Taxonomic Diversity and Selection of Functional Traits in Novel Ecosystems Developing on Coal-Mine Sedimentation Pools
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Collection of the Vegetation Data
2.3. Determining Plant Species’ Functional Trait Data and Establishing Ecological Groups of Species
2.4. Data Analyses
3. Results
3.1. The Floristic Differentiation of Plant Communities Occurring on Coal-Mine Sedimentation Pools
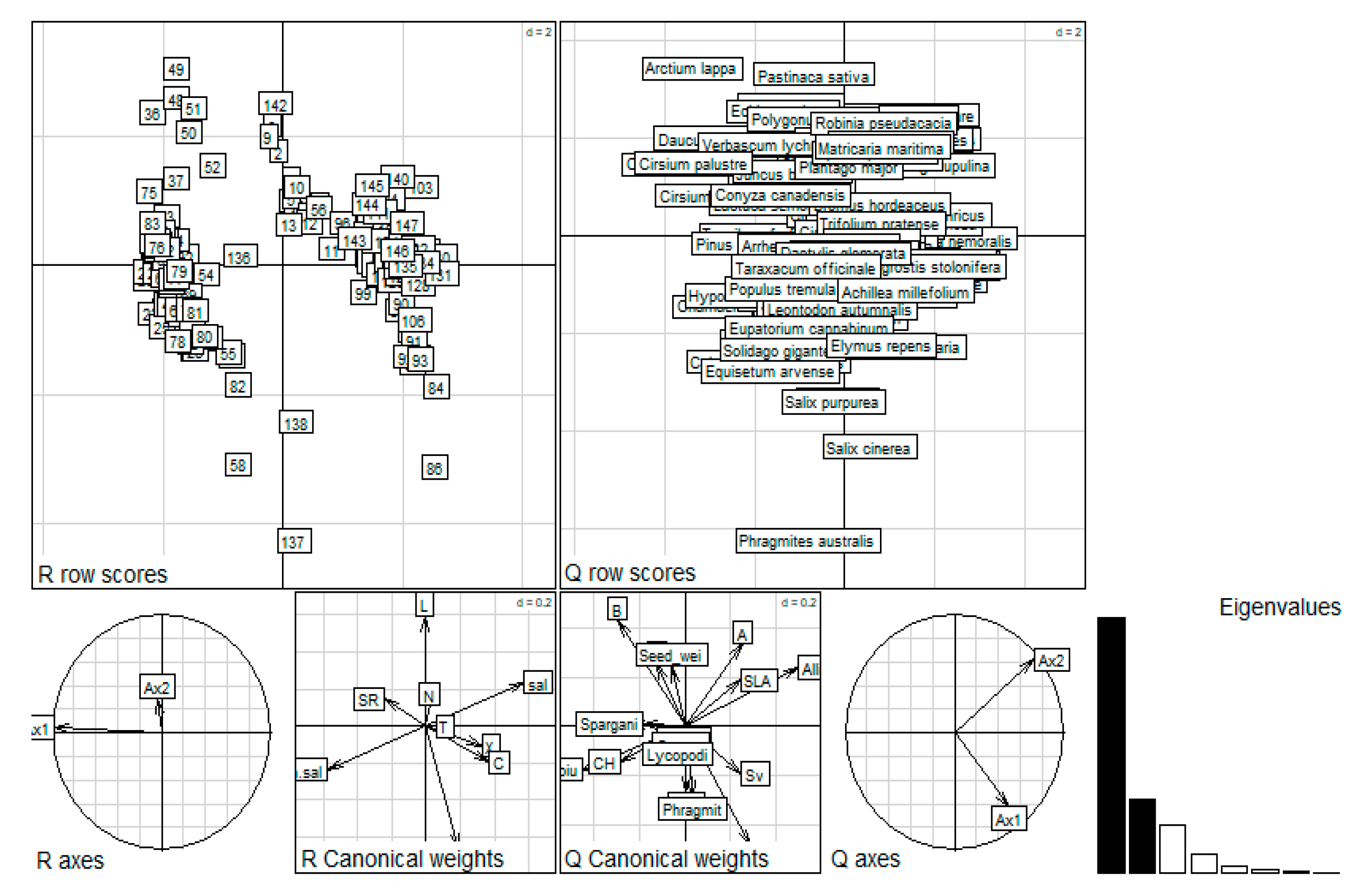
3.2. The Relationships between Vegetation That Developed in Coal-Mine Sedimentation Pools and Environmental Data
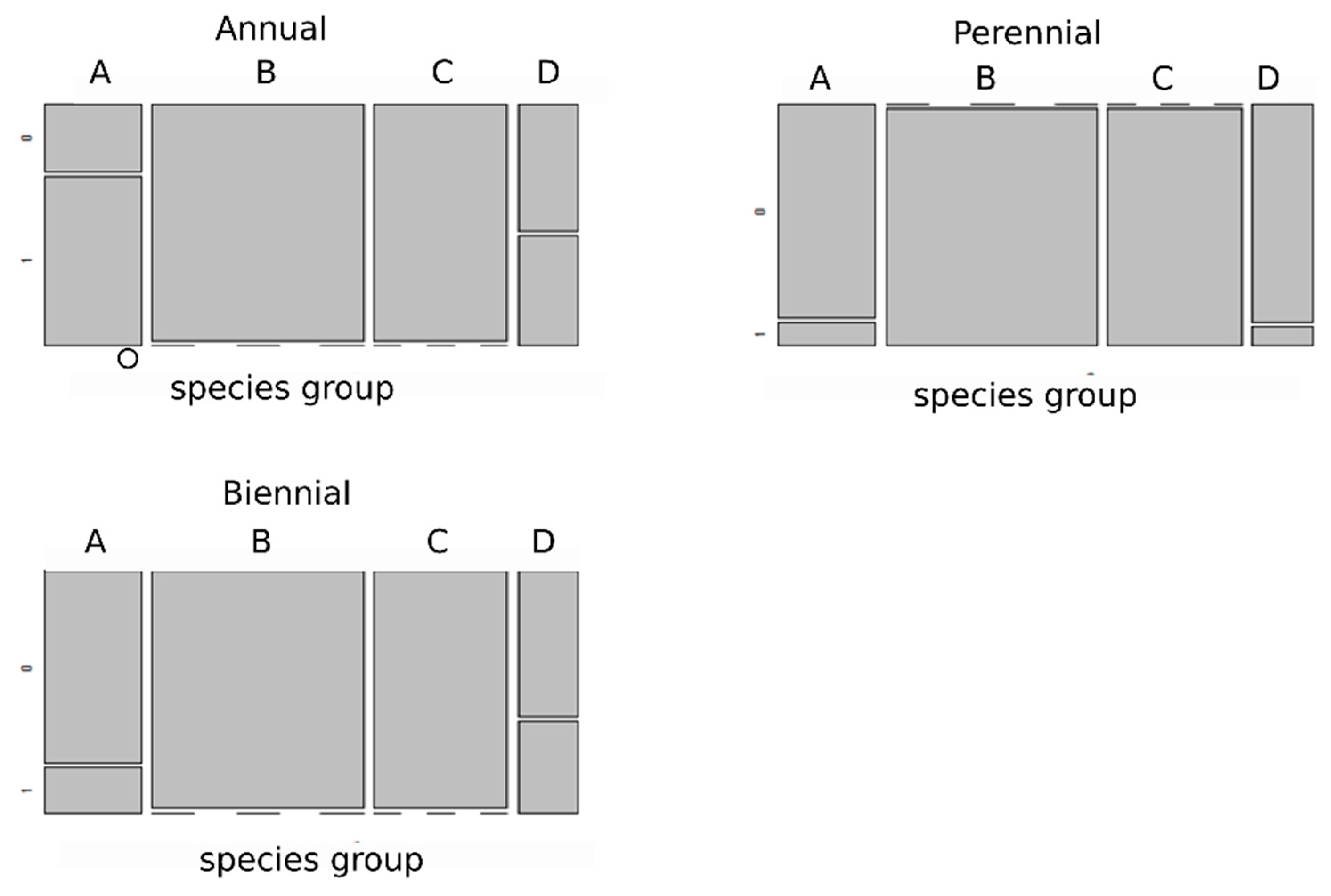
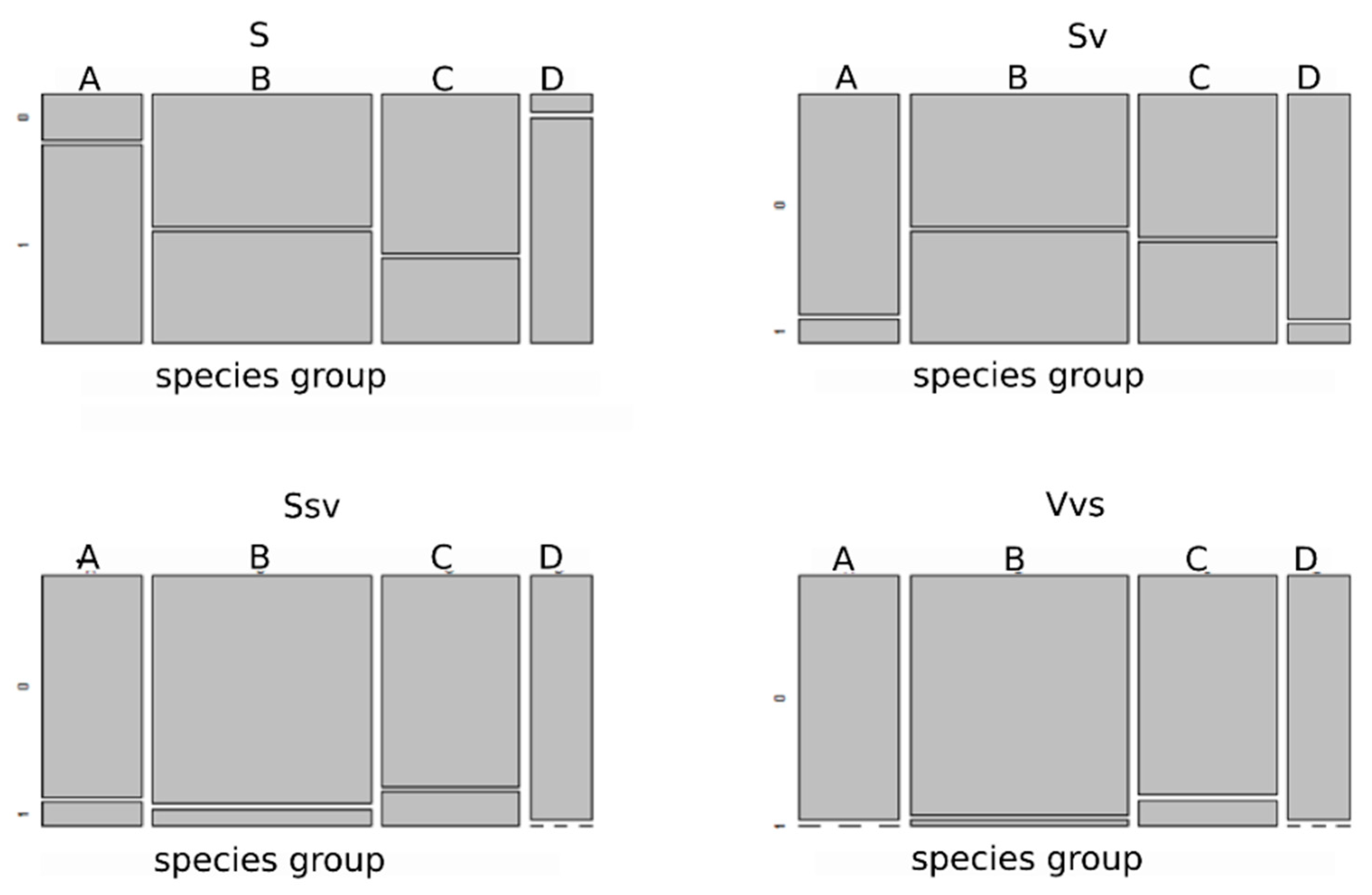
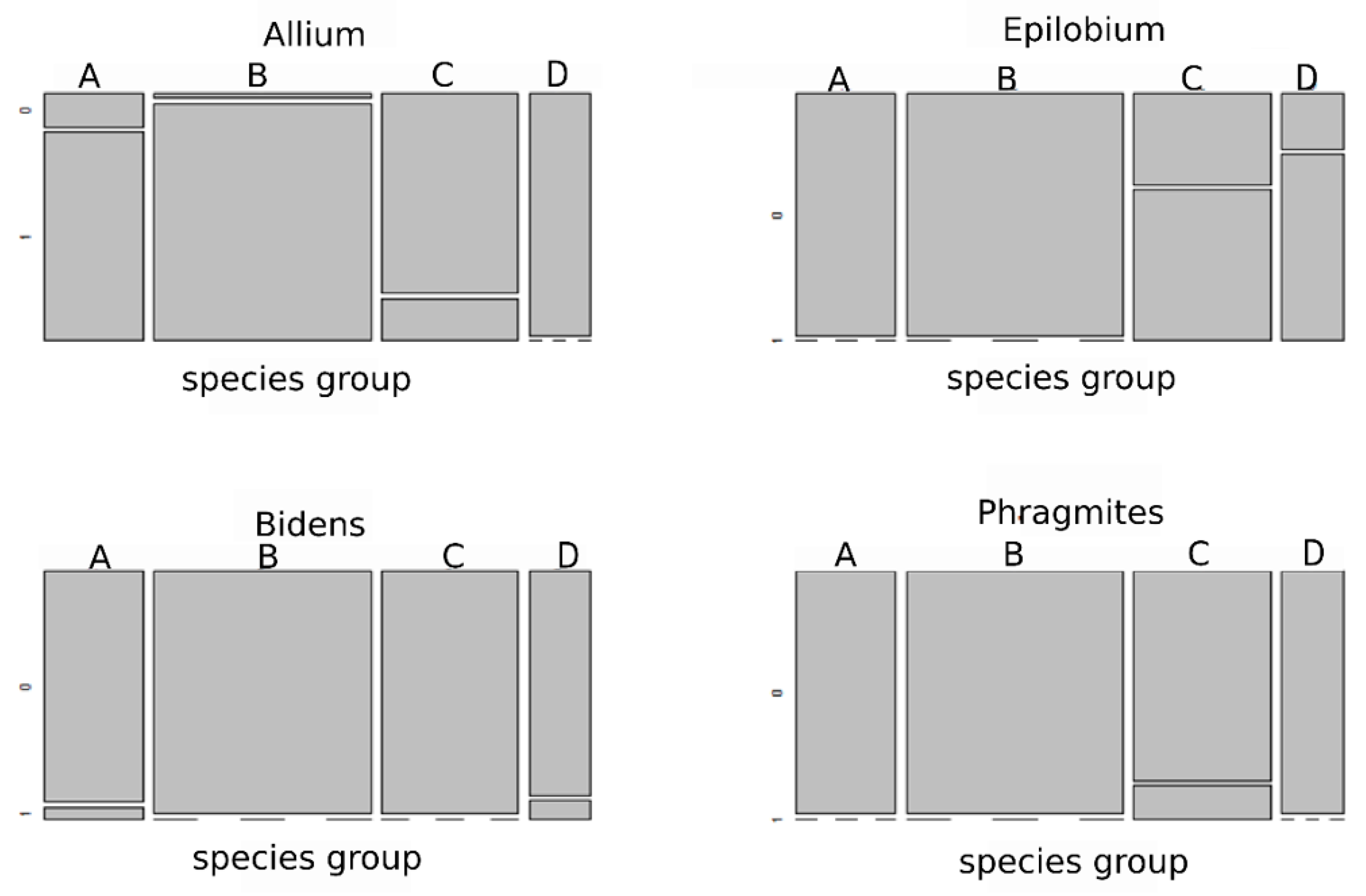
3.3. Selection of Species Traits along an Environmental Gradient
4. Discussion
4.1. Floristic Composition of Communities Occurring in Coal-Mine Sedimentation Pools
4.2. Abiotic Factors That Determine the Floristic Composition of Vegetation Patches
4.3. Selection of Functional Traits of Species along Environmental Gradients Occurring in Coal-Mine Sedimentation Pools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woźniak, G. Zróżnicowanie roślinności na zwałach pogórniczych Górnego Śląska; Instytut Botaniki im. W. Szafera, PAN: Kraków, Poland, 2010. [Google Scholar]
- Erskine, P.D.; Fletcher, A.T. Novel ecosystems created by coal mines in Central Queensland’s Bowen Basin. Ecol. Process. 2013, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, C.P.; Fernandes, C.O. Novel Ecosystems: A review of the concept in non-urban and urban contexts. Landsc. Ecol. 2020, 35, 23–39. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Bierza, W.; Błońska, A.; Sierka, E.; Magurno, F.; Chmura, D.; Besenyei, L.; Radosz, Ł.; Woźniak, G. Vegetation diversity on coal mine spoil heaps—How important is the texture of the soil substrate? Biologia 2019, 74, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Prach, K.; Hobbs, R.J. Spontaneous succession versus technical reclamation in the restoration of disturbed sites. Restor. Ecol. 2008, 16, 363–366. [Google Scholar] [CrossRef]
