Predicting Cu(II) Adsorption from Aqueous Solutions onto Nano Zero-Valent Aluminum (nZVAl) by Machine Learning and Artificial Intelligence Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cu(II) Solution
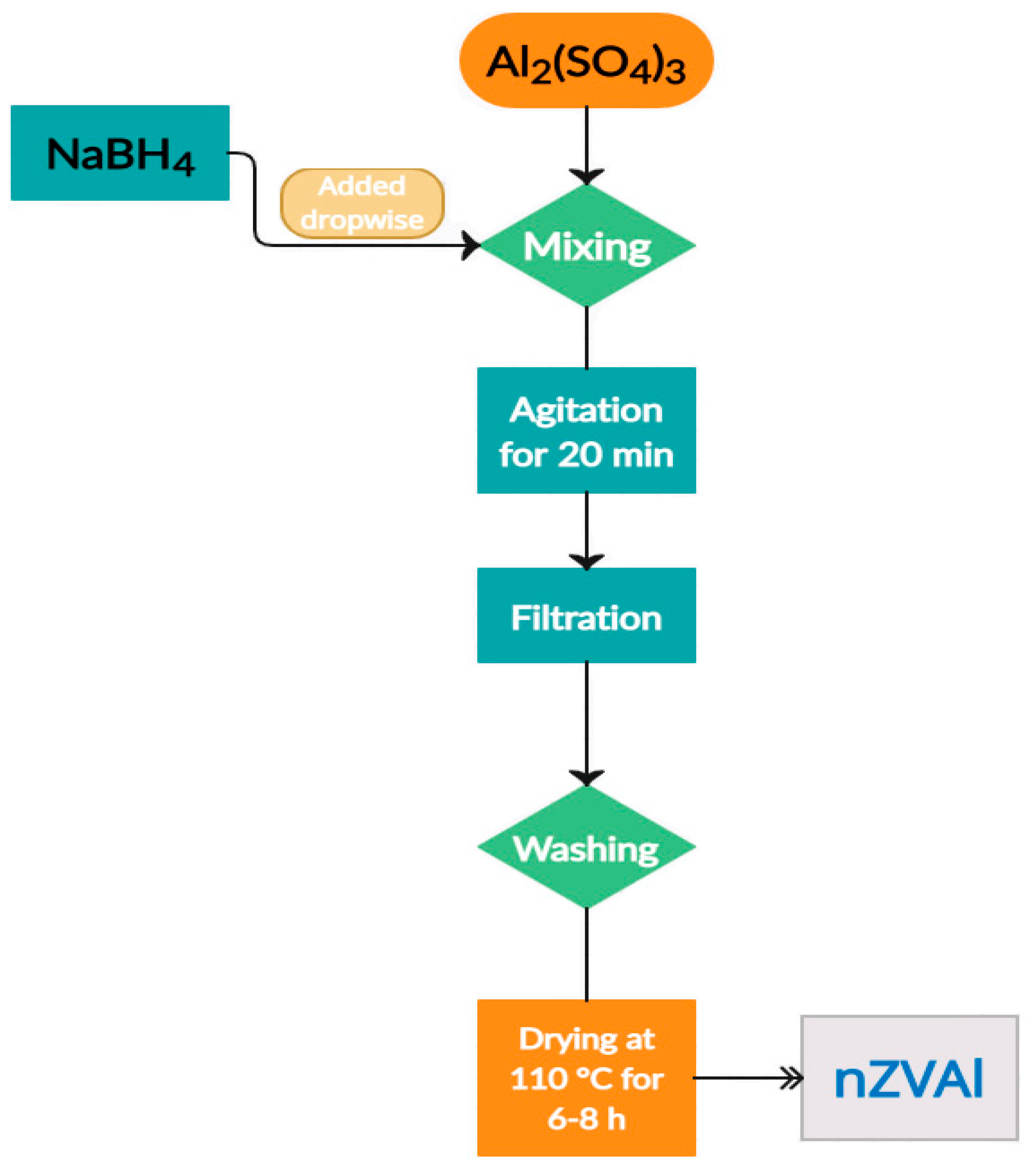
2.2. Synthesis of Adsorbent
2.3. Instrumentation
2.4. Experimental Setup
2.5. Modelling Techniques
2.5.1. Support Vector Regression (SVR)
2.5.2. Linear Regression
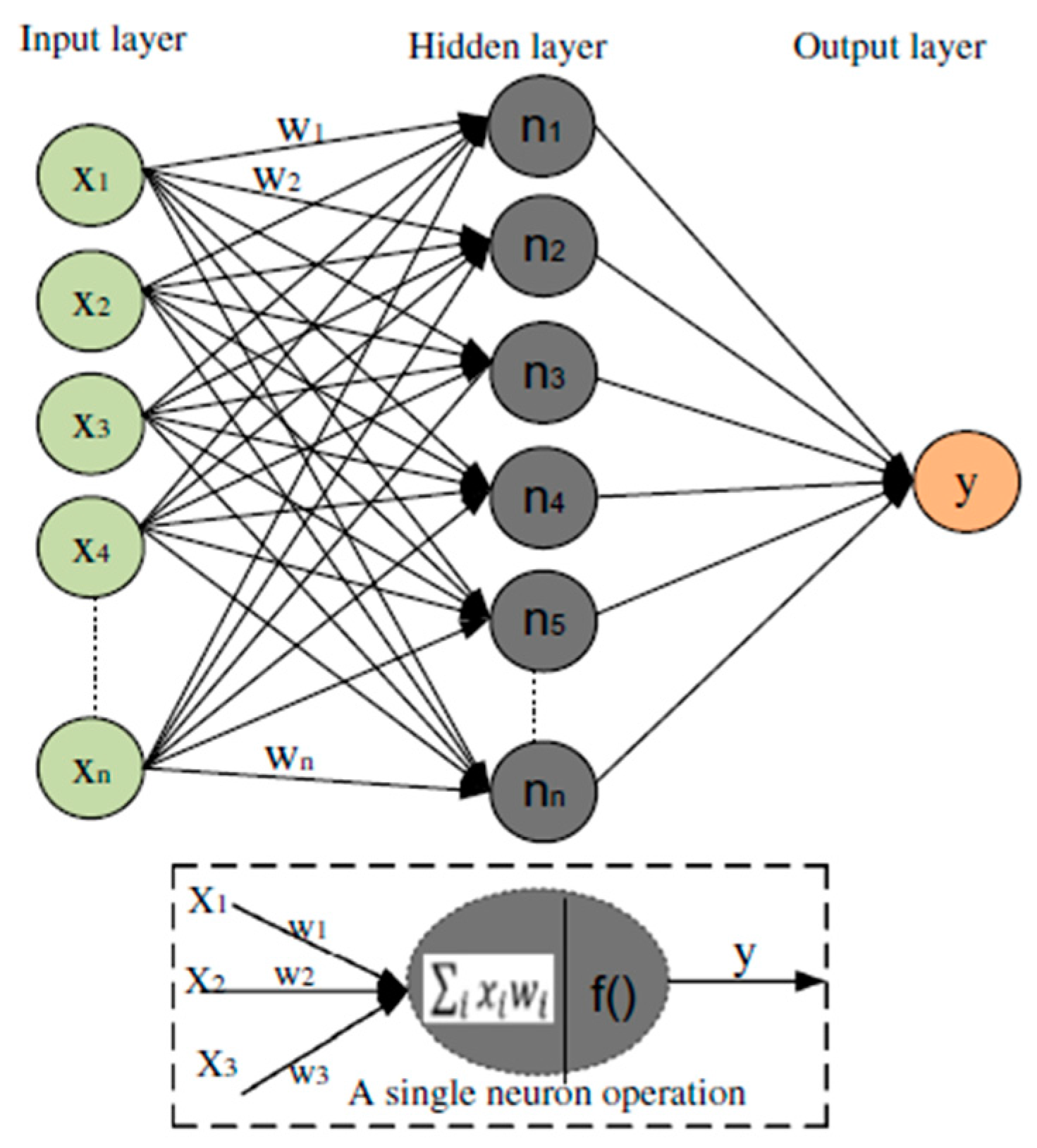
2.5.3. Artificial Neural Network (ANN)
3. Results and Discussion
3.1. Results of the Experimental Characterization
3.2. Effects of Operating Parameters on Cu(II) Removal
3.3. Cu(II) Removal by Various Adsorbents Mentioned in Previous Studies
3.4. Cu(II) Removal Mechanism by nZVAl
3.5. Isotherm and Kinetic Investigations
3.6. Thermodynamic Study
3.7. Modelling Evaluation Techniques
3.8. Future nZVAl-Based Potential Research Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Yuan, X.; Im, S.I.; Choi, S.W.; Lee, K.B. Removal of Cu(II) ions from aqueous solutions using petroleum coke-derived microporous carbon: Investigation of adsorption equilibrium and kinetics. Adsorption 2019, 25, 1205–1218. [Google Scholar] [CrossRef]
- Ma, J.; Qin, G.; Zhang, Y.; Sun, J.; Wang, S.; Jiang, L. Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J. Clean. Prod. 2018, 182, 776–782. [Google Scholar] [CrossRef]
- Macías-García, A.; Gómez Corzo, M.; Alfaro Domínguez, M.; Alexandre Franco, M.; Martínez Naharro, J. Study of the adsorption and electroadsorption process of Cu (II) ions within thermally and chemically modified activated carbon. J. Hazard. Mater. 2017, 328, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Parlayıcı; Pehlivan, E. Removal of metals by Fe3O4 loaded activated carbon prepared from plum stone (Prunus nigra): Kinetics and modelling study. Powder Technol. 2017, 317, 23–30. