Abstract
Lansium domesticum Corr. (L. domesticum), Meliaceae, has an economical fruit found throughout the southeast and has been reported to be used in traditional medicine. Therefore, this study aims to investigate the antimicrobial and cytotoxic potential of four extracts from the stembark of L. domesticum Corr. cv. Kokossan and isolated four terpenoid compounds. Antimicrobial testing was performed on two fungi, namely Malassezia furfur and Candida albicans, and two bacteria, Staphylococcus epidermidis and Klebsiella pneumoniae. Furthermore, antimicrobial activity was determined using the minimum inhibitory concentration (MIC). All isolated extracts were also tested on MCF-7 breast cancer cells. The results showed that butanol and n-hexane extracts have antimicrobial potential against K. pneumoniae bacteria with MIC values of 7.8125 mg/mL and 62.5 mg/mL, respectively, as well as against the dandruff fungus M. furfur. In addition to the antimicrobial results, the anticancer test results also showed that n-hexane has the most interesting cytotoxic value of all extracts, with an IC50 of 42.95 µg/mL than extracts of ethyl acetate, ethanol, and butanol with respective IC50 values of 72.84; 74.50, and 12088.33 µg/mL. The n-hexane and n-butanol extracts have anticancer and antimicrobial potential. These extracts can be studied further for other bioactivity.
1. Introduction
Maintaining a healthy body and protecting against various diseases are essential. Several studies aim to create healthier foods and products for everyday use, which has resulted in aggressive procedures during an investigation. Plants have many natural products that are used in the medical field; hence, detailed knowledge of the phytochemical composition, biological properties, safety profile, and environmental toxicity is required for the characterization of herbal products [1,2,3].
Lansium domesticum Corr., a sweet or acidic plant depending on the growing conditions and variety [4], has high-value fruit with significant nutritional and economic value that is widely distributed throughout Southeast Asia, particularly in the Philippines, Malaysia, Thailand, and Indonesia [5]. The delicious, succulent fruit can be eaten fresh after peeling, candied, or preserved in syrup [6]. Some previous studies on this species indicated the presence of terpenoid compounds [7,8,9,10]. In addition to having sweet fruit and being safe to consume, it has long been widely used for traditional therapeutic purposes, such as for treating fever, diarrhea or dysentery, as an antimalarial, worm medicine, and scorpion sting medication [11,12,13]. Due to its antioxidant properties, moisturizing, and skin-lightening effects, several studies on this plant have been conducted in the beauty field as a cosmetic additive [14].
This species produces many terpenoid compounds, such as onoceranoids, sesquiterpenoids, steroids, and limonoids. Several terpenoid compounds have been reported as having various activities, such as antimalarial and antimicrobial for limonoids, antifeedant for onoceranoids, and anticancer for sesquiterpenoids and steroids [5]. In Indonesia, there are three cultivars of this species, one of which is Lansium domesticum Corr. cv. Kokosan. A previous study has described the cytotoxic effect of sesquiterpenoids isolated from the stembark of Kokossan [7]. Despite the documented biological properties of Kokossan stembark, its antimicrobial properties are still poorly studied. The new antimicrobial identification is expected to resolve microbial infection suffered globally, where its treatment poses a serious risk to human life due to multi-drug resistance. According to Petrov, antibiotic resistance could kill 10 million people by 2050; hence, the development of new antibiotics is necessary to prevent the death of multidrug-resistant pathogens [15,16,17].
Candida albicans is the most well-studied and prevalent human fungal pathogen. It is an opportunistic pathogen that lives in the gut, genitourinary tract, and skin as a harmless commensal. However, under various host conditions, it can transform into an opportunistic pathogen, causing a decrease in immune competence or an imbalance of competing for bacterial microflora. Mucosal infections, such as thrush or vaginitis, are typically not lethal, but they can be an indicator of immunological suppression, as in HIV patients. According to [18,19], bloodstream Candida infections, which are associated with high mortality rates, are even more dangerous.
Malassezia furfur is a dimorphic lipophilic fungus found living on normal human skin [20]. In humans, they can cause various superficial skin diseases, such as Pityriasis Versicolor (PV), dandruff, Malassezia Folliculitis (MF), Seborrheic Dermatitis (SD), Atopic Dermatitis (AD), and psoriasis [21,22,23,24].
Klebsiella pneumoniae is another antibiotic-resistant bacterium that has long been recognized as a pathogen. It was first described as causing pneumonia by Carl Friedlander in 1882 and is still one of the most common nosocomial pathogens in the world. It is also a significant cause of neonatal sepsis [25,26,27].
Staphylococcus epidermidis is a common cause of infection in vulnerable individuals, such as immunocompromised people and newborns. Not only can it cause infections in sterile sites, such as native valve endocarditis, but it is also frequently linked to external bodies, like catheters and other medical devices. It is important to be aware of this bacteria and the risks it poses [28,29].