- Hodacova, D.; Prach, K. Spoil heaps from brown coal mining: Technical reclamation versus spontaneous revegetation. Restor. Ecol. 2003, 11, 385–391. [Google Scholar] [CrossRef]
- Bastl, M.; Kocar, P.; Prach, K.; Pysek, P. The effect of successional age and disturbance on the establishment of alien plants in man-made sites: An experimental approach. In Plant Invasions: Studies from North America and Europe; Brook, J.H., Wade, M., Pyšek, P., Green, D., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1997; pp. 191–201. [Google Scholar]
- Navarro-Ramos, S.E.; Sparacino, J.; Rodríguez, J.M.; Filippini, E.; Marsal-Castillo, B.E.; García-Cannata, L.; Renison, D.; Torres, R.C. Active revegetation after mining: What is the contribution of peer-reviewed studies? Heliyon 2022, 8, e09179. [Google Scholar] [CrossRef]
- Prach, K.; Lencová, K.; Řehounková, K.; Dvořáková, H.; Jírová, A.; Konvalinková, P.; Mudrák, O.; Novák, J.; Trnková, R. Spontaneous vegetation succession at different Central European mining sites: A comparison across seres. Environ. Sci. Pollut. Res. 2013, 20, 7680–7685. [Google Scholar] [CrossRef]
- Chmura, D.; Jagodziński, A.M.; Hutniczak, A.; Dyczko, A.; Woźniak, G. Novel ecosystems in the urban-industrial landscape–interesting aspects of environmental knowledge requiring broadening: A Review. Sustainability 2022, 14, 10829. [Google Scholar] [CrossRef]
- Korjus, H.; Laarmann, D.; Sims, A.; Paluots, T.; Kangur, A. Assessment of novel forest ecosystems on post-mining restoration site in Aidu, Estonia. In Local and Regional Challenges of Climate Change Adaptation and Green Technologies; University of West Hungary Press: Sopron, Hungary, 2014; pp. 35–44. [Google Scholar]
- Kompała-Bąba, A. Abiotic and Biotic Factors Affecting the Diversity of Ruderal Vegetation (Silesian Uplands Poland); Sorus: Poznań, Poland, 2013; ISBN 978-83-63768-08-9. [Google Scholar]
- Higgs, E. Novel and designed ecosystems. Restor. Ecol. 2017, 25, 8–13. [Google Scholar] [CrossRef]
