Differential Response of Brassica Cultivars to Potentially Toxic Elements and Their Distribution in Different Plant Parts Irrigated with Metal-Contaminated Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection, Preparation, and Analysis
2.2. Spiking of Irrigation Water with Potentially Toxic Elements
2.3. Treatment Setup and Crop Husbandry
2.4. Recording of Crop Physiological Attributes
2.5. Determination of Potentially Toxic Element Concentrations in Soil and Plant Samples
2.6. Calculation of Translocation and Bioaccumulation Factors
2.7. Statistical Analysis
3. Results
3.1. Agronomic Parameters
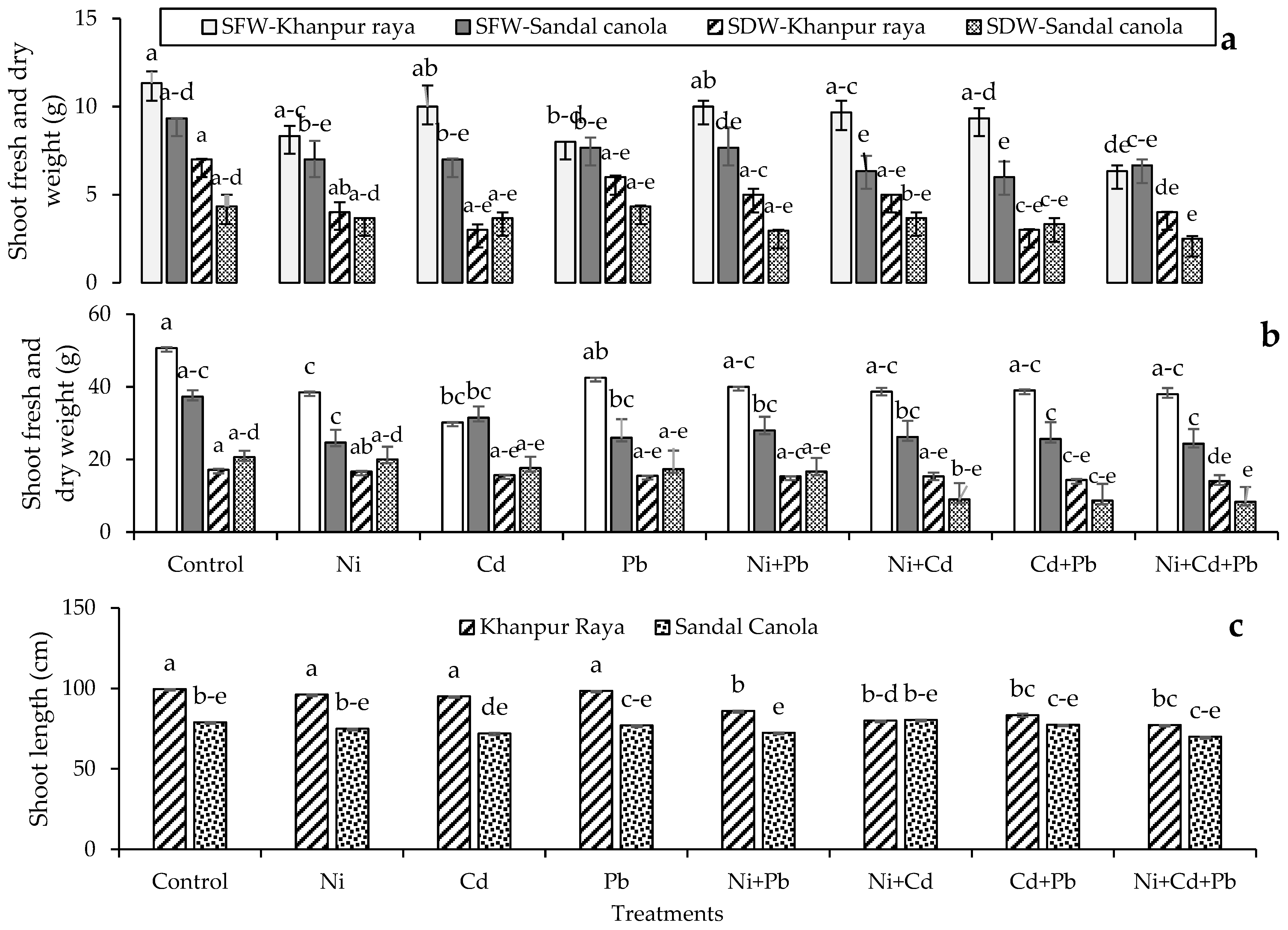
3.1.1. Shoot Fresh and Dry Weight (First Harvest)
3.1.2. Shoot Fresh and Dry Weight (Second Harvest)
3.1.3. Shoot Length
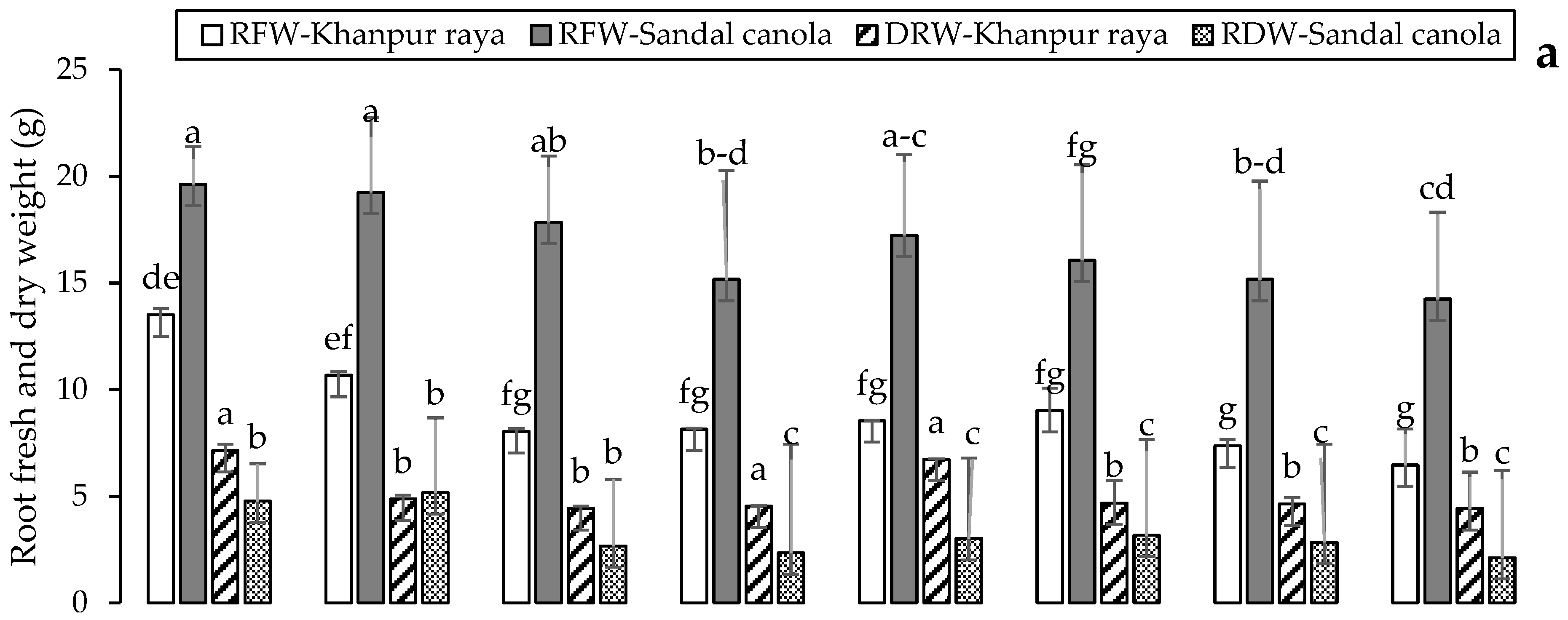
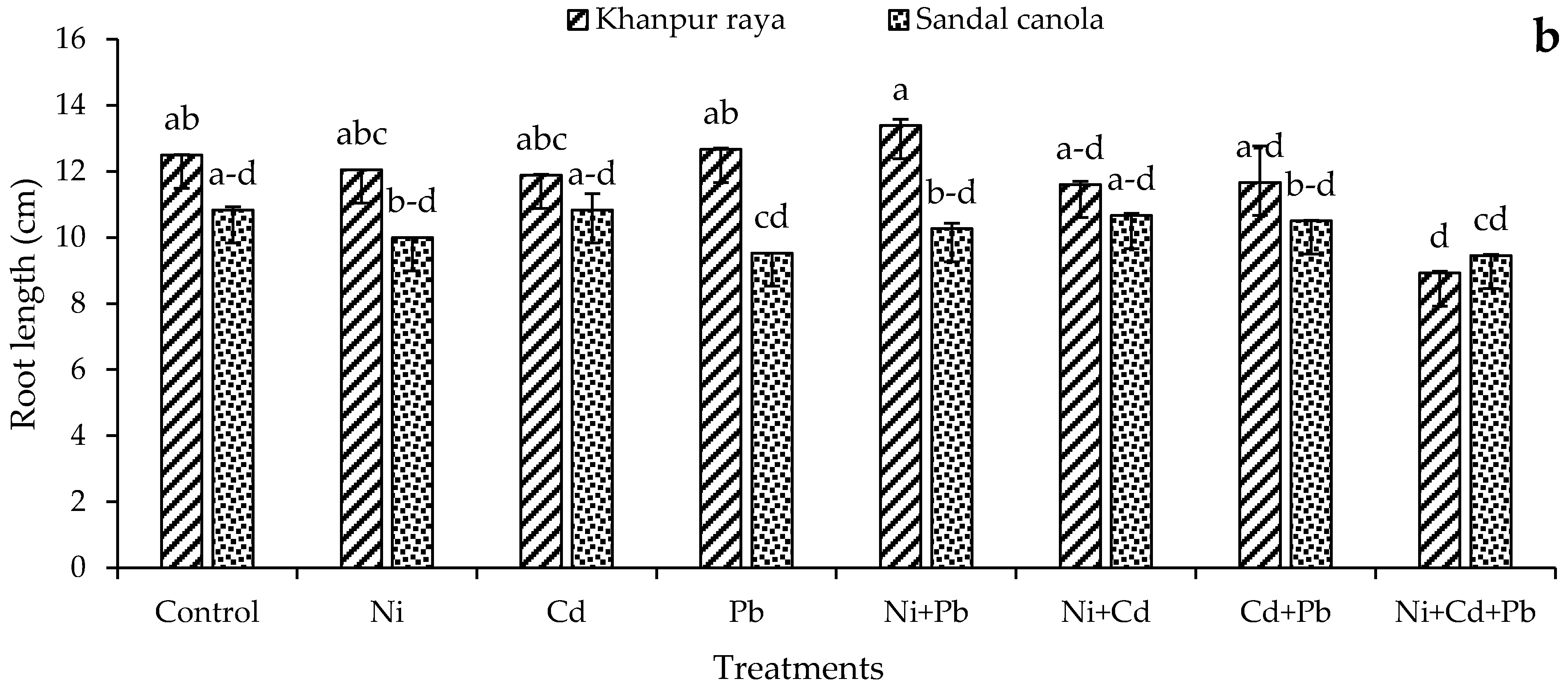
3.1.4. Root Length and Fresh and Dry Weight
3.2. Physiological Parameters
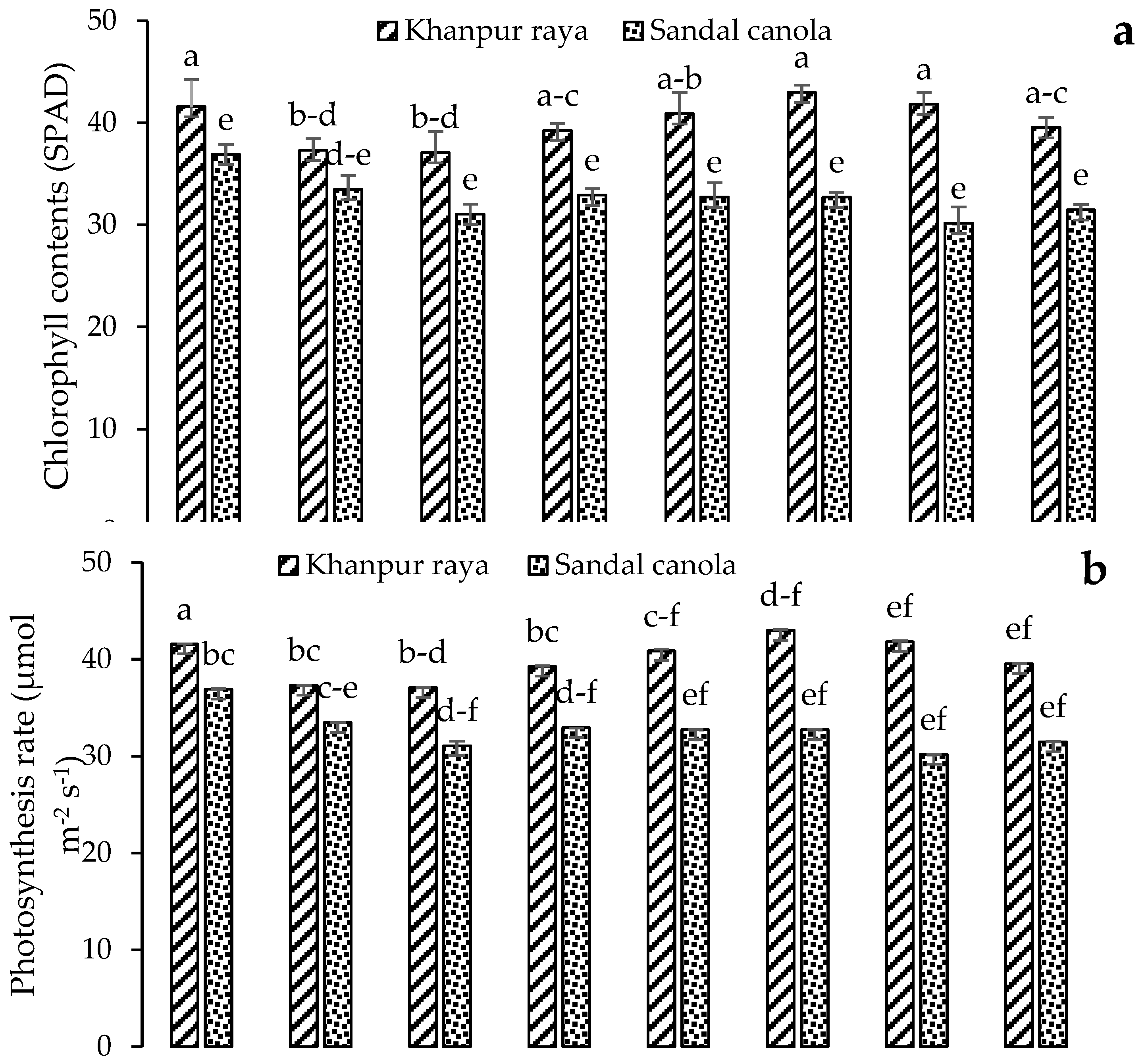
3.2.1. Chlorophyll Content (SPAD)
3.2.2. Photosynthetic Rate
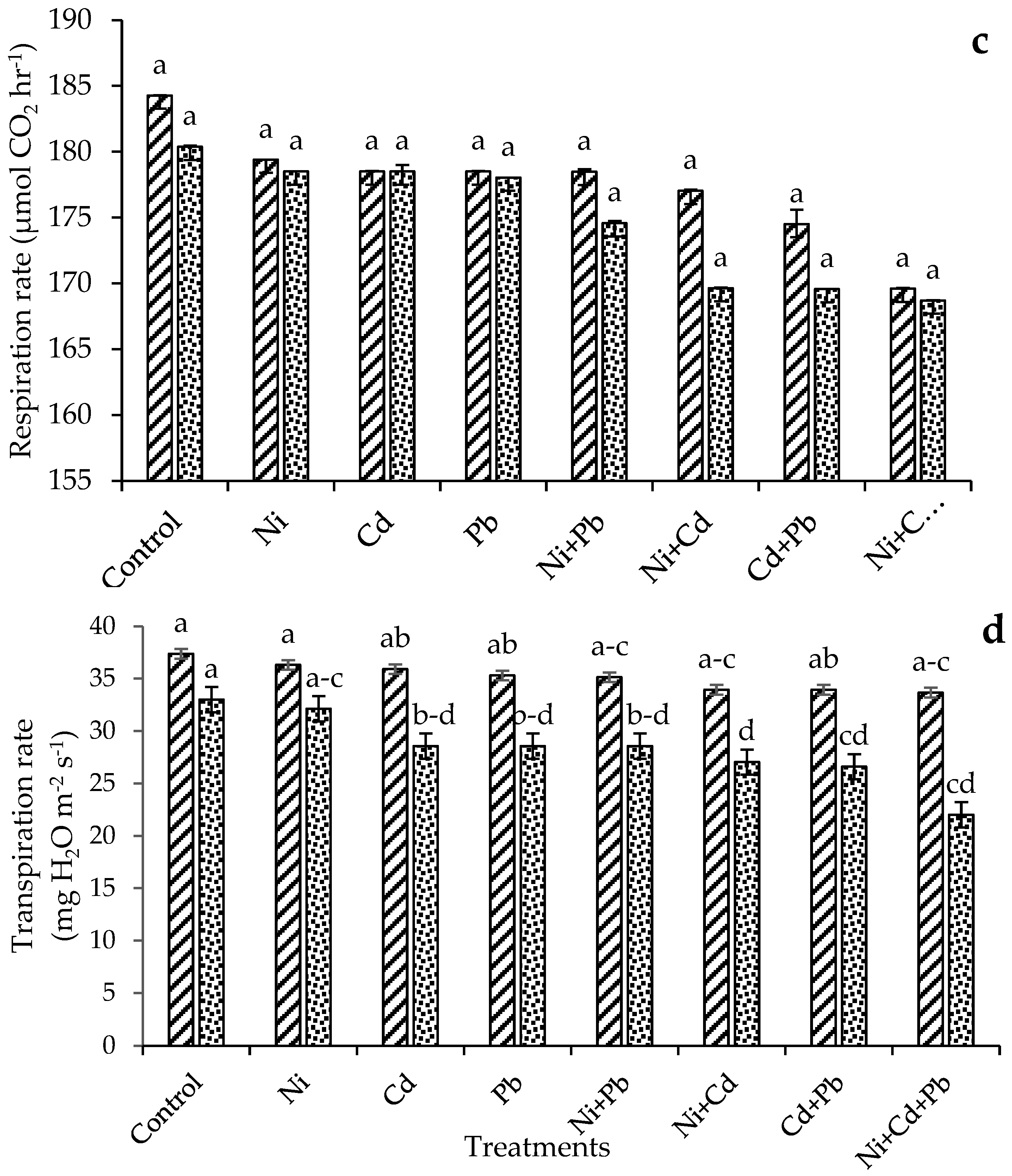
3.2.3. Respiration Rate
3.2.4. Transpiration Rate
3.3. Chemical Parameters
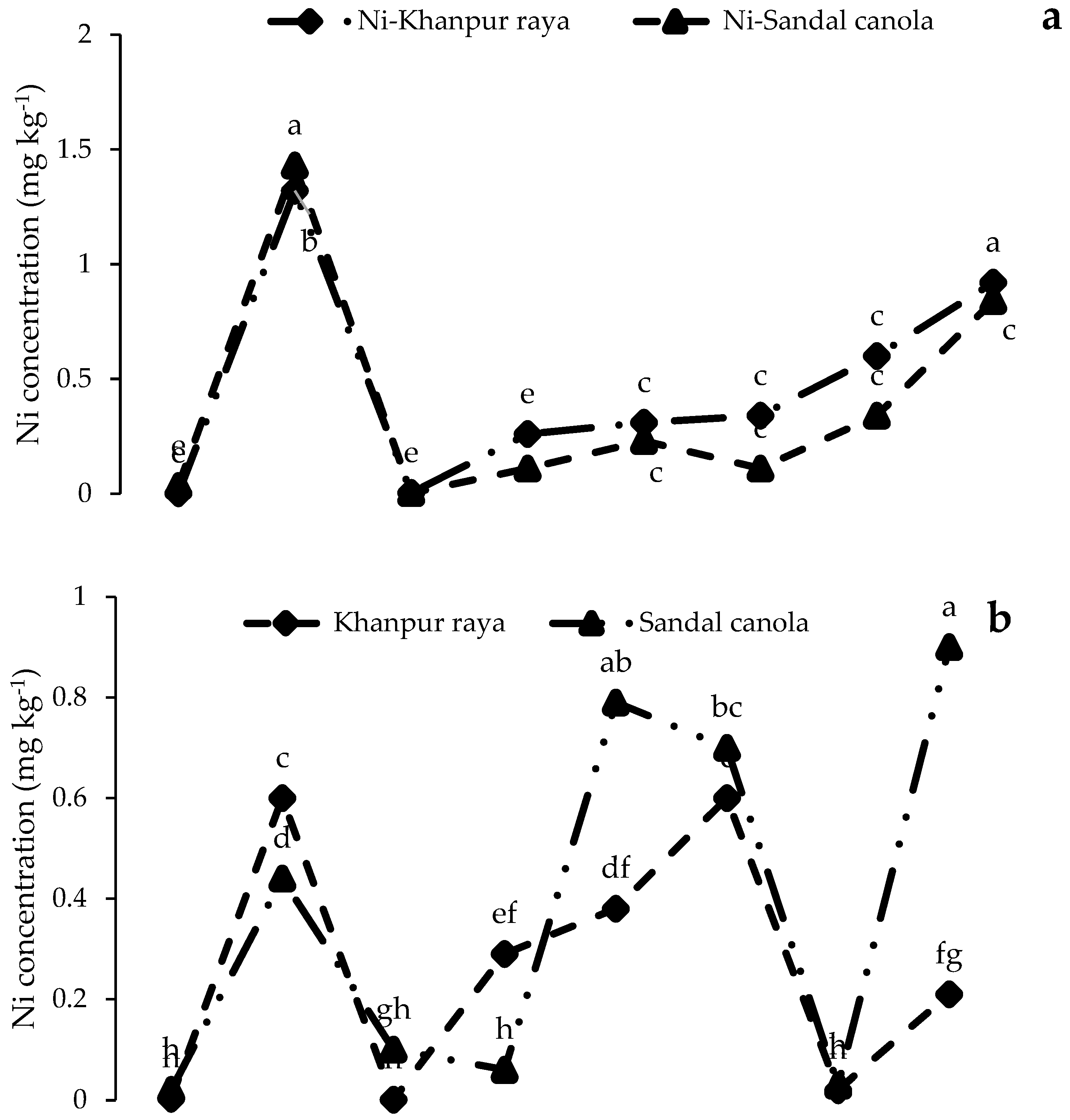
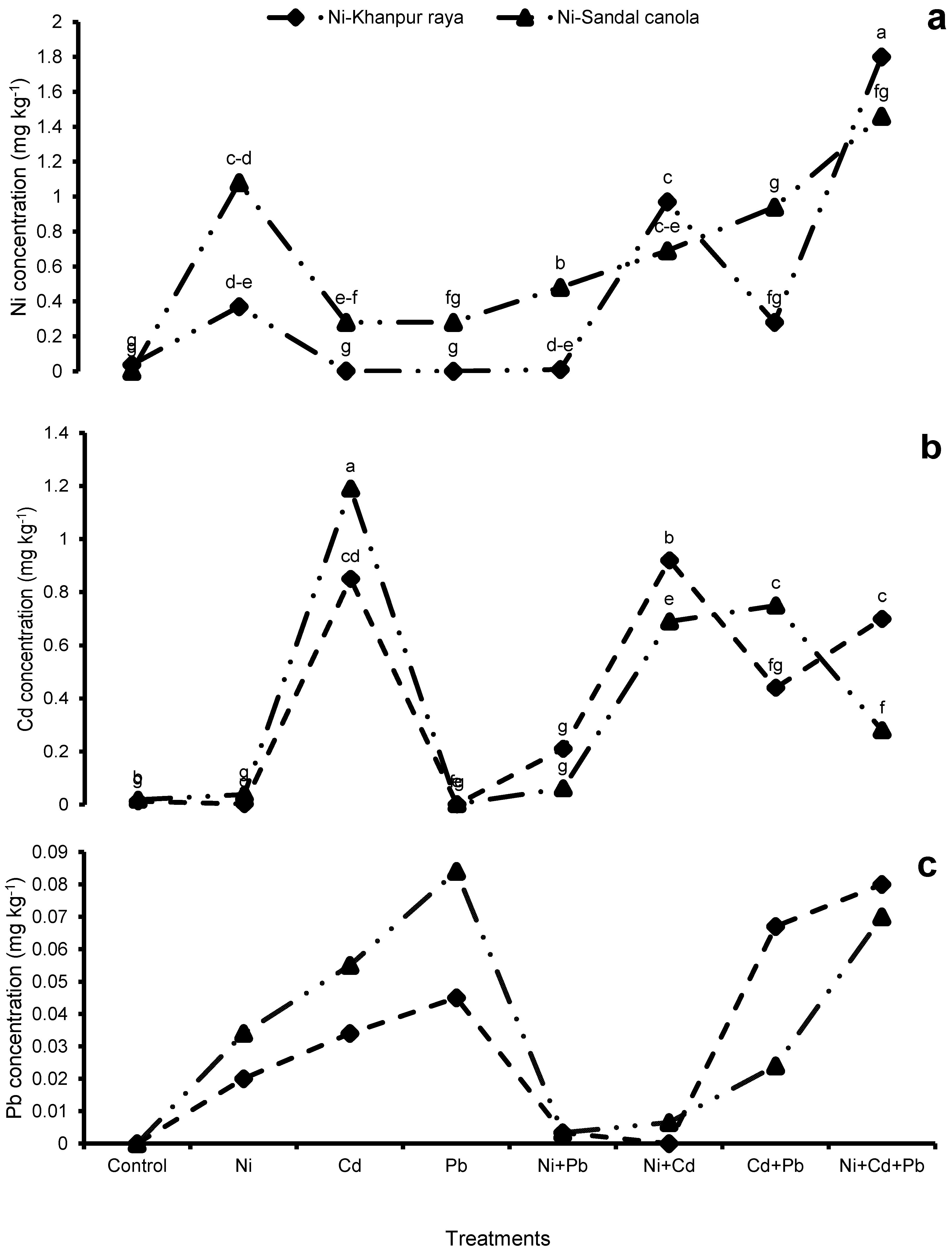
3.3.1. Ni Concentrations in Roots, Shoots, and Seeds
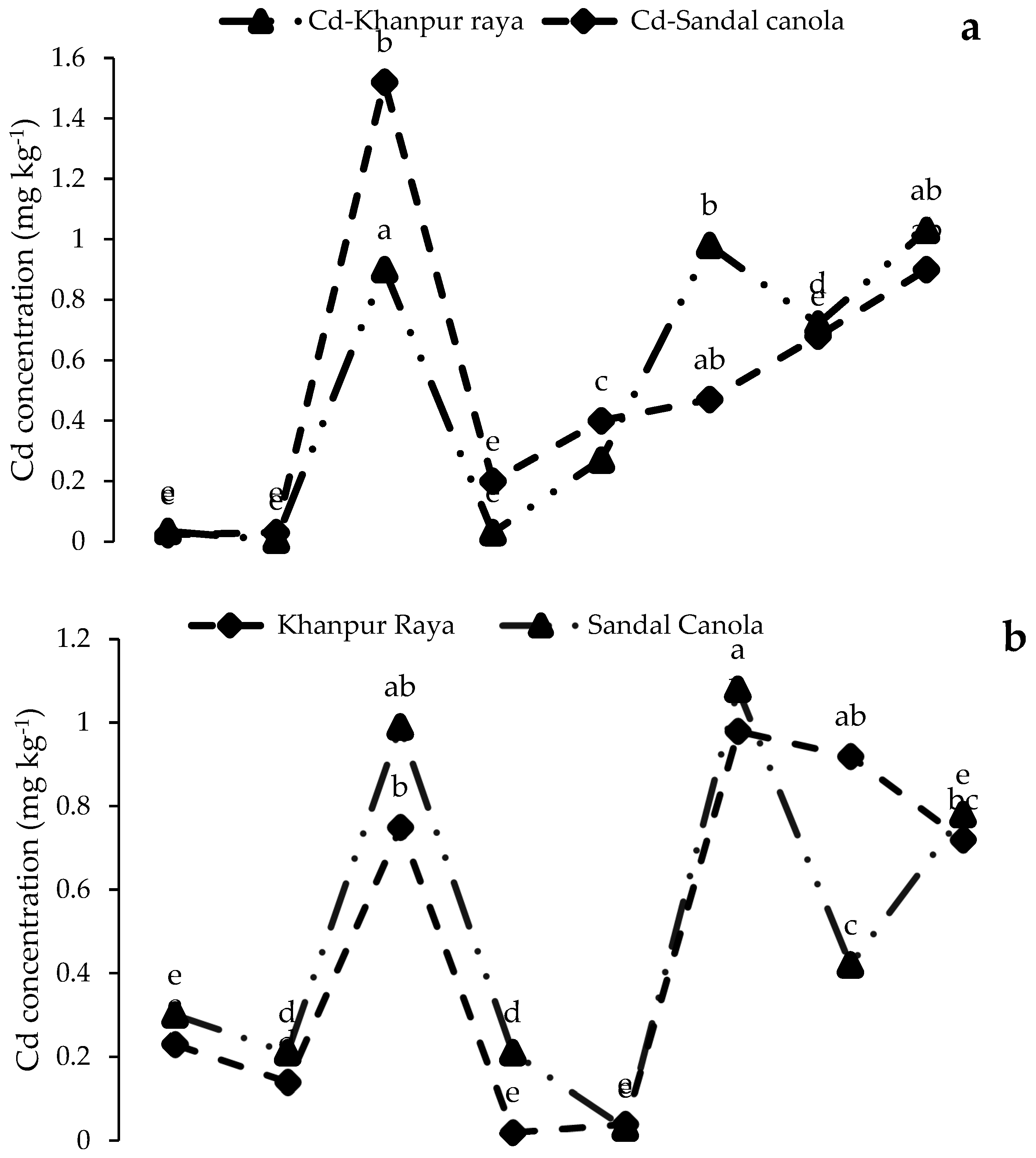
3.3.2. Cd Concentrations in Roots, Shoots, and Seeds
3.3.3. Pb concentrations in Roots, Shoots, and Seeds
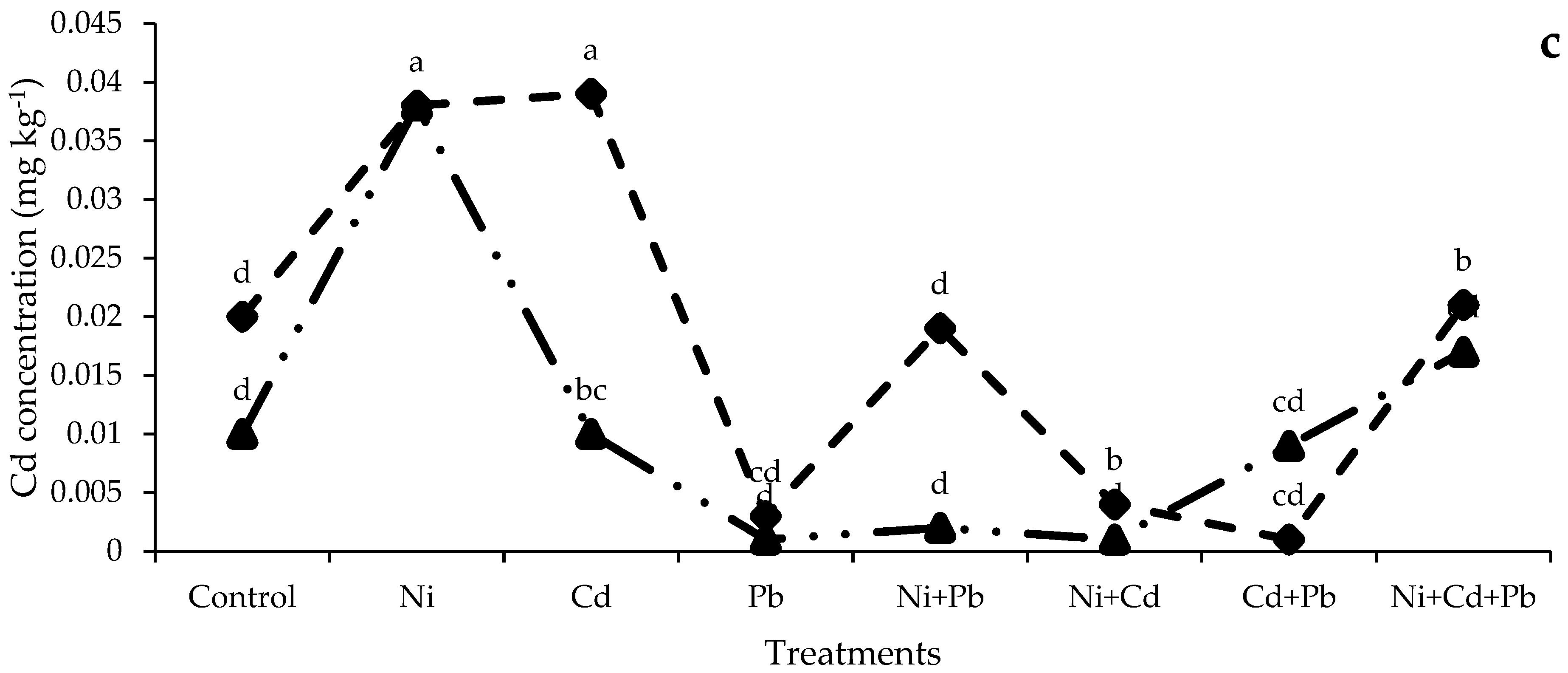
3.3.4. Ni, Cd, and Pb Concentrations (mg kg−1) in soil
3.3.5. Soil Quality and Contamination Indices
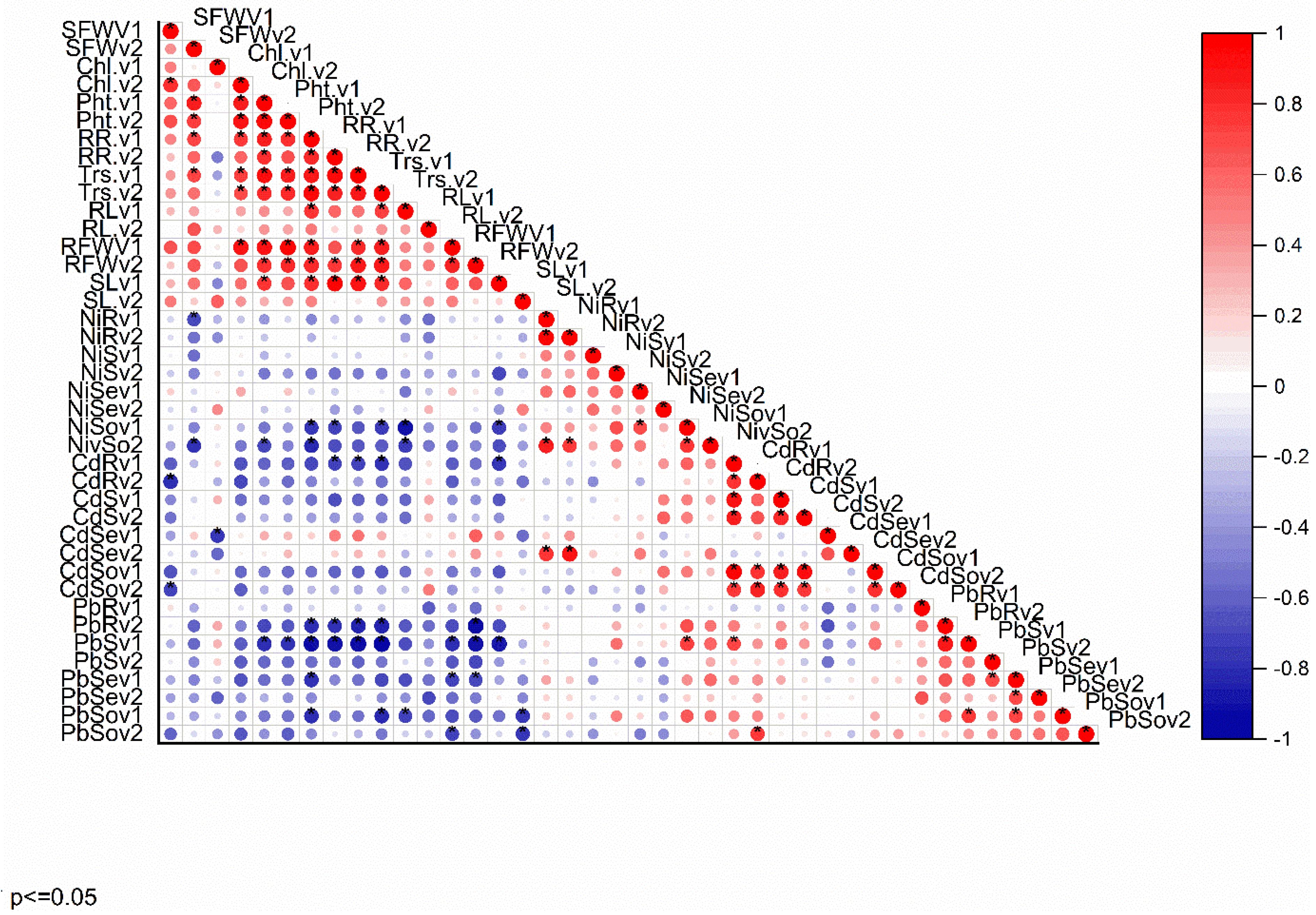
3.3.6. Correlation Analysis
4. Discussion
4.1. Plant Growth, Plant Yield, and Physiological Attributes
