Efficient Phosphate Removal from Wastewater by Ca-Laden Biochar Composites Prepared from Eggshell and Peanut Shells: A Comparison of Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biochar Production
2.3. P Adsorption
2.4. Adsorbents’ Regeneration
2.5. Characterization
3. Results and Discussion
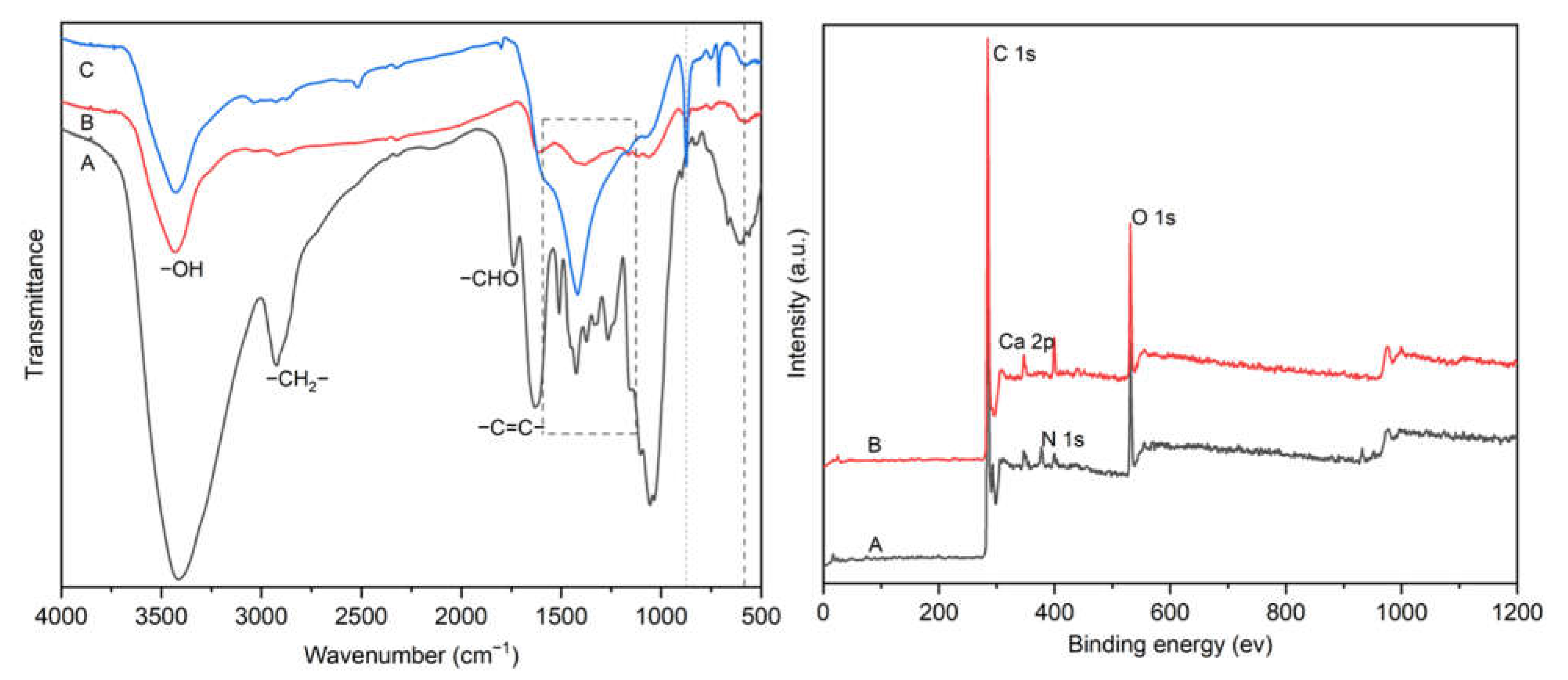
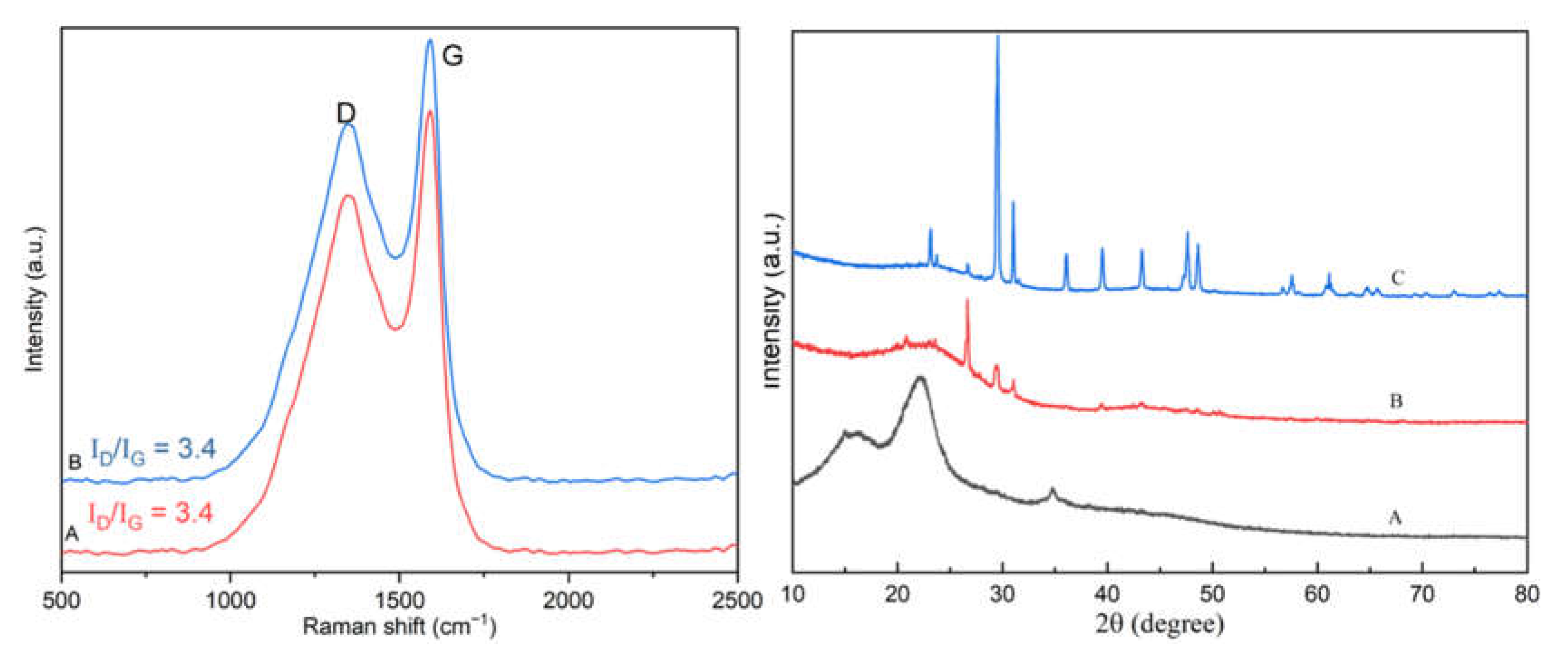
3.1. Characterization of Ca-Laden Biochar
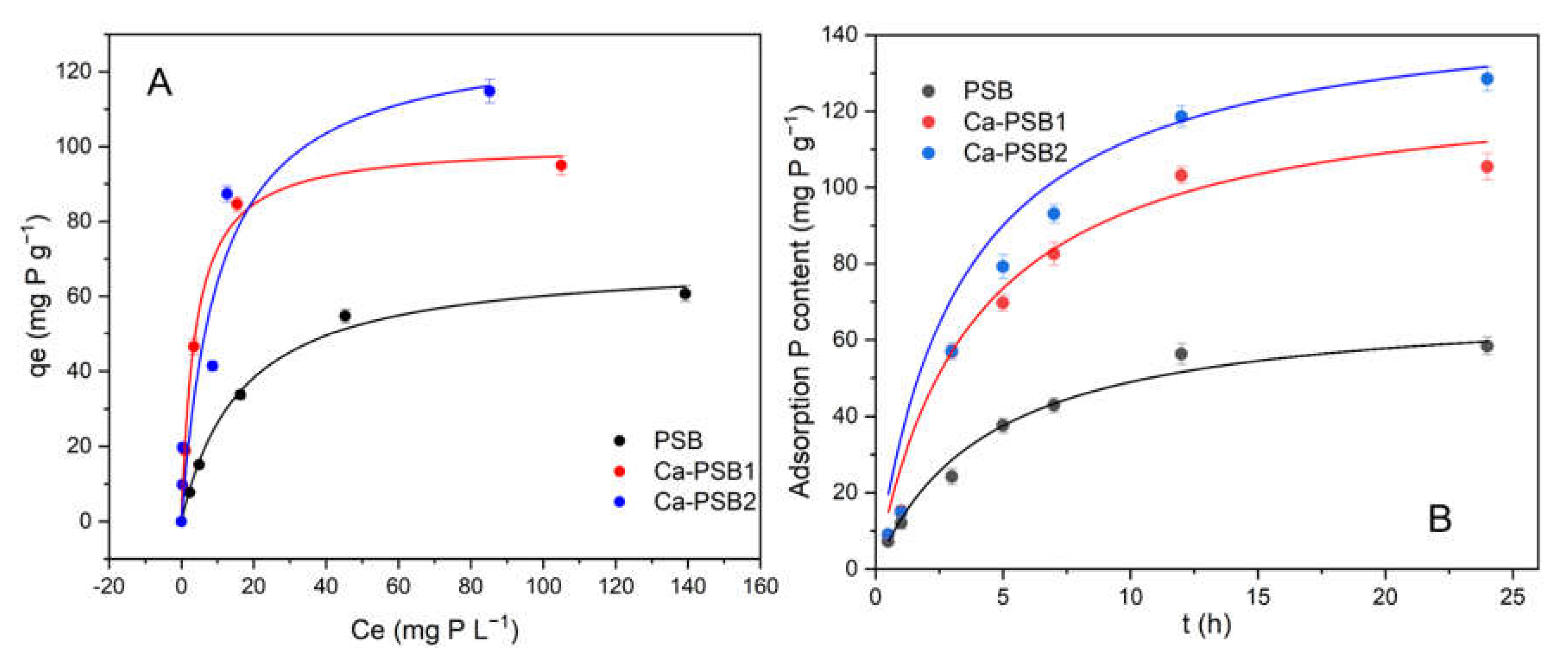
3.2. Adsorption of P onto PSB and Ca-PSB Samples
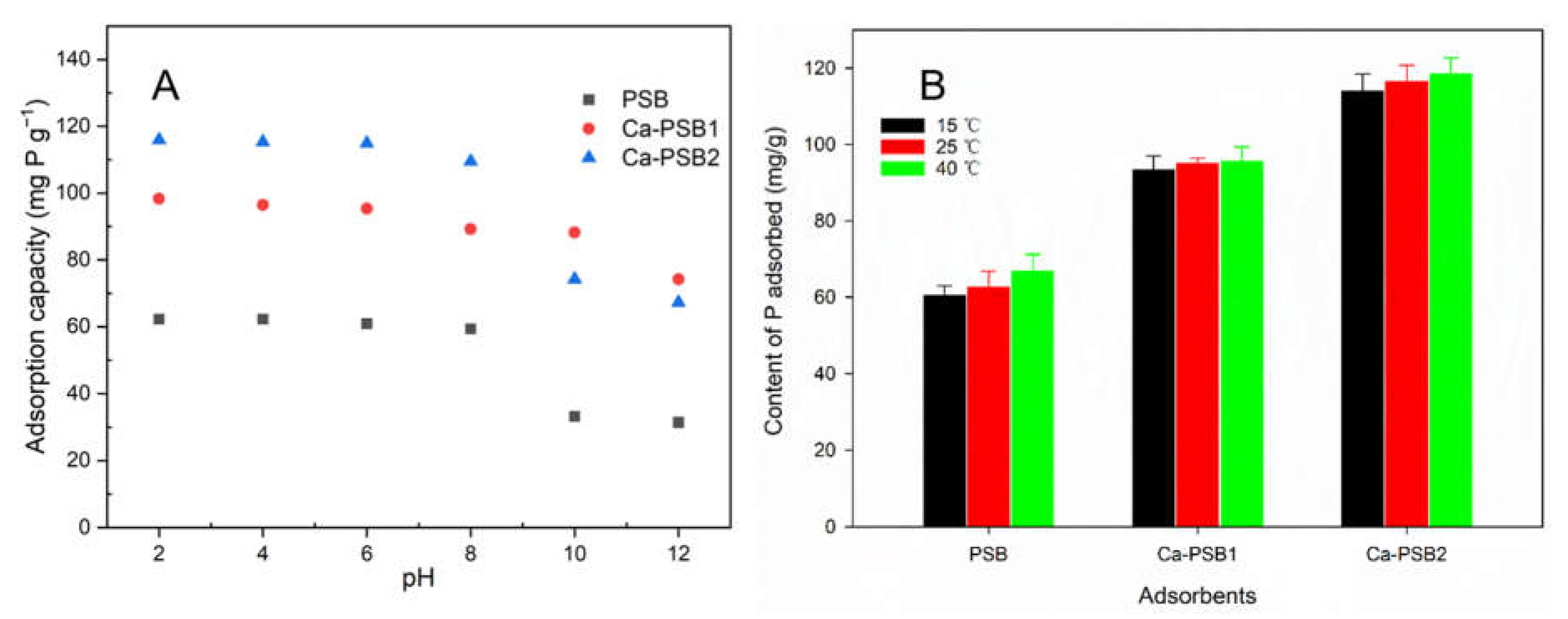
3.3. The Effects of Ambient Temperatures and Initial pH on P Adsorption
3.4. Recovery of Phosphate
3.5. Mechanism of the P Adsorption
3.6. Cost Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Wang, W.; Sun, F.; Shi, W.; Qi, D.; Wang, K.; Shi, R.; Cui, F.; Wang, C.; Chen, X. Highly Efficient Phosphate Scavenger Based on Well-Dispersed La(OH)3 Nanorods in Polyacrylonitrile Nanofibers for Nutrient-Starvation Antibacteria. ACS Nano 2015, 9, 9292–9302. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.; Elser, J.J.; Hilton, J.; Ohtake, H.; Schipper, W.J.; Van Dijk, K.C. Greening the global phosphorus cycle: How green chemistry can help achieve planetary P sustainability. Green Chem. 2015, 17, 2087–2099. [Google Scholar] [CrossRef] [Green Version]