In order to develop new drugs, it is important to study the increase in microbial resistance to various drugs related to the decrease in antimicrobial molecules and properties and to find natural compounds that have anticancer potential, especially in the breast, considering the biggest cause of death from cancer is breast cancer (around 30%). In this context, secondary metabolites produced by plants could be an excellent strategy for further investigation [30,31]. Therefore, this study aims to investigate the cytotoxic and antimicrobial properties of the stembark of L. domesticum Corr. cv. Kokossan in vitro and characterize the compounds isolated from this plant.
2. Materials and Methods
2.1. Plant Material
The stembark of Kokosan was gathered from Pangandaran Botanical Garden, Pangandaran, West Java Province, Indonesia, in March 2021. The plant was determined at Laboratory of Plant Taxonomy, Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor, West Java, and the voucher specimen was deposited at the Laboratory.
2.2. Chemicals and Reagents
Organic solvents, such as ethanol, n-hexane, ethyl acetate, methanol, n-butanol, methylene chloride, and acetone, were purchased from Kristata Gemilang Company, Bandung, in technical and distilled quality. Furthermore, Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, trypsinethylenediamine tetraacetic acid solution, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and purified water were acquired using the Milli-Qplus185 system (Millipore, Billerica, MA, USA). Müeller–Hinton Broth (MHB), Nutrient Broth (NB), and potato dextrose broth (PDB) were procured from Central Laboratory, Universitas Padjadjaran, Indonesia.
2.3. Extracts Preparations
First, the stembark of Kokossan was reduced in a dried sample, weighed up to 3.18 kg, then mashed using a mixer. The powder of the sample was macerated with 20 L ethanol at room temperature as long as 3 24 h. The extract was evaporated using a rotary evaporator at 40 °C under reduced pressure over time to obtain a residue of the concentrated ethanol extract (300 g). This residue was dissolved in water and partitioned with 8 L n-hexane, 7 L ethyl acetate, and 1.5 L n-butanol to yield 124 g, 54 g, and 15 g, respectively. This separation was guided by thin-layer chromatography and compound detection in ethanol with a 10% H2SO4 stain [7].
2.4. Isolation of Compounds from L. domesticum Corr. cv. Kokosan
Vacuum liquid chromatography was used to fractionate 54 g of ethyl acetate extract on silica gel G60 using 10% gradient elution of n-hexane: ethyl acetate: methanol to acquire 12 (A-L) subfractions. Fraction B (15.2 g) was further fractionated using the same method with 2% gradient elution of n-hexane:ethyl acetate to afford eight fractions.
Fraction B4 (7.8 g) was recrystallized with methanol to afford 3 g of compound 1. Furthermore, 8 g of fraction D fraction was separated using column chromatography on 230–400 mesh silica gel eluted with 2% gradient elution of n-hexane:EtOAc to afford 4 fractions, namely D1–D4. Fractions D1 (250 mg) and D2 (300mg) were recrystallized with methanol to afford compounds 2 (90 mg) and 3 (101 mg), respectively. Compound 4 was isolated from D4 (1.8 g) and washed with methanol. TLC was spotted on Silica Gel 60 F254 plate, then the purified compounds were elucidated to determine their structures using FT-IR (Shelton, Connecticut, USA), NaCl plate, HR-TOFMS (Waters, Milford, Massachusetts, USA), and NMR (nuclear magnetic resonance) spectrometry (JEOL JNM-ECX500R/S1 spectrometer Tokyo, Japan) in CDCl3 containing TMS as an internal standard. The analysis shows that the four compounds obtained from the n-hexane fraction of stembark were included in the triterpenoid group. They were 8,14-secogammacera-7,14-diene-3,21-dione (1), 8,14-secogammacera-7,14(27)-diene-3,21-dione (2), kokosanolide B (3), and 3-hidroksi-8,14-secogammacera-7,14-diene-21-one (4). The following are FT-IR, HR-TOFMS, 1H, and 13C-NMR data of four compounds:
- 8,14-secogammacera-7,14-diene-3,21-dione (1)
This compound has white crystals, m.p. 143–144 °C and the molecular formula are C30H46O2 (m/z 438.3745, [M + H]+); FT-IR, Vmax: 1708 cm−1 (C=O), 1662 cm−1 (C=C), 1430 and 1360 cm−1 (gem-dimethyl); 1H-NMR (CDCl3) δH (ppm) 1.46 (H-1a; H-19a), 2.09 (H-1b; H-19b), 2.24 (H-2a; H-20a), 2.70 (H-2b; H-20b),1.59 (H-5; H-17), 1.33 (H-6a; H-16a), 1.24 (H-6b; H-16b), 5.43 (H-7; H-15), 1.65 (H-9; H-13), 1.93 (H-11a; H-12a), 2.40 (H-11b; H-12b), 1.04 (H-23; H-29), 1.09 (H-24; H-30), 0.97 (H-25; H-28), 1.72 (H-26; H-27) 13C-NMR (CDCl3) δC (ppm) 38.5 (C-1; C-19), 34.8 (C-2; C-20), 216.9 (C-3; C-21), 47.6 (C-4; C-22), 51.6 (C-5; C-17), 30.1 (C-6; C-16), 122.1 (C-7; C-15), 135.3 (C-8; C-14), 55.6 (C-9; C-13), 36.7 (C-10; C-18), 24.2 (C-11; C-12), 25.1 (C-23; C-29), 22.3 (C-24; C-30), 13.5 (C-25; C-28), 22.5 (C-26; C-27).