4.2. Physiological Parameters
4.3. Ni, Cd, and Pb Contents in Soil and Plant Tissues
4.4. Phytoremediation and Soil Metal Quality Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Alghobar, M.A.; Suresha, S. Evaluation of metal accumulation in soil and tomatoes irrigated with sewage water from Mysore city, Karnataka, India. J. Saudi Soc. Agric. Sci. 2017, 16, 49–59. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer Science & Business Media: Dordrecht, The Netherland, 2012; Volume 22. [Google Scholar]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Kiran, B.; Rani, S.; Rani, A.; Kaur, B.; Mittal, N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, T.; Yao, S.; Liu, C.; Yin, Y.; Li, H.; Li, N. A real filed phytoremediation of multi-metals contaminated soils by selected hybrid sweet sorghum with high biomass and high accumulation ability. Chemosphere 2019, 237, 124536. [Google Scholar] [CrossRef]
- Priju, C.; Narayana, A. Spatial and temporal variability of trace element concentrations in a tropical lagoon, Southwest Coast of India: Environmental Implications. J. Coast. Res. 2006, II, 1053–1057. [Google Scholar]
- Vara Prasad, M.N.; de Oliveira Freitas, H.M. Metal hyperaccumulation in plants: Biodiversity prospecting for phytoremediation technology. Electron. J. Biotechnol. 2003, 6, 285–321. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.; Bashir, A.; Maqbool, A.; Tack, F.; Ok, Y.S. Cadmium phytoremediation potential of Brassica crop species: A review. Sci. Total Environ. 2018, 631, 1175–1191. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; LWW: Washington, DC, USA, 1954; Volume 78. [Google Scholar]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis. A Man. West Asia North Afr. Reg. 2013, 3, 65–119. [Google Scholar]
