 ± 0.1 | 3.01 ± 0.04 | 2.74 ± 0.02 | 0.092 ± 0.009 | <0.01 |
| C + A | 1.12 ± 0.03 | 6 ± 3 | 2.8 ± 0.2 | 2.67 ± 0.02 | 2.41 ± 0.03 | 0.085 ± 0.010 | <0.01 |
| O + C | 0.81 ± 0.02 | 5 ± 3 | 2.3 ± 0.1 | 2.42 ± 0.03 | 2.10 ± 0.02 | 0.068 ± 0.012 | <0.01 |
| O + C + A | 0.73 ± 0.03 | 5 ± 2 | 2.1 ± 0.1 | 2.00 ± 0.03 | 1.85 ± 0.02 | 0.054 ± 0.011 | <0.01 |
| Water Treatment Process | Zn2+ | Cu2+ | ||
|---|---|---|---|---|
| 0.5 mg/L | 1.0 mg/L | 0.5 mg/L | 1.0 mg/L | |
| C | 0.27 ± 0.02 | 0.57 ± 0.01 | 0.21 ± 0.01 | 0.45 ± 0.01 |
| A | 0.27 ± 0.01 | 0.59 ± 0.02 | 0.20 ± 0.01 | 0.54 ± 0.01 |
| C + A | 0.22 ± 0.02 | 0.48 ± 0.03 | 0.19 ± 0.02 | 0.41 ± 0.02 |
| O + C | 0.26 ± 0.02 | 0.50 ± 0.02 | 0.19 ± 0.01 | 0.43 ± 0.02 |
| O + C + A | 0.19 ± 0.01 | 0.46 ± 0.01 | 0.17 ± 0.01 | 0.38 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowska, B.; Sperczyńska, E. Coagulation Enhanced with Adsorption and Ozonation Processes in Surface Water Treatment. Sustainability 2023, 15, 16956. https://doi.org/10.3390/su152416956
Karwowska B, Sperczyńska E. Coagulation Enhanced with Adsorption and Ozonation Processes in Surface Water Treatment. Sustainability. 2023; 15(24):16956. https://doi.org/10.3390/su152416956
Chicago/Turabian StyleKarwowska, Beata, and Elżbieta Sperczyńska. 2023. "Coagulation Enhanced with Adsorption and Ozonation Processes in Surface Water Treatment" Sustainability 15, no. 24: 16956. https://doi.org/10.3390/su152416956