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rijith, S. Glutaraldehyde cross-linked epoxyaminated chitosan as an adsorbent for the removal and recovery of copper(II) from aqueous media. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 351, 52–59. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water. Air. Soil Pollut. 2020, 231, 1–17. [Google Scholar] [CrossRef]
- Madhava Rao, M.; Ramesh, A.; Purna Chandra Rao, G.; Seshaiah, K. Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls. J. Hazard. Mater. 2006, 129, 123–129. [Google Scholar] [CrossRef]
- Mostafa, M.K.; Mahmoud, A.S.; Mahmoud, M.S.; Nasr, M. Computational-Based Approaches for Predicting Biochemical Oxygen Demand (BOD) Removal in Adsorption Process. Adsorpt. Sci. Technol. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Kamar, M.T.; Elattar, H.; Mahmoud, A.S.; Peters, R.W.; Mostafa, M.K. A critical review of state-of-the-art technologies for electroplating wastewater treatment. Int. J. Environ. Anal. Chem. 2022, 1–34. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Hassan, G.K.; Abdel-Karim, A.; Al-Shemy, M.T.; Rojas, P.; Sanz, J.L.; Ismail, S.H.; Mohamed, G.G.; El-gohary, F.A.; Al-sayed, A. Harnessing Cu@Fe3O4 core shell nanostructure for biogas production from sewage sludge: Experimental study and microbial community shift. Renew. Energy 2022, 188, 1059–1071. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Deng, D.; Lamssali, M.; Aryal, N.; Ofori-Boadu, A.; Jha, M.K.; Samuel, R.E. Textiles wastewater treatment technology: A review. Water Environ. Res. 2020, 92, 1805–1810. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Khatri, J.; Anantha Singh, T.S.; Gandhimathi, R.; Ramesh, S.T. Review of zero-valent aluminium based water and wastewater treatment methods. Chemosphere 2018, 200, 621–631. [Google Scholar] [CrossRef]
- Ileri, B.; Dogu, I. Sono–degradation of Reactive Blue 19 in aqueous solution and synthetic textile industry wastewater by nanoscale zero–valent aluminum. J. Environ. Manag. 2022, 303, 114200. [Google Scholar] [CrossRef]