- Rostański, A. Spontaniczne kształtowanie się pokrywy roślinnej na zwałowiskach po górnictwie węgla kamiennego na Górnym Śląsku; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2006; ISBN 83-226-1536-1. [Google Scholar]
- Zhao, B.Q.; Gao, R.Z.; Xia, D.; Xia, L.; Zhu, W.Q.; Xu, W.N. Vegetation community characteristics under different vegetation eco-restoration techniques at Xiangjiaba Hydropower Station. Nat. Environ. Pollut. Technol. 2021, 20, 1381–1392. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Sierka, E.; Dyderski, M.K.; Bierza, W.; Magurno, F.; Besenyei, L.; Błońska, A.; Ryś, K.; Jagodziński, A.M.; Woźniak, G. Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Woźniak, G.; Chmura, D.; Błońska, A.; Sierka, E.; Tokarska-Guzik, B. Applicability of functional groups concept in analysis of spatiotemporal vegetation changes on manmade habitats. Polish J. Environ. Stud. 2011, 20, 623–631. [Google Scholar]
- Řehounková, K.; Prach, K. Life-history traits and habitat preferences of colonizing plant species in long-term spontaneous succession in abandoned gravel–sand pits. Basic Appl. Ecol. 2010, 11, 45–53. [Google Scholar] [CrossRef]
- Piekarska-Stachowiak, A.; Szary, M.; Ziemer, B.; Besenyei, L.; Woźniak, G. An application of the plant functional group concept to restoration practice on coal mine spoil heaps. Ecol. Res. 2014, 29, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, R.J.; Higgs, E.; Hall, C.M.; Bridgewater, P.; Chapin, F.S.; Ellis, E.C.; Ewel, J.J.; Hallett, L.M.; Harris, J.; Hulvey, K.B.; et al. Managing the whole landscape: Historical, hybrid, and novel ecosystems. Front. Ecol. Environ. 2014, 12, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Kompała-Bąba, A.; Woźniak, G. Returning coal mine sedimentation pools to nature: Technical versus natural processes in population, vegetation and landscape contexts. In Green Scenarios: Mining Industry Responses to Environmental Challenges of the Anthropocene Epoch; CRC Press: Boca Raton, FL, USA, 2022; pp. 103–113. [Google Scholar]