- Farooqi, Z.U.R.; Murtaza, G.; Bibi, S.; Sabir, M.; Owens, G.; Saifullah; Ahmad, I.; Zeeshan, N. Immobilization of cadmium in soil-plant system through soil and foliar applied silicon. Int. J. Phytoremediation 2022, 24, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Alam, M.K.; Miah, M.J.; Majed, N. Removal of heavy metals in subsurface flow constructed wetlands: Application of effluent recirculation. Environ. Sustain. Indic. 2021, 12, 100146. [Google Scholar] [CrossRef]
- Sabir, A.; Naveed, M.; Bashir, M.A.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Núñez-Delgado, A.; Saeed, Q. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Ahmed, Z.; Howladar, M.F. Evaluation of heavy metal contamination in water, soil and plant around the open landfill site Mogla Bazar in Sylhet, Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100311. [Google Scholar] [CrossRef]
- Sheetal, K.; Singh, S.; Anand, A.; Prasad, S. Heavy metal accumulation and effects on growth, biomass and physiological processes in mustard. Indian J. Plant Physiol. 2016, 21, 219–223. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Chaturvedi, A.K.; Prasad, P. Effect of enhanced lead and cadmium in soil on physiological and biochemical attributes of Phaseolus vulgaris L. Nat. Sci. 2009, 7, 63–75. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Lugon-Moulin, N.; Ryan, L.; Donini, P.; Rossi, L. Cadmium content of phosphate fertilizers used for tobacco production. Agron. Sustain. Dev. 2006, 26, 151–155. [Google Scholar] [CrossRef]
- Ha, K.C.; Park, D.-U.; Yoon, C.S. Cadmium Concentrations in Environmental Tobacco Smoke of Indoor Environments. Anal. Sci. Technol. 2003, 16, 299–308. [Google Scholar]