- Sadek, A.H.; Mostafa, M.K. Preparation of nano zero-valent aluminum for one-step removal of methylene blue from aqueous solutions: Cost analysis for scaling-up and artificial intelligence. Appl. Water Sci. 2022, 13, 1–23. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Farag, R.S.; Elshfai, M.M.; Mohamed, L.A.; Ragheb, S.M. Nano Zero-Valent Aluminum (nZVAl) Preparation, Characterization, and Application for the Removal of Soluble Organic Matter with Artificial Intelligence, Isotherm Study, and Kinetic Analysis. Air Soil Water Res. 2019, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, S.K.; Pyrgaki, K.; Salih, S.Q.; Tiyasha, T.; Beyaztas, U.; Shahid, S.; Yaseen, Z.M. Prediction of copper ions adsorption by attapulgite adsorbent using tuned-artificial intelligence model. Chemosphere 2021, 276, 130162. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Alavi Moghaddam, M.R.; Kowsari, E. A systematic and critical review on development of machine learning based-ensemble models for prediction of adsorption process efficiency. J. Clean. Prod. 2022, 379, 134588. [Google Scholar] [CrossRef]
- Hafsa, N.; Rushd, S.; Al-Yaari, M.; Rahman, M. A Generalized Method for Modeling the Adsorption of Heavy Metals with Machine Learning Algorithms. Water 2020, 12, 3490. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Farag, R.S.; Elshfai, M.M. Reduction of organic matter from municipal wastewater at low cost using green synthesis nano iron extracted from black tea: Artificial intelligence with regression analysis. Egypt. J. Pet. 2020, 29, 9–20. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mohamed, N.Y.; Mostafa, M.K.; Mahmoud, M.S. Effective Chromium Adsorption From Aqueous Solutions and Tannery Wastewater Using Bimetallic Fe/Cu Nanoparticles: Response Surface Methodology and Artificial Neural Network. Air Soil Water Res. 2021, 14. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Peters, R.W. A prototype of textile wastewater treatment using coagulation and adsorption by Fe/Cu nanoparticles: Techno-economic and scaling-up studies. Nanomater. Nanotechnol. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- Yasmin, N.S.A.; Wahab, N.A.; Ismail, F.S.; Musa, M.J.; Halim, M.H.A.; Anuar, A.N. Support Vector Regression Modelling of an Aerobic Granular Sludge in Sequential Batch Reactor. Membranes 2021, 11, 554. [Google Scholar] [CrossRef]
- Mundi, G.; Zytner, R.G.; Warriner, K.; Bonakdari, H.; Gharabaghi, B.; Ayed, L.B.; Golomazou, E.; Karanis, P.; Scheid, P.; Tzoraki, O.; et al. Machine Learning Models for Predicting Water Quality of Treated Fruit and Vegetable Wastewater. Water 2021, 13, 2485. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Shi, P.; Bi, S.; Wang, C. RegCNN: A Deep Multi-output Regression Method for Wastewater Treatment. In Proceedings of the 2019 IEEE 31st Int. Conf. Tools with Artif Intell, Portland, OR, USA, 4–6 November 2019; Volume 2019, pp. 816–823. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Mahmoud, A.S. Wastewater treatment using nano bimetallic iron/copper, adsorption isotherm, kinetic studies, and artificial intelligence neural networks. Emergent Mater. 2021, 4, 1455–1463. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mahmoud, M.S.; Noureldin, A.M.; Peters, R.W.; Mostafa, M.K. (423f) Effective Adsorption of Chromium from Tannery Wastewater Using Green Synthesis Nano-Zero Valent Iron (GT-nZVI) | AIChE Academy. In Proceedings of the 2021 Annual Meeting, 7–19 November 2021; Available online: https://www.aiche.org/academy/conferences/aiche-annual-meeting/2021/proceeding/paper/423f-effective-adsorption-chromium-tannery-wastewater-using-green-synthesis-nano-zero-valent-iron-gt (accessed on 13 October 2022).