- 2.
- 8,14-secogammacera-7,14(27)-diene-3,21-dione (2)
This compound has needle-like crystals and the molecular formula are C30H46O2 m/z 438.3745, [M + H]+; FT-IR, Vmax 1667 cm−1 (C=C), 1454 and 1384 cm−1 (gem-dimethyl); 1H-NMR (CDCl3) δH (ppm) 0.95 (3H, s), 0.97 (3H, s), 1.04 (3H, s), 1.08 (3H, s), 1.09 (3H, s), 1.10 (3H, s), 1.20 (1H, m), 1.22 (3H, s), 1.24 (1H, dd, J = 5, 10 Hz), 1.33 (1H, dd, J = 7, 10 Hz), 1.40 (1H, m), 1.46 (1H, m), 1.50 (1H, m), 1.59 (1H, dd, J = 5, 7 Hz), 1.63 (1H, m), 1.65 (1H, m), 1.72 (3H, s), 1.79 (1H, m), 1.83 (1H, m), 1.93 (1H, m), 2.04 (1H, m), 2.09 (1H, m), 2.15 (1H, m), 2.24 (1H, m), 2.35 (1H, m), 2.40 (1H, m), 2.45 (1H, m), 2.70 (1H, m), 5.12 (1H, J = 10.5 Hz), 5.43 (1H, m), 5.45 (1H, J = 10.5 Hz); 13C-NMR (CDCl3) δC (ppm) 13.5, 22.3, 22.5, 24.2, 25.1, 30.1, 34.8, 34.9, 36.7, 38.5, 47.6, 51.2, 51.6, 55.6, 122.1, 135.3, 216.90 (C-3), 217.0 (C-21).
- 3.
- Kokosanolid B (3)
The compound has white cubic crystal and its molecular formula was determined to be C30H48O3 (HR-TOF-MS m/z 456.6892, [M + H]+); FT-IR, Vmax 3749 cm−1 (OH), 1705 cm−1 (C=O), 1384 and 1261 cm−1 (gem-dimethyl); 1H-NMR (CDCl3) δH (ppm) 1.91 (H-1a), 2.08 (H-1b), 2.23 (H-2a), 2.41 (H-2b), 1.57 (H-5), 1.12 (H-6a), 2.56 (H-6b), 5.41 (H-7), 1.59 (H-9), 1.61 (H-11a), 2.41 (H-11b), 1.62 (H-12a), 1.76 (H-12b), 1.12 (H-13), 1.46 (H-15a), 2.23 (H-15b), 1.51 (H-16a), 1.84 (H-16b), 1.42 (H-17), 1.78 (H-19a), 2.10 (H-19b), 2.26 (H-20a), 2.73 (H-20b), 1.04 (H-23), 1.08 (H-24), 0.96 (H-25), 1.77 (H-26), 1.21 (H-27), 0.93 (H-28), 1.02 (H-29), 1.09 (H-30); 13C-NMR (CDCl3) δC (ppm) 38.5 (C-1), 34.8 (C-2), 216.9 (C-3), 47.6 (C-4), 51.6 (C-5), 28.9 (C-6), 121.7 (C-7), 135.3 (C-8), 55.51 (C-9), 36.6 (C-10), 21.5 (C-11; C-12), 61.8 (C-13), 74.0 (C-14), 44.2 (C-15), 31.4 (C-16), 55.2 (C-17), 36.6 (C-18), 38.4 (C-19), 34.1 (C-20), 217.1 (C-21), 47.6 (C-22), 25.1 (C-23), 22.3 (C-24), 13.4 (C-25), 22.3 (C-26), 24.2 (C-27), 15.1 (C-28), 21.4 (C-29), 26.5 (C-30).
- 4.
- 3-hidroksi-8,14-secogammacera-7,14-diene-21-one (4)
The compound has white crystal and the molecular formula are C30H48O2Na (HR-TOF-MS m/z 463.3569 [M + Na]+); FT-IR νmax (cm−1): 3533 (OH), 2932 (sp3), 1700 (C=O), 1456 (C=C); 1H-NMR (CDCl3) δH (ppm) 1.14; 1.86 (H-1a; H-1b), 1.65 (H-2), 3.29 (H-3), 1.62 (H-5), 1.35; 1.50 (H-6a; H-6b), 5.40 (H-7), 1.66 (H-9), 1.99 (H-11), 1.98 (H-12), 1.59 (H-13), 5.40 (H-15), 1.35 (H-16), 1.20 (H-17), 1.51 (H-19), 2.29 (H-20), 1.00 (H-23), 0.87 (H-24), 0.99 (H-25), 1.75 and 1.72 (H-26; H-27), 0.76 (H-28), 1.12 (H-29), and 1.08 (H-30); 13C-NMR (CDCl3) δC (ppm) 37.5 (C-1), 27.4 (C-2), 79.1 (C-3), 38.7 (C-4), 51.5 (C-5), 29.8 (C-6), 121.7 (C-7), 135.4 (C-8), 55.3 (C-9), 36.5 (C-10), 24.1 (C-11), 23.5 (C-12), 56.0 (C-13), 134.9 (C-14), 122.3 (C-15), 29.9 (C-16), 49.6 (C-17), 36.5 (C-18), 38.4 (C-19), 34.7 (C-20), 217.0 (C-21), 47.5 (C-22), 27.9 (C-23), 15.1 (C-24), 13.3 (C-25), 22.3 (C-26), 22.4 (C-27), 13.6 (C-28), 22.1 (C-29), 25.0 (C-30).