- Tan, L.; Qu, M.; Zhu, Y.; Peng, C.; Wang, J.; Gao, D.; Chen, C. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 function synergistically in zinc/cadmium uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef]
- Cornu, J.-Y.; Bussiere, S.; Coriou, C.; Robert, T.; Maucourt, M.; Deborde, C.; Moing, A.; Nguyen, C. Changes in plant growth, Cd partitioning and xylem sap composition in two sunflower cultivars exposed to low Cd concentrations in hydroponics. Ecotoxicol. Environ. Saf. 2020, 205, 111145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, Y.; Zeng, H.; Zhou, X.; Peng, L.; Zeng, Q. Phytoextraction of cadmium-contaminated soil and potential of regenerated tobacco biomass for recovery of cadmium. Sci. Rep. 2017, 7, 7210. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Watanabe, Y.; Matsunaka, H.; Seki, M.; Fujita, M.; Kawada, N.; Hatta, K.; Nakajima, T. Differences in cadmium accumulation and root morphology in seedlings of Japanese wheat varieties with distinctive grain cadmium concentration. Plant Prod. Sci. 2011, 14, 148–155. [Google Scholar] [CrossRef]
- Shafi, M.; Zhang, G.; Bakht, J.; Khan, M.A.; Islam, U.; Khan, M.D.; Raziuddin, G. Effect of cadmium and salinity stresses on root morphology of wheat. Pak. J. Bot. 2010, 42, 2747–2754. [Google Scholar]
- Qin, P.; Wang, H.; Yang, X.; He, L.; Müller, K.; Shaheen, S.M.; Xu, S.; Rinklebe, J.; Tsang, D.C.; Ok, Y.S. Bamboo-and pig-derived biochars reduce leaching losses of dibutyl phthalate, cadmium, and lead from co-contaminated soils. Chemosphere 2018, 198, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Bagheri, M.; Zhang, W.; Chen, Z.; Burken, J.G.; Ma, X. Using artificial neural network to investigate physiological changes and cerium oxide nanoparticles and cadmium uptake by Brassica napus plants. Environ. Pollut. 