- Mahmoud, M.S.; Mahmoud, A.S.; El-Said, M.A.; Mostafa, M.K. Comparison of aluminum and iron nanoparticles for chromium removal from aqueous solutions and tannery wastewater, empirical modeling and prediction. Emergent Mater. 2022, 5, 1729–1744. [Google Scholar] [CrossRef]
- Farag, R.S.; Elshfai, M.M.; Mahmoud, A.S.; Mostafa, M.K.; Peters, R.W. (592d) Green Synthesis of Nano Iron Carbide: Preparation, Characterization and Application for Removal of Phosphate from Aqueous Solutions. In Proceedings of the 2018 AIChE Annual Meeting, Lawrence Convention Center, Pittsburgh, PA, USA, 28 October–2 November 2018; Available online: https://www.aiche.org/conferences/aiche-annual-meeting/2018/proceeding/paper/592d-green-synthesis-nano-iron-carbide-preparation-characterization-and-application-removal (accessed on 13 October 2022).
- Fan, M.; Hu, J.; Cao, R.; Xiong, K.; Wei, X. Modeling and prediction of copper removal from aqueous solutions by nZVI/rGO magnetic nanocomposites using ANN-GA and ANN-PSO. Sci. Rep. 2017, 7, 18040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, F.; Papirio, S.; Esposito, G.; Gargano, R.; de Marinis, G. Machine Learning Algorithms for the Forecasting of Wastewater Quality Indicators. Water 2017, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, K.; Bonakdari, H.; Ebtehaj, I.; Delatolla, R.; Zinatizadeh, A.A.; Gharabaghi, B. A novel stochastic wastewater quality modeling based on fuzzy techniques. J. Environ. Health Sci. Eng. 2020, 18, 1099. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, K.; Bonakdari, H.; Ebtehaj, I.; Mjalli, F.S.; Zeynoddin, M.; Delatolla, R.; Gharabaghi, B. Predicting wastewater treatment plant quality parameters using a novel hybrid linear-nonlinear methodology. J. Environ. Manag. 2019, 240, 463–474. [Google Scholar] [CrossRef]
- Sadek, A.H.; Mostafa, M. PCT/EG2017/000029 Preparation of Zerovalent Aluminium Nanoparticles at Room Temperature and Uses Thereof. WIPO. 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019057262 (accessed on 14 October 2022).
- Muniz, F.; Miranda, M.; Santos, C.M.D.; Sasaki, J. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, 385–390. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Chii, Y.Y.; Siddique, B.M. Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on sawdust of Meranti wood. Desalination 2009, 247, 636–646. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Endud, C.S.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Samanta, B.; Al-Balushi, K.R.; Al-Araimi, S.A. Bearing Fault Detection Using Artificial Neural Networks and Genetic Algorithm. EURASIP J. Appl. Signal Process. 2004, 3, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Yaqub, M.; Eren, B.; Eyupoglu, V. Soft computing techniques in prediction Cr(VI) removal efficiency of polymer inclusion membranes. Environ. Eng. Res. 2020, 25, 418–425. [Google Scholar] [CrossRef]