- Hellal, F.; El-Sayed, S.; Zewainy, R.; Amer, A. Importance of phosphate pock application for sustaining agricultural production in Egypt. Bull. Natl. Res. Cent. 2019, 43, 11. [Google Scholar] [CrossRef]
- Tomei, M.C.; Stazi, V.; Daneshgar, S.; Capodaglio, A.G. Holistic Approach to Phosphorus Recovery from Urban Wastewater: Enhanced Biological Removal Combined with Precipitation. Sustainability 2020, 12, 575. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour Conserv Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact to 2050. Glob. Food Sec. 2020, 26, 100426. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- UN. United Nations Millennium Declaration. Millennium Summit, held in United Nations Headquarters in New York, from 6–8 September. 2000. Available online: http://www.unsceb.org/content/un-millennium-declaration (accessed on 12 October 2013).
- Hermassi, M.; Valderrama, C.; Moreno, N.; Font, O.; Querol, X.; Batis, N.H.; Cortina, J.L. Fly ash as reactive sorbent for phosphate removal from treated waste water as a potential slow release fertilizer. J. Environ. Chem. Eng. 2017, 5, 160–169. [Google Scholar] [CrossRef]
- Ogata, T.; Morisada, S.; Oinuma, Y.; Seida, Y.; Nakano, Y. Preparation of adsorbent for phosphate recovery from aqueous solutions based on condensed tannin gel. J. Hazard. Mater. 2011, 192, 698–703. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate From Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of phosphorus recovery from wastewater by crystallization processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Kong, L.; Liu, X. Emerging electrochemical processes for materials recovery from wastewater: Mechanisms and prospects. Front. Environ. Sci. Eng. 2020, 14, 90. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- Dai, H.; Lu, X.; Peng, Y.; Yang, Z.; Zhsssu, H. Effects of supersaturation control strategies on hydroxyapatite (HAP) crystallization for phosphorus recovery from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 5791–5799. [Google Scholar] [CrossRef]
- Dai, H.; Lu, X.; Peng, Y.; Zou, H.; Shi, J. An efficient approach for phosphorus recovery from wastewater using series-coupled air-agitated crystallization reactors. Chemosphere 2016, 165, 211–220. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total value of phosphorus recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.F.; Wang, Y.C. Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef] [Green Version]