Compound 1 was included in the triterpenoid group, which is 8,14-secogammacera-7,14-diene-3,21-dione with molecular formula C30H46O2. Its 1H-NMR and 13C-NMR spectrum show that there are 15 carbon atoms at chemical shifts 38.50, 34.80, 216.90, 47.60, 51.60, 30.10, 122.10, 135.30, 55.60, 36.70, 24.20, 25.10, 22.30, 13.50, 22.50. Furthermore, this compound has a symmetrical structure with carbonyl and a double bond (Figure 1).
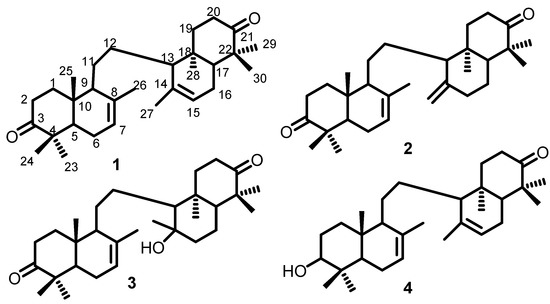
Figure 1.
Compounds 1–4 isolated from the stembark of L. domesticum Corr. cv. Kokosan; 8,14-secogammacera-7,14-diene-3,21-dione (1); 8,14-secogammacera-7,14(27)-diene-3,21-dione (2); Kokosanolide B (3); 3-hidroksi-8,14-secogammacera-7,14-diene-21-one (4).
Compound 2 is also included in the triterpenoid group with formula C30H46O2 m/z 438.3745. Based on 13C-NMR data spectrum, there are two carbonyl groups (C=O) at chemical shift ppm 216.90 (C-3), 217.00 (C-21). IR data also shows the presence of 1708 cm−1, carbonyl, 1662 cm−1 carbon double bond, as well as 1430 and 1360 cm−1 gem-dimethyl. The 1H and 13C-NMR spectra of compound 2 were similar to those of 1, except for the appearance of methylene protons and sp2 carbon at C-27 [δH 5.12 (1H, J = 10.5 Hz), 5.45 (1H, J = 10.5 Hz), δC 122.1], indicating that 2 is an isomer of 1. The structure was elucidated using a single-crystal X-diffraction analysis (Figure 1).
Compound 3 was included in the triterpenoid group that has partial structure with 1 and 2. It is a hydroxy derivative of compound 1, which is proven with the hydroxyl group at C-14 (74.01 ppm) and from the FT-IR data (Vmax 3749 cm−1). Compound 4 was in the terpenoid group having a partial structure of 1. Their difference is at the position of carbonyl, where compound 4 changed with hydroxyl group at C-3, thereby C-3 appears at 79.12 ppm. The structures of compounds 1–4 are shown in Figure 1 below:
2.5. Antimicrobial Test of Extracts
2.5.1. Preparation of Bacterial and Fungal Culture
Two bacterial strains were used in this study, namely, Klebsiella pneumoniae ATCC 2357 and Staphylococcus epidermidis ATCC 12228, which are Gram-negative and Gram-positive bacteria, respectively. The fungi which were used are Candida albicans and Malassezia furfur. The tools and materials were sterilized in an autoclave at 121 °C for 15 min. Afterward, bacterial and fungal cultures were grown on agar media, namely Müeller–Hinton Agar (MHA), Nutrient Agar (NA), and Potato Dextrose Broth (PDA), by selecting several bacterial and fungal colonies with an inoculation loop and incubating them for 24 h at 37 °C. Then, the bacterial and fungal cultures were suspended in a liquid medium, namely, Müeller-Hinton Broth (MHB), Nutrient Broth (NB), and Potato Dextrose Broth (PDB) for S. epidermidis, K. pneumoniae, and as well as the C. albicans and M. furfur, respectively. After incubating at 37 °C for 24 h, the suspended bacterial culture was standardized to 0.5 Mc Farland (2 × 108 CFU mL−1), and the cultures were diluted to 5 105 CFU mL−1 concentration [32,33,34].