- Patrzałek, A. Właściwości gleby inicjalnej powstającej na zwałowisku odpadów karbońskich. Zesz. Nauk. Politech. Śląskiej. Ser. Górnictwo 2001, 248, 151–156. [Google Scholar]
- Kent, M. Vegetation Description and Data Analysis. A Practical Approach, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Dzwonko, Z. Przewodnik do badań fitosocjologicznych; Vademecum Geobotanicum; Sorus: Poznań, Poland, 2007. [Google Scholar]
- Latzel, V.; Klimešová, J.; Doležal, J.; Pyšek, P.; Tackenberg, O.; Prach, K. The association of dispersal and persistence traits of plants with different stages of succession in Central European man-made habitats. Folia Geobot. 2011, 46, 289–302. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; Van Groenendael, J.M.; Klime, L.; Klimešová, J.; et al. The LEDA traitbase: A database of life-history traits of the Northwest European Flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Klotz, S.; Durka, W. BIOLFLOR—Eine Datenbank mit Biologisch-Ökologischen Merkmalen zur Flora von Deutschland; Bundesamt für Naturschutz: Bonn, Germany, 2002; Volume 38, pp. 1–334. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Checklist. Biodiversity of Poland 1; Mirek, Z., Ed.; W. Szafer: Kraków, Poland, 2022. [Google Scholar]
- Oberdorfer, E. Pflanzensoziologische Exkursionsflora; Verlag Eug: Stuttgart, Germany, 1990. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do oznaczania zbiorowisk roślinnych Polski; Wydawnictw: Warszawa, Poland, 2001. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Dull, R.; Wirth, V.; Werner, W.; Paullisen, W. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1991, 38, 248. [Google Scholar]
- Chytrý, M.; Danihelka, J.; Kaplan, Z.; Wild, J.; Holubová, D.; Novotný, P.; Reznícková, M.; Rohn, M.; Drevojan, P.; Grulich, V.; et al. Pladias database of the Czech Flora and Vegetation. Preslia 2021, 93, 1–87. [Google Scholar] [CrossRef]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różańska, W.; Szeląg, W.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plant of Poland. Biodiversity of Poland 2; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- Tichý, L.; Holt, J.; Nejezchlebová, M. JUICE Program for Management, Analysis and Classification of Ecological Data; Vegetation: Brno, Czech Republic, 2011. [Google Scholar]