- Pathy, A.; Ray, J.; Paramasivan, B. Challenges and opportunities of nutrient recovery from human urine using biochar for fertilizer applications. J. Clean. Prod. 2021, 304, 127019. [Google Scholar]
- Chakraborty, A.; Pal, A.; Saha, B.B. A Critical Review of the Removal of Radionuclides from Wastewater Employing Activated Carbon as an Adsorbent. Materials 2022, 15, 8818. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Thu, K.; Mitra, S.; EI-Sharkawy, I.I.; Saha, B.B.; Kil, H.S.; Yoon, S.H.; Miyawaki, J. Study on biomass derived activated carbons for adsorptive heat pump application. Int. J. Heat Mass Transf. 2017, 110, 7–19. [Google Scholar] [CrossRef]
- Nobaharan, K.; Bagheri Novair, S.; Asgari Lajayer, B.; van Hullebusch, E.D. Phosphorus removal from wastewater: The potential use of biochar and the key controlling factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Shyam, S.; Arun, J.; Gopinath, K.P.; Ribhu, G.; Ashish, M.; Ajay, S. Biomass as source for hydrochar and biochar production to recover phosphates from wastewater: A review on challenges, commercialization, and future perspectives. Chemosphere 2022, 286, 131490. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; We, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2018, 276, 183–189. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Wang, H.; Xue, J.; Lv, Q.; Pang, G. Efficient Recovery of Phosphate from Aqueous Solution Using Biochar Derived from Co-pyrolysis of Sewage Sludge with Eggshell. J. Environ. Chem. Eng. 2021, 9, 105354. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Qi, X. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Feng, L.; Wu, L.; Lv, J.; Du, W. Characteristics and Mechanisms of Phosphorous Adsorption by Peanut Shell-Derived Biochar Modified with Magnesium Chloride by Ultrasonic-Assisted Impregnation. ACS Omega 2022, 7, 43102–43110. [Google Scholar] [CrossRef]
- Lopez-Tenllado, F.J.; Motta, I.L.; Hill, J.M. Modification of biochar with high-energy ball milling: Development of porosity and surface acid functional groups. Bioresour. Technol. Rep. 2021, 15, 100704. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Zhao, D.; Fan, Y.; Bi, J.; Shan, R.; Yang, J.; Luo, B.; Yuan, H.; Ling, X.; et al. Calcium-Loaded Municipal Sludge-Biochar as an Efficient and Stable Catalyst for Biodiesel Production from Vegetable Oil. ACS Omega 2020, 5, 17471–17478. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Marinovic, A.; Jorge, A.B.; Mansor, N.; Brett, D.J.L.; Fuertes, A.B.; Sevilla, M.; Titirici, M.M. Fe–N-Doped Carbon Capsules with Outstanding Electrochemical Performance and Stability for the Oxygen Reduction Reaction in Both Acid and Alkaline Conditions. ACS Nano 2016, 10, 5922–5932. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Liu, Y.; Qian, F.; Shang, H.; Wei, X.; Zhang, S.; Chen, J.; Jason Ren, Z. Carbon transmission of CO2 activated nano-MgO carbon composites enhances phosphate immobilization. J. Mater. Chem. A 2018, 6, 3705–3713. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhan, Y. Effect of precipitation pH and coexisting magnesium ion on phosphate adsorption onto hydrous zirconium oxide. J. Environ. Sci. 2019, 76, 167–187. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Xu, K.; Lin, F.; Dou, X.; Zheng, M.; Tan, W.; Wang, C. Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides. J. Clean. Prod. 2018, 187, 205–214. [Google Scholar] [CrossRef]
- Jiao, G.J.; Ma, J.; Li, Y.; Jin, D.; Guo, Y.; Zhou, J.; Sun, R. Enhanced adsorption activity for phosphate removal by functional lignin-derived carbon-based adsorbent: Optimization, performance and evaluation. Sci Total Environ. 2021, 761, 143217. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Wu, F.; Yao, Y.; Yuan, Y.; Bi, X.; Luo, X.; Shahbazian-Yassar, R.; Zhang, C.; Amine, K. Mesocarbon Microbead Carbon-Supported Magnesium Hydroxide Nanoparticles: Turning Spent Li-ion Battery Anode into a Highly Efficient Phosphate Adsorbent for Wastewater Treatment. ACS Appl. Mater. 2016, 8, 21315–21325. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Li, Q.; Xu, M.; Guo, Y.; Li, J.; Wu, T. pH effect on Re(VII) and Se(IV) diffusion in compacted GMZ bentonite. Appl. Geochem. 2016, 73, 1–7. [Google Scholar] [CrossRef]



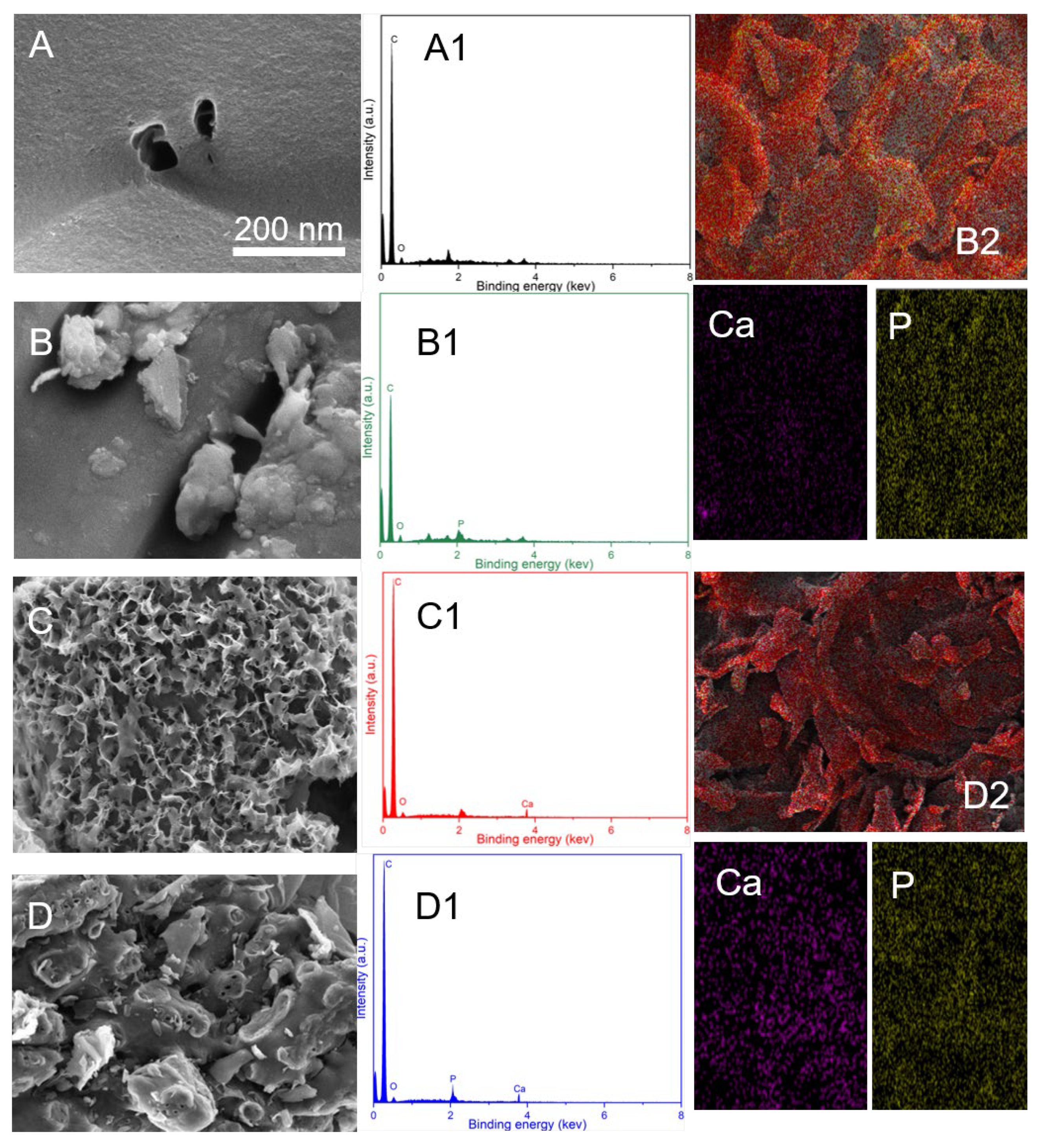
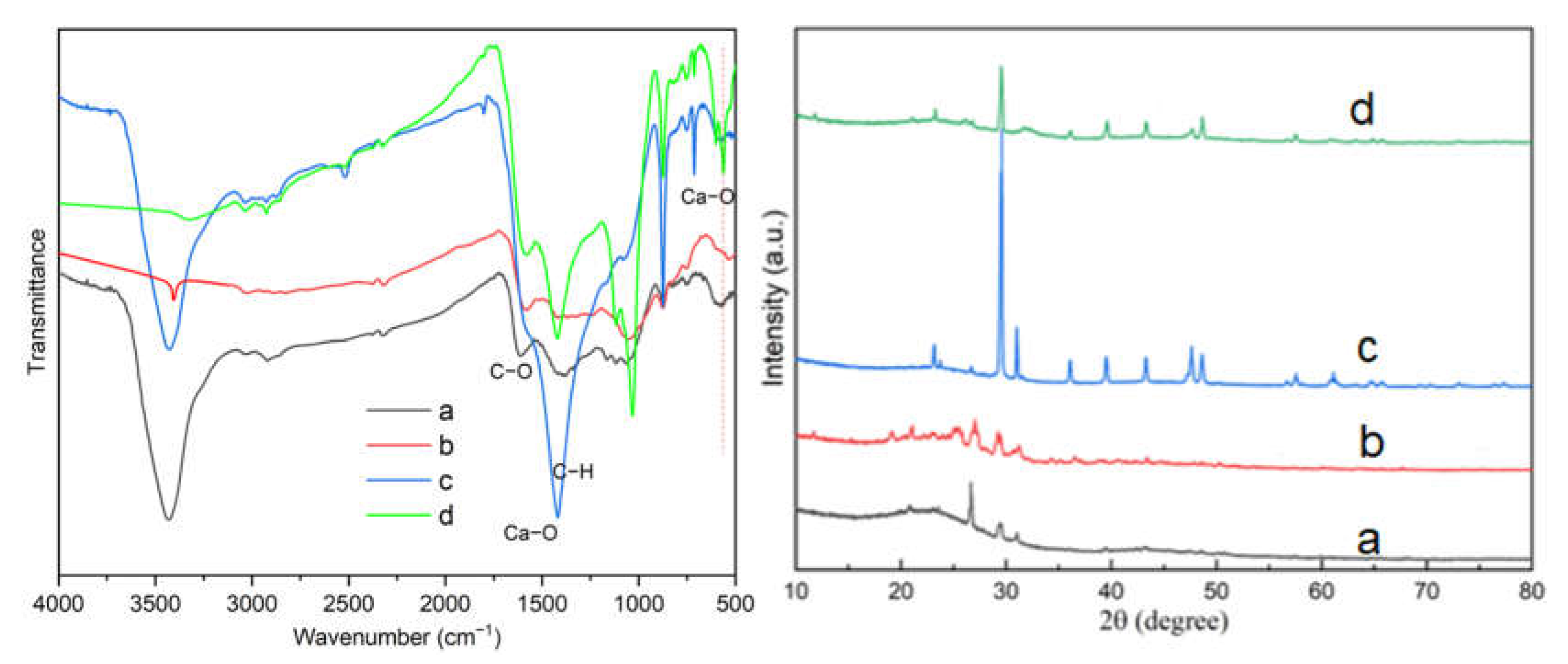
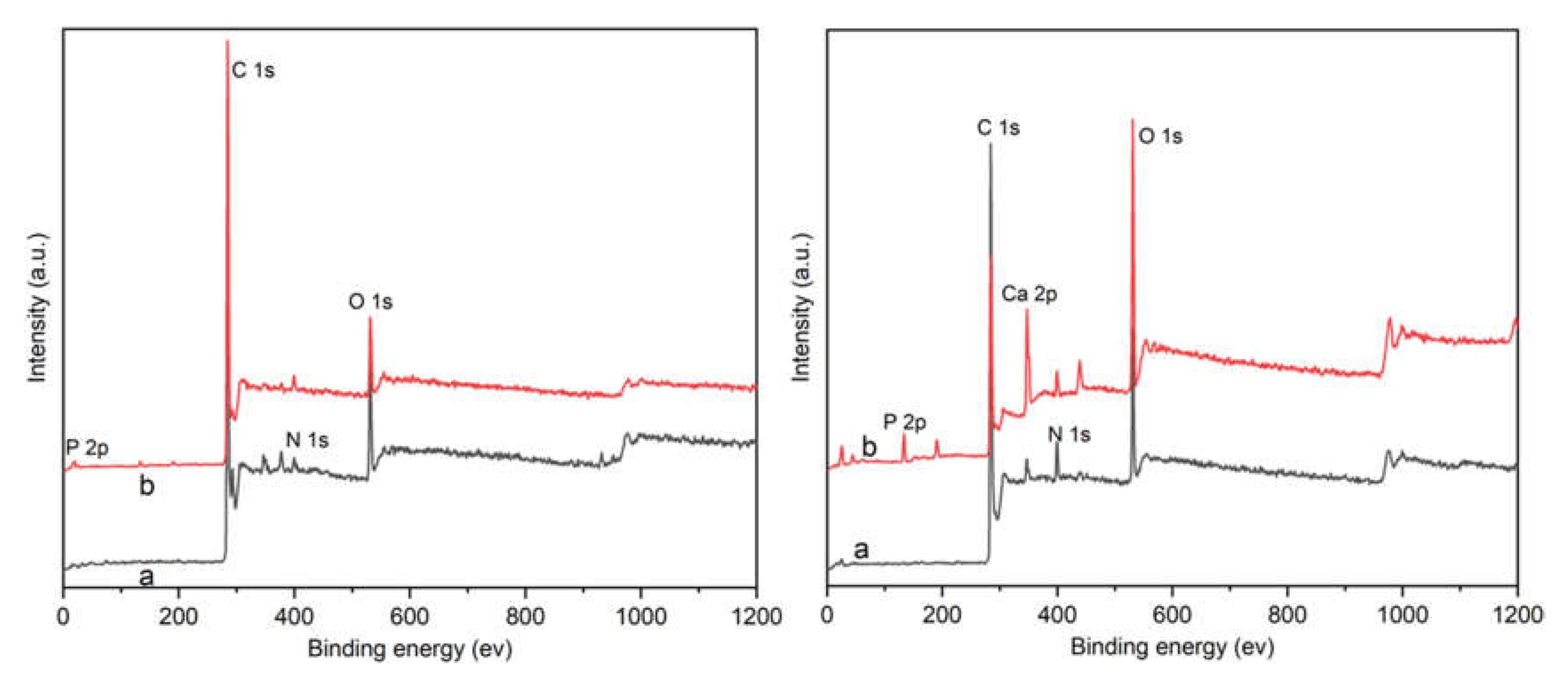
| Sample | C Element (%) | N Element (%) | H Element (%) | S Element (%) |
|---|---|---|---|---|
| Peanut shells | 47.81 | 0.66 | 5.54 | 0.05 |
| PSB | 77.59 | 1.49 | 2.293 | 0.27 |
| Sample | BET Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| PSB | 183 | 0.24 | 14.81 |
| Ca-PSB1 | 147 | 0.22 | 16.34 |