2.5.2. MIC Determination of the Extracts from Kokossan Stembark
Four extracts, namely, ethanol, n-hexane, ethyl acetate, and n-butanol, were dissolved in 2% DMSO at a concentration of 500 mg mL−1, and four triterpenoid compounds and positive control were dissolved in 2% DMSO at a concentration of 1 mg mL−1. The positive controls used in this test were ciprofloxacin, nystatin, and ketoconazole, while 2% DMSO was a negative control. Antimicrobial concentrations tested ranged from 0.12 to 250 mg/mL for four extracts and 0.24 to 500 μg/mL for compounds, and positive control was incubated on a 96-well microplate at 37 °C for 18–24 h. Visual reading of the plate was performed after incubation, and the growth of each microbe at various sample concentrations was evaluated. The MIC was determined as the lowest sample concentration, resulting in a turbidity reduction. Spectrometric measurements supported test results analysis at a wavelength of 600 nm [35,36].
2.6. Cytotoxic Activity Test by the MTT Assay
Cell viability was determined using the MTT reagent (Thermo Fisher Scientific, Uppsala, Sweden), which measures the reduction of resazurin (blue) as a function of redox potential. Actively respiring cells converted the water-soluble MTT to an insoluble purple formazan, which is then dissolved, and its concentration is calculated using an optical density whereas normal cells may reduce the cytotoxicity for normal cell. The therapeutic index (TI) is typically considered as the ratio of the highest exposure to the drug that results in no toxicity to the exposure that produces the desired efficacy. The TI index which is used as parameter is 50% of inhibition concentration (IC50). The first step was to wash 80% confluent MCF-7 cell cultures twice with 1 mL 1X PBS. Then, 1 mL of trypsin EDTA was added and incubated for 3 min until the cells were released. The cells were then transferred to a Falcon tube containing 5 mL of culture medium and centrifuged for 4 min at 1200 rpm. After discarding the supernatant, they were then resuspended in 1 mL of culture medium, counted with a hemocytometer, and planted in 96-well plates with a serial number for the standard curve, which is 6× replications and 3× repetitions for the treatment. Subsequently, 100 µL of the medium was added before incubating for 24 h at 37 °C with 5% CO2.
The medium was replaced with 180 µL of new medium and 20 µL of ethanol, n-hexane, ethylacetate, and n-butanol extracts at 1, 10, 100, 250, and 500 ppm, and 0.5–2.5% co-solvent in PBS was added (Uppsala, Sweden). The solution was incubated for 24 h at 37 °C with 5% CO2. The cells were then treated with 20 L MTT and incubated for 3 h at 37 °C with 5% CO2. Cisplatin was used as a positive control in this trial, the absorbance was measured at 570 nm, and the IC50 value was calculated by comparing the percentage of cytotoxicity to untreated cells. According to the study [7], all assays and analyses were performed in duplicate and averaged such that a plot of cytotoxicity versus sample concentration was used to calculate the concentration, which indicates 50% cytotoxicity (IC50) [37,38].
3. Results
The antimicrobial activity of ethanol, n-hexane, ethyl acetate, and n-butanol extracts of Kokossan stembark was screened using Gram-positive and Gram-negative bacteria, namely, S. epidermidis and K. pneumoniae, respectively, as well as two fungi, namely, C. albicans and M. furfur. The antimicrobial screening results of Kokossan extracts, namely, ethanol, n-hexane, ethyl acetate, and n-butanol, are shown in Table 1.

Table 1.
Antimicrobial activities of extracts and compounds.
In addition to being antimicrobial, all Kokossan extracts were also tested for breast cancer cells, namely, MCF-7, and the cisplatin was used as positive control. The results showed n-hexane extract has good activity. Table 2 presents the results of the cytotoxic evaluation of these extracts.

Table 2.
The results of cytotoxic evaluations against MCF-7 Cell Cancer lines.
4. Discussions
Table 1 shows that the Kokossan extracts have more antibacterial potential than antifungal potential. Furthermore, the n-hexane and n-butanol extracts had very active antibacterial activity against K. pneumoniae with MIC 7.81 mg/mL but moderate against S. epidermidis bacteria. Ethanol and ethyl acetate had less activity with a MIC of 125 mg/mL against K. pneumoniae than the previous extracts and less activity with 250 mg/mL MIC against S. epidermidis.
In general, medicinal plant extracts are more effective as an antibacterial against Gram-positive bacteria than Gram-negative. This is due to the differences in the membranes of the two types of bacteria. Gram-negative bacteria have a double membrane or effective permeability barrier that can keep certain drugs, antibiotics, and other antibacterial agents from penetrating cells, making them more resistant than the Gram-positive. Furthermore, Gram-positive bacteria are known to have a peptidoglycan layer like a net, which is more easily penetrated by extracts, but this study showed a contradicting result. It is suspected that the lipophilic properties of the n-hexane extract facilitate the penetration of cells into K. pneumoniae compared to S. epidermidis [39,40,41,42,43].
The only extract among the list obtained from Kokossan having the potential as an antifungal with a moderate MIC of 62.5 mg/mL against M. furfur is n-butanol. Its several polar chemical constituents are presumably the leading cause of this antifungal activity. Furthermore, this extract is not active against C. albicans, which shows its selectivity only against fungi that cause dandruff, such as M. furfur. Its antibacterial potential also looks more attractive in this extract. This study indicates that additional investigation is required to determine the chemical components in the extract responsible for antimicrobials.