- McCune, B.; Mefford, M. PC-ORD. Multivariate Analysis of Ecological Data; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Chytrý, M.; Tichý, L.; Holt, J.; Botta-Dukát, Z. Determination of diagnostic species with statistical fidelity measures. J. Veg. Sci. 2002, 13, 79–90. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Kompała-Bąba, A.; Bierza, W.; Sierka, E.; Błońska, A.; Besenyei, L.; Woźniak, G. The role of plants and soil properties in the enzyme activities of substrates on hard coal mine spoil heaps. Sci. Rep. 2021, 11, 5155. [Google Scholar] [CrossRef] [PubMed]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Zelený, D.; Schaffers, A.P. Too Good to Be True: Pitfalls of Using Mean Ellenberg Indicator Values in Vegetation Analyses. J. Veg. Sci. 2012, 23, 419–431. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Römermann, C.; Nuske, R.; Parth, A.; Klotz, S.; Schmidt, W.; Stadler, J. On the identification of the most suitable traits for plant functional trait analyses. Oikos 2008, 117, 1533–1541. [Google Scholar] [CrossRef]
- Thioulouse, J.; Dray, S.; Dufour, A.; Siberchicot, A.; Jombart, T.; Pavoine, S. Multivariate Analysis of Ecological Data with Ade4; Springer: New York, NY, USA, 2018; pp. 1–284. [Google Scholar] [CrossRef]
- Kleyer, M.; Dray, S.; Bello, F.; Lepš, J.; Pakeman, R.J.; Strauss, B.; Thuiller, W.; Lavorel, S. Assessing species and community functional responses to environmental gradients: Which multivariate methods? J. Veg. Sci. 2012, 23, 805–821. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Zhang, C.; Pei, Z. Reclaimed soil properties and weathered gangue change characteristics under various vegetation types on gangue pile. Acta Ecol Sin. 2011, 31, 6429–6441. [Google Scholar]