2019, 246, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A. Modulation of phytoremediation and plant growth by the treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int. J. Phytoremediation 2016, 18, 1258–1269. [Google Scholar] [CrossRef]
- Ebbs, S.; Uchil, S. Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) Czern] involves preferential loss of chlorophyll b. Photosynthetica 2008, 46, 49–55. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Jiang, C.-Y.; Sheng, X.-F.; Qian, M.; Wang, Q.-Y. Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 2008, 72, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhu, K.; Zhang, Y.; Chai, T. Growth and cadmium accumulation of Solanum nigrum L. seedling were enhanced by heavy metal-tolerant strains of Pseudomonas aeruginosa. Water Air Soil Pollut. 2016, 227, 459. [Google Scholar] [CrossRef]
- Shankar, V.; Thekkeettil, V.; Sharma, G.; Agrawal, V. Alleviation of heavy metal stress in Spilanthes calva L.(antimalarial herb) by exogenous application of glutathione. Vitr. Cell. Dev. Biol.-Plant 2012, 48, 113–119. [Google Scholar] [CrossRef]
- Ashfaque, F.; Inam, A.; Iqbal, S.; Sahay, S. Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). South Afr. J. Bot. 2017, 111, 153–160. [Google Scholar] [CrossRef]
- Bitton, G.; Dutka, B.J. Toxicity Testing Using Microorganisms; CRC Press: Boca Raton, FL, USA, 2019; Volume 1. [Google Scholar]
- Barman, S.; Kisku, G.; Bhargava, S. Accumulation of heavy metals in vegetables, pulses and wheat grown in fly ash amended soil. J. Environ. Biol. 1999, 20, 15–18. [Google Scholar]