- Niu, G.; Yi, X.; Chen, C.; Li, X.; Han, D.; Yan, B.; Huang, M.; Ying, G. A novel effluent quality predicting model based on genetic-deep belief network algorithm for cleaner production in a full-scale paper-making wastewater treatment. J. Clean. Prod. 2020, 265, 121787. [Google Scholar] [CrossRef]
- Yaqub, M.; Eren, B.; Eyupoglu, V. Prediction of heavy metals removal by polymer inclusion membranes using machine learning techniques. Water Environ. J. 2021, 35, 1073–1084. [Google Scholar] [CrossRef]
- Yang, Y.; Gai, W.Z.; Zhou, J.G.; Deng, Z.Y. Surface modified zero-valent aluminum for Cr(VI) removal at neutral pH. Chem. Eng. J. 2020, 395, 125140. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, S.; Liu, J.; Ren, T.; Zhang, Y.; Sun, X. Degradation of hexabromocyclododecane (HBCD) by nanoscale zero-valent aluminum (nZVAl). Chemosphere 2020, 244, 125536. [Google Scholar] [CrossRef]
- Moreno-Piraján, J.C.; Giraldo, L. Adsorption of copper from aqueous solution by activated carbons obtained by pyrolysis of cassava peel. J. Anal. Appl. Pyrolysis 2010, 87, 188–193. [Google Scholar] [CrossRef]
- Bouhamed, F.; Elouear, Z.; Bouzid, J. Adsorptive removal of copper(II) from aqueous solutions on activated carbon prepared from Tunisian date stones: Equilibrium, kinetics and thermodynamics. J. Taiwan Inst. Chem. Eng. 2012, 43, 741–749. [Google Scholar] [CrossRef]
- Alharby, N.F.; Almutairi, R.S.; Mohamed, N.A. Adsorption Behavior of Methylene Blue Dye by Novel CrossLinked O-CM-Chitosan Hydrogel in Aqueous Solution: Kinetics, Isotherm and Thermodynamics. Polymers 2021, 13, 3659. [Google Scholar] [CrossRef]
- Benzaoui, T.; Selatnia, A.; Djabali, D. Adsorption of copper (II) ions from aqueous solution using bottom ash of expired drugs incineration. Adsorpt. Sci. Technol. 2018, 36, 114–129. [Google Scholar] [CrossRef]
- Pavan Kumar, G.V.S.R.; Komal, A.M.; Yerra, B.; Rao, K.S. Removal of Cu(II) using three low-cost adsorbents and prediction of adsorption using artificial neural networks. Appl. Water Sci. 2019, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, S.; Azis, R.S.; Ismail, I.; Man, H.C.; Yusof, K.F.M.; Abba, M.U.; Katibi, K.K. Adsorptive Removal of Copper (II) Ions from Aqueous Solution Using a Magnetite Nano-Adsorbent from Mill Scale Waste: Synthesis, Characterization, Adsorption and Kinetic Modelling Studies. Nanoscale Res. Lett. 2021, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ezati, F.; Sepehr, E.; Ahmadi, F. The efficiency of nano-TiO2 and γ-Al2O3 in copper removal from aqueous solution by characterization and adsorption study. Sci. Rep. 2021, 11, 18831. [Google Scholar] [CrossRef] [PubMed]
- Andelescu, A.; Nistor, M.A.; Muntean, S.G.; Rădulescu-Grad, M.E. Adsorption studies on copper, cadmium, and zinc ion removal from aqueous solution using magnetite/carbon nanocomposites. Sep. Sci. Technol. 2018, 53, 2352–2364. [Google Scholar] [CrossRef]