- Burghardt, W.; Niggemeyer, M.; Braunersreuther, M. Ways of soil development on stony substrate from hard coal mining spoil. Soil Sci. Annu. 2020, 71, 382–394. [Google Scholar] [CrossRef]
- Kang, B.; Liu, S.R.; Cai, D.X.; Lu, L.H.; He, R.M.; Gao, Y.X.; Di, W.Z. Soil physical and chemical characteristics under different vegetation restoration patterns in China south subtropical area. J. Appl. Ecol. 2010, 21, 2479–2486. [Google Scholar]
- Alday, J.G.; Marrs, R.H.; Martínez Ruiz, C. Vegetation succession on reclaimed coal wastes in Spain: The influence of soil and environmental factors. Appl. Veg. Sci. 2011, 14, 84–94. [Google Scholar] [CrossRef]
- Nyssen, J.; Vermeersch, D. Slope aspect affects geomorphic dynamics of coal mining spoil heaps in Belgium. Geomorphology 2010, 123, 109–121. [Google Scholar] [CrossRef] [Green Version]
- López-Marcos, D.; Turrión, M.B.; Martínez-Ruiz, C. Linking soil variability with plant community composition along a mine-slope topographic gradient: Implications for restoration. Ambio 2020, 49, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Kucharski, J.; Lajszner, W. Enzymatic activities in different soils contaminated with copper. Polish J. Environ. Stud. 2005, 14, 659–664. [Google Scholar]
- Rahmonov, O.; Czajka, A.; Nádudvari, Á.; Fajer, M.; Spórna, T.; Szypuła, B. Soil and vegetation development on coal-waste dump in southern Poland. Int. J. Environ. Res. Public Health 2022, 19, 9167. [Google Scholar] [CrossRef]
- Godefroid, S.; Monbaliu, D.; Koedam, N. The role of soil and microclimatic variables in the distribution patterns of urban wasteland Flora in Brussels, Belgium. Landsc. Urban Plan. 2007, 80, 45–55. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.; Niu, S.; Bai, Z. The reclaimed coal mine ecosystem diverges from the surrounding ecosystem and reaches a new self-sustaining state after 20–23 years of succession in the Loess Plateau Area, China. Sci. Total Environ. 2020, 727, 138739. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xia, G.; Wei, Y.; Aili, A.; Yuan, K. Responses of Vegetation and Soil to Artificial Restoration Measures in Abandoned Gold Mining Areas in Altai Mountain, Northwest China. Diversity 2022, 14, 427. [Google Scholar] [CrossRef]