- Qayyum, M.F.; ur Rehman, M.Z.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 2017, 174, 515–523. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Majid, S.N.; Khwakaram, A.I.; Rasul, G.A.M.; Ahmed, Z.H. Bioaccumulation, enrichment and translocation factors of some heavy metals in Typha angustifolia and Phragmites australis Species growing ALONG Qalyasan stream in Sulaimani City/IKR. J. Zankoy Sulaimani-Part A 2014, 16, 93–109. [Google Scholar] [CrossRef]
- Takarina, N.D.; Pin, T.G. Bioconcentration factor (BCF) and translocation factor (TF) of heavy metals in mangrove trees of Blanakan fish farm. Makara J. Sci. 2017, 21, 4. [Google Scholar] [CrossRef]
- Kaushik, A.; Kansal, A.; Kumari, S.; Kaushik, C. Heavy metal contamination of river Yamuna, Haryana, India: Assessment by metal enrichment factor of the sediments. J. Hazard. Mater. 2009, 164, 265–270. [Google Scholar] [CrossRef]


| Soil Properties | Units | Values |
|---|---|---|
| soil texture | - | Sandy Clay loam |
| Sand | % | 61.01 |
| Silt | % | 18.70 |
| Clay | % | 20.29 |
| Saturation percentage (SP) | % | 32.50 |
| pHs | - | 7.30 |
| Ece | dS m−1 | 3.42 |
| TSS | mmolcL−1 | 34.2 |
| CO32− | mmolcL−1 | 1.00 |
| HCO3− | mmolcL−1 | 7.20 |
| Cl- | mmolcL−1 | 13.0 |
| Ca2++Mg2+ | mmolcL−1 | 23.0 |
| SO42− | mmolcL−1 | 14.0 |
| Na+ | mmolcL−1 | 12.2 |
| SAR | (mmolcL−1)1/2 | 12.0 |
| Symbol | Description |
|---|---|
| T0 | Control |
| T1 | Ni (Nickel nitrate) 90 mg kg−1 |
| T2 | Cd (Cadmium chloride) 20 mg kg−1 |
| T3 | Pb (Lead nitrate) 500 mg kg−1 |
| T4 | Ni + Pb (90 + 500) mg kg−1 |
| T5 | Ni + Cd (90 + 20) mg kg−1 |
| T6 | Cd + Pb (20 + 500) mg kg−1 |
| T7 | Cd + Pb + Ni (20 + 500 + 90) mg kg−1 |