Mayanti et al. in 2011 [44] studied the bark of Kokossan and isolated compound 1–3 from a n-hexane fraction, while Zulfikar et al. in 2020 [8] reported that they succeeded in isolating a new compound (4) from the fruit peels of the same plants. Four triterpenoids were isolated in this study, in which compounds 1–4, often found in large quantities, were isolated from ethyl acetate fraction of stembark Kokossan.
Compounds 1–3 were identified as the same as compounds reported by Mayanti et. al., such as 8,14-secogammacera-7,14-diene-3,21-dione (a), 8,14-secogammacera-7,14(27)-diene-3,21-dione (b), and kokosanolide B (c). This compound was isolated successfully from the n-hexane extract of Kokosan stem bark. The 13C-NMR spectrum of compound a revealed 15 signals that were similar to the compound c, indicating that compound a has a symmetrical structure. The absence of a hydroxyl group and the appearance of a double bond [5.43 (1H, m), H 122.1 and 135.3 ppm] and fifteen carbon signals distinguish the NMR spectra of compounds a and c, indicating that compound a was a dehydroxy derivative of compound c [44].
Compound 2 reported in this study is a compound with the same structure as compound b reported by Mayanti et al. The 1H and 13C-NMR spectra of b are similar to those of a, except that the appearance of the methylene proton and sp2 carbon at C-27 [δH 5.12 (1H, J = 10.5 Hz), 5.45 (1H, J = 10.5 Hz), δC 122.1], indicates that b is an isomer of a. These structures were elucidated using single-crystal X-ray diffraction analysis. Therefore, it can be confirmed that compounds 1–3 are the same compounds as those isolated by Mayanti. This shows that the ethyl acetate extract most likely contains the same compound as the n-hexane extract [44].
Compound 4 is confirmed to be the same as the compound isolated by Zulfikar et al. from kokosan fruit peels. This confirmation is proven by the emergence of signals in chemical shifts approaching each other, as seen from the existing NMR data. 13C NMR data with DEPT and HSQC experiments revealed the presence of a total of 30 carbon signals, classified as eight methyls, eight methylenes, seven methines (two olefinics and one oxygenated), and seven quaternary carbons (two olefinic sand one carbonyl). Two substituted double bonds and a carbonyl in a system with seven degrees of unsaturation indicate that this compound has a tetracyclic structure and called with onoceranoid. The chemical shift data reported by Zulfikar et al. has similarities with the proton and carbon NMR data in this study. This shows that the fruit peel has some of the same compounds as the stem bark. The structures of compounds 1–4 are shown in Figure 1 below. Compounds such as sesquiterpenoids, steroids, and limonoids have also been isolated [7,45]. Compounds 1–4 showed no antibacterial or antifungal activity, which is consistent with the results of Ragasa et al. [10]. Furthermore, all the terpenoid compounds obtained from L. domesticum did not show good activity against microbes. This cytotoxic test was reported because the IC50 of Kokossan extracts against MCF-7 cancer cells was not reported in previous studies. Zulfikar et al. [8] and Tri Mayanti et al. [44] studied onoceranoids and reported that these compounds have no cytotoxic activity against MCF-7 breast cancer cells with IC50 > 700 µg/mL. Therefore, the good cytotoxic value of n-hexane is not caused by onoceranoid compounds but by other terpenoids. They include eudesman-type sesquiterpenoids, isolated by Siska et al. [7], and limonoids (kokosanolide A and C) which were examined for their molecular docking of breast cancer cells by Purwani et al. [46].
5. Conclusions
This study is the first to report the antimicrobial and cytotoxic activity of the stembark of L. domesticum Corr. cv. Kokossan. The n-hexane and n-butanol extracts showed promising results as an antibacterial against K. pneumoniae ATCC 2357. Similarly, n-butanol had good activity against M. furfur fungus, which is known to cause dandruff. These results also showed that the stembark extract of Kokossan has good cytotoxic value, especially n-hexane extract with IC50 42.95 µg/mL against MCF-7 breast cancer cells. Studies on the presence of phytochemicals need to be carried out mainly on n-butanol and n-hexane extracts, which have potential as antimicrobials and cytotoxic compounds, respectively. Furthermore, more studies are required to assess their bioactivity, toxicity profile, and clinical investigations for developing new drugs based on the bioactive chemicals identified in Kokossan.