- Feng, T.; Wei, W.; Chen, L.; Cerdà, A.; Yang, L.; Yu, Y. Combining land preparation and vegetation restoration for optimal soil eco-hydrological services in the Loess Plateau, China. Sci. Total Environ. 2019, 657, 535–547. [Google Scholar] [CrossRef]
- Alday, J.G.; Marrs, R.H.; Martínez-Ruiz, C. Soil and vegetation development during early succession on restored coal wastes: A six-year permanent plot study. Plant Soil 2012, 353, 305–320. [Google Scholar] [CrossRef]
- Markowicz, A.; Woźniak, G.; Borymski, S.; Piotrowska-Seget, Z.; Chmura, D. Links in the functional diversity between soil microorganisms and plant communities during natural succession in coal mine spoil heaps. Ecol. Res. 2015, 30, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.C.; Zheng, H.X.; Liu, W.S.; Liu, C.; Guo, M.N.; Huot, H.; Morel, J.L.; Qiu, R.L.; Chao, Y.; Tang, Y.T. Plant-soil feedbacks for the restoration of degraded mine lands: A Review. Front. Microbiol. 2022, 12, 751794. [Google Scholar] [CrossRef]
- Mudrák, O.; Řehounková, K.; Vítovcová, K.; Tichý, L.; Prach, K. Ability of plant species to colonise human-disturbed habitats: Role of phylogeny and functional traits. Appl. Veg. Sci. 2021, 24, e12528. [Google Scholar] [CrossRef]
- Woźniak, G.; Markowicz, A.; Borymski, S.; Piotrowska-Seget, Z.; Chmura, D.; Besenyei, L. The relationship between successional vascular plant assemblages and associated microbial communities on coal mine spoil heaps. Community Ecol. 2015, 16, 23–32. [Google Scholar] [CrossRef]





| Community | Number of Species | Dominance | Shannon–Wiener Diversity Index | Evenness |
|---|---|---|---|---|
| Chenopodium glaucum-Chenopodium rubrum com. | 7.43 ± 0.36 | 0.35 ± 0.11 | 1.39 ± 0.42 | 0.59 ± 0.11 |
| Deschampsia caespitosa-Cirsium arvense com. | 6.33 ± 0.58 | 0.45 ± 0.09 | 1.16 ± 0.10 | 0.51 ± 0.09 |