| TF | ||||||
|---|---|---|---|---|---|---|
| Treatments | Khanpur Raya | Sandal Canola | ||||
| Ni | Cd | Pb | Ni | Cd | Pb | |
| Control | 11.00 | 0.75 | 0.00 | 3.67 | 1.33 | 0.00 |
| Ni | 0.75 | 1.46 | 2.89 | 0.99 | 0.33 | 1.07 |
| Cd | 684.83 | 0.18 | 4.52 | 0.02 | 0.03 | 1.04 |
| Pb | 0.52 | 1.00 | 0.15 | 139.05 | 0.03 | 0.24 |
| Ni + Pb | 2.29 | 0.00 | 1.85 | 0.43 | 1.18 | 1.64 |
| Ni + Cd | 0.86 | 0.57 | 4.65 | 0.66 | 1.43 | 0.08 |
| Cd + Pb | 0.68 | 1.48 | 0.97 | 0.00 | 1.20 | 1.98 |
| Ni + Cd + Pb | 3.84 | 0.03 | 1.58 | 0.70 | 44.20 | 0.99 |
| BAF | ||||||
| Treatments | Khanpur Raya | Sandal Canola | ||||
| Ni | Cd | Pb | Ni | Cd | Pb | |
| Control | 0.06 | 1.82 | 0.00 | 0.03 | 0.02 | 0.00 |
| Ni | 1.13 | 0.26 | 0.05 | 1.01 | 0.17 | 1.73 |
| Cd | 1.13 | 0.78 | 0.00 | 0.42 | 0.74 | 0.36 |
| Pb | 21.41 | 0.02 | 3.34 | 0.06 | 0.00 | 1.35 |
| Ni + Pb | 0.73 | 0.39 | 1.05 | 1.68 | 3.64 | 1.21 |
| Ni + Cd | 1.41 | 1.20 | 0.61 | 1.63 | 0.74 | 0.27 |
| Cd + Pb | 0.03 | 2.00 | 1.38 | 3.17 | 0.26 | 2.01 |
| Ni + Cd + Pb | 0.48 | 1.87 | 1.82 | 1.24 | 0.93 | 1.62 |
| EF | ||||||
| Treatments | Khanpur Raya | Sandal Canola | ||||
| Ni | Cd | Pb | Ni | Cd | Pb | |
| Control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ni | 0.01 | 0.00 | 0.00 | 0.03 | 0.01 | 0.00 |
| Cd | 0.00 | 0.19 | 0.02 | 0.01 | 0.27 | 0.03 |
| Pb | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.02 |
| Ni + Pb | 0.00 | 0.05 | 0.02 | 0.02 | 0.01 | 0.02 |
| Ni + Cd | 0.03 | 0.21 | 0.00 | 0.02 | 0.16 | 0.00 |
| Cd + Pb | 0.01 | 0.10 | 0.01 | 0.03 | 0.17 | 0.01 |
| Ni + Cd + Pb | 0.06 | 0.16 | 0.06 | 0.05 | 0.06 | 0.02 |
| PLI | ||||||
| Treatments | Khanpur Raya | Sandal Canola | ||||