Author Contributions
Conceptualization, S.E.S. and M.F.; methodology, S.E.S. and M.F.; validation, U.S.; formal analysis, S.E.S., M.F., T.M. and U.S.; investigation, S.E.S.; resources, S.E.S. and T.M.; data curation, S.E.S.; writing—original draft preparation, S.E.S.; writing—review and editing, U.S.; supervision, T.M. and U.S.; project administration, U.S.; funding acquisition, U.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Universitas Padjadjaran Academic Leadership Grant (ALG) with Academic Leadership Grant, Universitas Padjadjaran. No: 1959/UN6.3.1/PT.00/2021 by Unang Supratman. The APC was funded by Universitas Padjadjaran.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the facilities of the central laboratory, Universitas Padjadjaran, Indonesia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal teas and their health benefits: A scoping review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Effects of different phenols extraction conditions on antioxidant activity of almond (prunus dulcis) fruits. J. Food Biochem. 2009, 33, 763–776. [Google Scholar] [CrossRef]
- Nunes, A.R.; Flores-Félix, J.D.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Anti-inflammatory and antimicrobial activities of Portuguese Prunus Avium L. (sweet cherry) by-products extracts. Nutrients. 2022, 14, 4576. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Lansium domesticum ‘langsat-lonkong group’. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; pp. 269–277. [Google Scholar]
- Mayanti, T.; Sinaga, S.E.; Supratman, U. Phytochemistry and biological activity of Lansium domesticum corr. species: A review. J. Pharm. Pharmacol. 2022, 74, 1568–1587. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R.M. Lansium domesticum—A Fruit with multi-benefits: Traditional uses, phytochemicals, nutritional value, and bioactivities. Nutrients 2022, 14, 1531. [Google Scholar] [CrossRef]
- Sinaga, S.E.; Mayanti, T.; Naini, A.A.; Harneti, D.; Nurlelasari, N.; Maharani, R.; Farabi, K.; Supratman, U.; Fajriah, S.; Azmi, M.N. Sesquiterpenoids from the stem bark of Lansium domesticum Corr. cv. Kokossan and their cytotoxic activity against MCF-7 breast cancer cell lines. Indones. J. Chem. 2022, 22, 1035–1042. [Google Scholar] [CrossRef]
- Zulfikar; Putri, N.K.; Fajriah, S.; Yusuf, M.; Maharani, R.; Anshori, J.A.; Supratman, U.; Mayanti, T. 3-Hydroxy-8,14-secogammacera-7,14-dien-21-one: A New Onoceranoid Triterpenes from Lansium domesticum Corr. cv kokossan. Molbank 2020, 2020, M1157. [Google Scholar] [CrossRef]
- Rudiyansyah; Alimuddin, A.H.; Masriani; Muharini, R.; Proksch, P. New tetranortriterpenoids, langsatides a and b from the seeds of Lansium domesticum Corr. (Meliaceae). Phytochem. Lett. 2018, 23, 90–93. [Google Scholar] [CrossRef]
- Ragasa, C.; Ragasa, C.Y.; Labrador, P.; Rideout, J.A. Antimicrobial terpenoids from Lansium domesticum. Philipp. Agric. Sci. 2006, 89, 101. [Google Scholar]
- Saewan, N.; Sutherland, J.D.; Chantrapromma, K. Antimalarial tetranortriterpenoids from the seeds of Lansium domesticum Corr. Phytochemistry. 2006, 67, 2288–2293. [Google Scholar] [CrossRef]
- Heyne, K. Tumbuhan berguna indonesia. Badan Penelit. Dan Pengemb. Kehutanan. Dep. Kehutan. 1987, 2, 1188–1189. [Google Scholar]
- Hayati, I.; Wiyono, S.; Widodo, W.; Sobir, S. Variability of agronomic characters related to resistance to stem canker (phytophthora palmivora) on duku (Lansium domesticum) along Batanghari River, Sumatra, Indonesia. Biodiversitas 2019, 20, 1127–1132. [Google Scholar] [CrossRef]
- Tilaar, M.; Wih, W.L.; Ranti, A.S.; Wasitaatmadja, S.M.; Suryaningsih, S.; Junardy, F.D.; Maily, M. Review of Lansium domesticum Corrêa and its use in cosmetics. Bol. Latinoam Caribe Plantas Med. Aromat. 2008, 7, 183–189. [Google Scholar]
- Petrov, M.M. Antimicrobial resistance and antibiotic consumption. Int. J. Infect. Dis. Epidemiol. Antimicrob. 2022, 3, 3–4. [Google Scholar] [CrossRef]
- Marfori, E.C.; Kajiyama, S.I.; Fukusaki, E.; Kobayashi, A. Lansioside D, a new triterpenoid glycoside antibiotic from the fruit peel of Lansium domesticum Correa. J. Pharmacogn. Phytochem. 2015, 3, 140–143. [Google Scholar]
- Arenas-Chávez, C.A.; de Hollanda, L.M.; Arce-Esquivel, A.A.; Alvarez-Risco, A.; Del-Aguila-Arcentales, S.; Yáñez, J.A.; Vera-Gonzales, C. Antibacterial and antifungal activity of functionalized cotton fabric with nanocomposite based on silver nanoparticles and carboxymethyl chitosan. Processes 2022, 10, 1088. [Google Scholar] [CrossRef]
- Berman, J. Candida albicans. Curr. Biol. 2012, 22, R620–R622. [Google Scholar] [CrossRef]