| Chenopodium album com. | 7.40 ± 3.92 | 0.44 ± 0.24 | 1.24 ± 0.61 | 0.58 ± 0.15 |
| Plantago intermedia com. | 4.50 ± 0.32 | 0.43 ± 0.11 | 1.02 ± 0.32 | 0.70 ± 0.16 |
| Atriplex prostrata spp. prostrata com. | 6.13 ± 2.68 | 0.42 ± 0.18 | 1.20 ± 0.44 | 0.63 ± 0.16 |
| Puccinellia distans com. | 5.88 ± 3.28 | 0.61 ± 0.26 | 0.87 ± 0.59 | 0.49 ± 0.13 |
| Chamomilla suaveolens-Polygonum aviculare com. | 11.00 ± 3.67 | 0.39 ± 0.18 | 1.49 ± 0.44 | 0.45 ± 0.19 |
| Medicago lupulina com. | 16.63 ± 7.80 | 0.27 ± 0.10 | 1.96 ± 0.46 | 0.47 ± 0.13 |
| Melilotus alba com. | 7.00 ± 1.41 | 0.53 ± 0.16 | 1.04 ± 0.29 | 0.44 ± 0.15 |
| Daucus carota com. | 12.67 ± 3.86 | 0.27 ± 0.16 | 1.91 ± 0.52 | 0.58 ± 0.19 |
| Artemisia vulgaris-Tanacetum vulgare com. | 11.73 ± 9.08 | 0.37 ± 0.15 | 1.51 ± 0.55 | 0.4 ± 0.11 |
| Calamagrostis epigejos com. | 6.25 ± 3.10 | 0.56 ± 0.26 | 0.95 ± 0.56 | 0.48 ± 0.09 |
| Phragmites australis-Solidago canadensis com. | 8.25 ± 0.96 | 0.28 ± 0.13 | 1.64 ± 0.29 | 0.65 ± 0.18 |
| Tussilago farfara com. | 5.17 ± 2.21 | 0.58 ± 0.18 | 0.89 ± 0.40 | 0.5 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kompała-Bąba, A.; Bąba, W.; Ryś, K.; Hanczaruk, R.; Radosz, Ł.; Prostański, D.; Woźniak, G. Taxonomic Diversity and Selection of Functional Traits in Novel Ecosystems Developing on Coal-Mine Sedimentation Pools. Sustainability 2023, 15, 2094. https://doi.org/10.3390/su15032094
Kompała-Bąba A, Bąba W, Ryś K, Hanczaruk R, Radosz Ł, Prostański D, Woźniak G. Taxonomic Diversity and Selection of Functional Traits in Novel Ecosystems Developing on Coal-Mine Sedimentation Pools. Sustainability. 2023; 15(3):2094. https://doi.org/10.3390/su15032094
Chicago/Turabian StyleKompała-Bąba, Agnieszka, Wojciech Bąba, Karolina Ryś, Robert Hanczaruk, Łukasz Radosz, Dariusz Prostański, and Gabriela Woźniak. 2023. "Taxonomic Diversity and Selection of Functional Traits in Novel Ecosystems Developing on Coal-Mine Sedimentation Pools" Sustainability 15, no. 3: 2094. https://doi.org/10.3390/su15032094