- Duddridge, J.E.; Wainwright, M. Heavy metals in river sediments—calculation of metal adsorption maxima using Langmuir and Freundlich isotherms. Environ. Pollut. Ser. B Chem. Phys. 1981, 2, 387–397. [Google Scholar] [CrossRef]
- Eloussaief, M.; Jarraya, I.; Benzina, M. Adsorption of copper ions on two clays from Tunisia: pH and temperature effects. Appl. Clay Sci. 2009, 46, 409–413. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Mathias, P.M. The Gibbs–Helmholtz Equation in Chemical Process Technology. Ind. Eng. Chem. Res. 2016, 55, 1076–1087. [Google Scholar] [CrossRef]








| Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | Cr2O3 | ZnO | FeO or Fe2O3 | CuO | Loss of Ignition | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Cu(II) ion adsorption | 77.07 ± 3.36 | 2.46 ± 0.17 | 0.69 ± 0.03 | 11.16 ± 0.58 | 2.89 ± 0.14 | 0.22 ± 0.01 | 0.31 ± 0.01 | 0.04 ± 0.02 | 0.037 ± 0.002 | 0 | 0 | 5.12 ± 0.27 |
| After Cu(II) ion adsorption | 70.12 ± 3.80 | 2.42 ± 0.15 | 0.55 ± 0.02 | 5.07 ± 0.25 | 4.55 ± 0.17 | 0 | 0.28 ± 0.01 | 0 | 0.012 ± 0.002 | 0.06 ± 0.008 or 0.07 ± 0.009 | 2.94 ± 0.12 | 14 ± 0.723 or 13.98 ± 0.724 |
| Adsorbent | Adsorbent Dosage (g/L) | pH | Co (mg/L) | Time (min) | Temp. (°C) | Stirring Rate (rpm) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| nZVAl | 0.25 | 5 | 10 | 10 | 30 | 150 | 45.9 | This study |
| Groundnut seed cake powder (GNCSP) | 0.25 | 5 | 10 | 30 | 40 | N/A | 43 | [54] |
| Sesame seed cake powder (SSCP) | 0.25 | 5 | 10 | 30 | 40 | N/A | 40 | [54] |
| Coconut cake powder (CCP) | 0.25 | 5 | 10 | 30 | 40 | N/A | 41 | [54] |
| Magnetite Nano-Adsorbent from Mill Scale Waste | 0.05 | 5.4 | 10 | 30 | 25 | N/A | 49 | [55] |
| Titanium oxide (TiO2) nanosorbents | 0.05 | 6 | 10 | 120 | 25 | 1000 | 98 | [56] |
| Bottom ash of expired drugs incineration (BAEDI) | 1.0 | 5 | 50 | 15 | 25 | N/A | 22 | [53] |
| Magnetite/carbon nanocomposites | 0.5 | 6 | 10 | 240 | 25 | N/A | 68 | [57] |
| Model | Parameter | Fitting Accuracy (R2) |
|---|---|---|
| Langmuir isotherm | qm = 5.892 mg/g KL = 0.921 L/mg | 0.9248 |
| Freundlich isotherm | 1/n = 0.2282 KF = 3.178 (mg/g)·(L/mg)1/n | 0.8952 |
| Pseudo-first-order | qe = 5.2 mg/g k1 = 0.0826/min | 0.7895 |
| Pseudo-second-order | qe = 7.4 mg/g k2 = 0.00477 g/mg/min | 0.9957 |
| T (K) | K° (mL/g) | ln(K°) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol/K) | R2 |
|---|---|---|---|---|---|---|
| 303 | 316.32 | 5.76 | −14.50 | 5.77 | 66.92 | 0.997 |
| 323 | 276.72 | 5.62 | −15.10 | 46.75 | ||
| 333 | 256.70 | 5.55 | −15.36 | 46.13 |
| ANN Model Parameters | |
|---|---|
| Hidden layers | 3 |
| Activation function | rectified linear unit (ReLU) |
| Optimizer | Stochastic gradient descent (SGD) |
| Loss function | Mean Squared Error (MSE) |
| No. iterations | 250 |
| Batch size | 32 |
| Weight initializer | Uniform initialization |
| SVR model parameters | |
| Kernel type | polynomial (Poly) |
| Kernel degree | 3 |
| ‘scale’ | |
| 0.1 | |