| Ca-PSB2 | 261 | 0.40 | 16.32 |
| Treatment | Q (mg P g−1) | K | R2 | (MBC) |
|---|---|---|---|---|
| PSB | 72.23 | 6.016 × 10−2 | 0.9875 | 4.345 |
| Ca-PSB1 | 105.12 | 0.2572 | 0.9789 | 27.036 |
| Ca-PSB2 | 130.57 | 0.6612 | 0.9672 | 86.33 |
| Treatment | R2 | K (g/(mg·min)) | Qe (mg P g−1) |
|---|---|---|---|
| PSB | 0.9765 | 3.14 × 10−3 | 73.32 |
| Ca-PSB1 | 0.9984 | 1.36 × 10−2 | 118.58 |
| Ca-PSB2 | 0.9853 | 1.69 × 10−2 | 138.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lv, J. Efficient Phosphate Removal from Wastewater by Ca-Laden Biochar Composites Prepared from Eggshell and Peanut Shells: A Comparison of Methods. Sustainability 2023, 15, 1778. https://doi.org/10.3390/su15031778
Liu X, Lv J. Efficient Phosphate Removal from Wastewater by Ca-Laden Biochar Composites Prepared from Eggshell and Peanut Shells: A Comparison of Methods. Sustainability. 2023; 15(3):1778. https://doi.org/10.3390/su15031778
Chicago/Turabian StyleLiu, Xiaoqi, and Jialong Lv. 2023. "Efficient Phosphate Removal from Wastewater by Ca-Laden Biochar Composites Prepared from Eggshell and Peanut Shells: A Comparison of Methods" Sustainability 15, no. 3: 1778. https://doi.org/10.3390/su15031778