| Control | 1.10839 × 10−8 | 0 | ||||
| Ni | 1.95927 × 10−8 | 1.4 × 10−6 | ||||
| Cd | 3.47222 × 10−7 | 0.000113 | ||||
| Pb | 0 | 1.14 × 10−7 | ||||
| Ni + Pb | 5.24237 × 10−7 | 7.31 × 10−6 | ||||
| Ni + Cd | 2.6328 × 10−5 | 9.36 × 10−6 | ||||
| Cd + Pb | 2.19014 × 10−5 | 0.000125 | ||||
| Ni + Cd + Pb | 0.000857842 | 0.000108 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlawi, S.; Sadiq, M.; Sabir, M.; Farooqi, Z.U.R.; Saifullah; Qadir, A.A.; Faraj, T.K. Differential Response of Brassica Cultivars to Potentially Toxic Elements and Their Distribution in Different Plant Parts Irrigated with Metal-Contaminated Water. Sustainability 2023, 15, 1966. https://doi.org/10.3390/su15031966
Dahlawi S, Sadiq M, Sabir M, Farooqi ZUR, Saifullah, Qadir AA, Faraj TK. Differential Response of Brassica Cultivars to Potentially Toxic Elements and Their Distribution in Different Plant Parts Irrigated with Metal-Contaminated Water. Sustainability. 2023; 15(3):1966. https://doi.org/10.3390/su15031966
Chicago/Turabian StyleDahlawi, Saad, Muhammad Sadiq, Muhammad Sabir, Zia Ur Rahman Farooqi, Saifullah, Ayesha Abdul Qadir, and Turki Kh Faraj. 2023. "Differential Response of Brassica Cultivars to Potentially Toxic Elements and Their Distribution in Different Plant Parts Irrigated with Metal-Contaminated Water" Sustainability 15, no. 3: 1966. https://doi.org/10.3390/su15031966
APA StyleDahlawi, S., Sadiq, M., Sabir, M., Farooqi, Z. U. R., Saifullah, Qadir, A. A., & Faraj, T. K. (2023). Differential Response of Brassica Cultivars to Potentially Toxic Elements and Their Distribution in Different Plant Parts Irrigated with Metal-Contaminated Water. Sustainability, 15(3), 1966. https://doi.org/10.3390/su15031966