| pH | Time | Initial Concentration (mg/L) | nZVAl Dose (g/L) | Stirring Rate (rpm) | Temp (°C) | Removal Efficiency (%) | ANN R% | LR R% | SVR R% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 50 | 0.25 | 150 | 30 | 18.1 | 18.0691 | 17.6548 | 18.0466 |
| 3 | 10 | 50 | 0.25 | 150 | 30 | 18.8 | 18.7668 | 18.3520 | 18.7438 |
| 5 | 10 | 50 | 0.25 | 150 | 30 | 27.9 | 27.9118 | 27.4970 | 27.8888 |
| 7 | 10 | 50 | 0.25 | 150 | 30 | 40.0 | 39.9914 | 39.5766 | 39.9684 |
| 5 | 10 | 50 | 0.25 | 150 | 30 | 27.9 | 27.9118 | 27.4970 | 27.8888 |
| 5 | 20 | 50 | 0.25 | 150 | 30 | 29.7 | 29.6872 | 29.2724 | 29.6642 |
| 5 | 30 | 50 | 0.25 | 150 | 30 | 30.9 | 30.8730 | 30.4582 | 30.8500 |
| 5 | 40 | 50 | 0.25 | 150 | 30 | 32.2 | 32.2402 | 31.8254 | 32.2172 |
| 5 | 50 | 50 | 0.25 | 150 | 30 | 34.7 | 34.6550 | 34.2402 | 34.6320 |
| 5 | 60 | 50 | 0.25 | 150 | 30 | 34.8 | 34.7724 | 34.3576 | 34.7494 |
| 5 | 10 | 10 | 0.25 | 150 | 30 | 45.9 | 45.9084 | 45.4936 | 45.8854 |
| 5 | 10 | 20 | 0.25 | 150 | 30 | 32.6 | 32.5549 | 32.1401 | 32.5319 |
| 5 | 10 | 30 | 0.25 | 150 | 30 | 29.9 | 29.8781 | 29.4633 | 29.8550 |
| 5 | 10 | 40 | 0.25 | 150 | 30 | 26.1 | 26.1029 | 25.6881 | 26.0799 |
| 5 | 10 | 50 | 0.5 | 150 | 30 | 39.3 | 39.3284 | 38.9136 | 39.3054 |
| 5 | 10 | 50 | 0.75 | 150 | 30 | 47.7 | 47.6452 | 47.2304 | 47.6222 |
| 5 | 10 | 50 | 1.0 | 150 | 30 | 53.2 | 53.1846 | 52.7698 | 53.1616 |
| 5 | 10 | 50 | 0.25 | 50 | 30 | 21.8 | 21.7426 | 21.3278 | 21.7196 |
| 5 | 10 | 50 | 0.25 | 100 | 30 | 21.9 | 21.9176 | 21.5028 | 21.8946 |
| 5 | 10 | 50 | 0.25 | 200 | 30 | 30.0 | 29.9914 | 29.5766 | 29.9684 |
| 5 | 10 | 50 | 0.25 | 150 | 50 | 29.6 | 29.5456 | 29.1308 | 29.5226 |
| 5 | 10 | 50 | 0.25 | 150 | 60 | 30.5 | 30.4466 | 30.0318 | 30.4236 |
| MSE | ˂10−5 | 0.01 | 10−3 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadek, A.H.; Fahmy, O.M.; Nasr, M.; Mostafa, M.K. Predicting Cu(II) Adsorption from Aqueous Solutions onto Nano Zero-Valent Aluminum (nZVAl) by Machine Learning and Artificial Intelligence Techniques. Sustainability 2023, 15, 2081. https://doi.org/10.3390/su15032081
Sadek AH, Fahmy OM, Nasr M, Mostafa MK. Predicting Cu(II) Adsorption from Aqueous Solutions onto Nano Zero-Valent Aluminum (nZVAl) by Machine Learning and Artificial Intelligence Techniques. Sustainability. 2023; 15(3):2081. https://doi.org/10.3390/su15032081
Chicago/Turabian StyleSadek, Ahmed H., Omar M. Fahmy, Mahmoud Nasr, and Mohamed K. Mostafa. 2023. "Predicting Cu(II) Adsorption from Aqueous Solutions onto Nano Zero-Valent Aluminum (nZVAl) by Machine Learning and Artificial Intelligence Techniques" Sustainability 15, no. 3: 2081. https://doi.org/10.3390/su15032081
APA StyleSadek, A. H., Fahmy, O. M., Nasr, M., & Mostafa, M. K. (2023). Predicting Cu(II) Adsorption from Aqueous Solutions onto Nano Zero-Valent Aluminum (nZVAl) by Machine Learning and Artificial Intelligence Techniques. Sustainability, 15(3), 2081. https://doi.org/10.3390/su15032081