- Berman, J.; Hadany, L. Does stress induce (para)sex? implications for Candida albicans evolution. Trends Genet. 2012, 28, 197–203. [Google Scholar] [CrossRef]
- Ingham, E.; Cunningham, C. Malassezia furfur. J. Med. Vet. 1993, 31, 265–288. [Google Scholar] [CrossRef]
- Tragiannidis, A.; Bisping, G.; Koehler, G.; Groll, A.H. Minireview: Malassezia infections in immunocompromised patients. Mycoses 2009, 53, 187–195. [Google Scholar] [CrossRef]
- Harada, K.; Saito, M.; Sugita, T.; Tsuboi, R. Malassezia species and their associated skin diseases. J. Dermatol. 2015, 42, 250–257. [Google Scholar] [CrossRef]
- Dolenc-volj, M. Diseases caused by Malassezia species in human beings. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 2, pp. 77–91. [Google Scholar]
- Ljubojevic, S.; Skerlev, M.; Lipozencic, J.; Basta-Juzbasic, A. The role of Malassezia furfur in dermatology. Clin. Dermatol. 2002, 20, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the eskape pathogens. Expert Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sedjati, S.; Ambariyanto, A.; Trianto, A.; Ridlo, A.; Supriyantini, E.; Sabdono, A.; Radjasa, O.K.; Firmansyah, T. Antibacterial activity of the fungal metabolite Trichoderma longibrachiatum against multidrug-resistant Klebsiella pneumoniae and methicillin-resistant Staphylococcus aureus. Jordan. J. Biol. Sci. 2022, 15, 107–113. [Google Scholar] [CrossRef]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef]
- Uckay, I.; Pittet, D.; Vaudaux, P.; Sax, H.; Lew, D.; Waldvogel, F. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 2009, 41, 109–119. [Google Scholar] [CrossRef]
- Abedini, A.; Colin, M.; Hubert, J.; Charpentier, E.; Angelis, A.; Bounasri, H.; Bertaux, B.; Kotland, A.; Reffuveille, F.; Nuzillard, J.M.; et al. Abundant Extractable metabolites from temperate tree barks: The specific antimicrobial activity of Prunus avium extracts. Antibiotics 2020, 9, 111. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Shi, X.W.; Gao, J.M. Antifungal and antibacterial metabolites from an endophytic Aspergillus Sp. associated with Melia azedarach. Nat. Prod. Res. 2014, 28, 1388–1392. [Google Scholar] [CrossRef]
- Maharani, R.; Napitupulu, O.I.; Dirgantara, J.M.; Hidayat, A.T.; Sumiarsa, D.; Harneti, D.; Supratman, U.; Fukase, K. Synthesis of cyclotetrapeptide analogues of c-plai and evaluation of their antimicrobial properties. R. Soc. Open Sci. 2021, 8, 201822. [Google Scholar] [CrossRef]
- Panjaitan, J.R.H.; Monica, S.; Gozan, M. Biological evaluation of certain substituted hydantoins and benzalhydantoins against microbes biological evaluation of certain substituted hydantoins and benzalhydantoins against microbes. Mater. Sci. Eng. 2016, 107, 6–13. [Google Scholar] [CrossRef]
- Bradford, P.A.; Petersen, P.J.; Young, M.; Jones, C.H.; Tischler, M.; Connell, J.O.; Al, B.E.T. Tigecycline MIC testing by broth dilution requires use of fresh medium or addition of the biocatalytic oxygen-reducing reagent oxyrase to standardize the test method. Antimicrob. Agents Chemother. 2005, 49, 3903–3909. [Google Scholar] [CrossRef] [PubMed]
- Iatta, R.; Puttilli, M.R.; Immediato, D.; Otranto, D.; Cafarchia, C. The role of drug efflux pumps in Malassezia pachydermatis and Malassezia furfur defence against Azoles. Mycoses 2017, 60, 178–182. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.Q.; Song, H.Y.; Zhang, Q.; Liu, G.F. Chemical analysis and in Vitro antimicrobial effects and mechanism of action of Trachyspermum copticum essential oil against Escherichia coli. Asian Pac. J. Trop. Med. 2017, 10, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Senthilraja, P.; Kathiresan, K. In Vitro Cytotoxicity MTT Assay in Vero, HepG2 and MCF-7 cell lines study of marine yeast. J. Appl. Pharm. Sci. 2015, 5, 80–84. [Google Scholar] [CrossRef]
- Rahmayanti, I.; Nurlelasari; Harneti, D.; Maharani, R.; Darwati; Shiono, Y.; Supratman, U. Two limonoids from the seeds of Chisocheton macrophyllus and their cytotoxic activity against MCF-7 breast cancer cells. Molekul 2021, 16, 117–124. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Qa’dan, F.; Thewaini, A.J.; Ali, D.A.; Afifi, R.; Elkhawad, A.; Matalka, K.Z. The Antimicrobial activities of Psidium guajava and Juglans regia Leaf extracts to acne-developing organisms. Am. J. Chin. Med. 2005, 33, 197–204. [Google Scholar] [CrossRef]
- Rameshkumar, K.B.; George, V.; Shiburaj, S. Chemical constituents and antibacterial activity of the leaf oil of Cinnamomum chemungianum mohan et Henry. J. Essent. Oil Res. 2007, 19, 98–100. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Cervi, A.C.; Ito, I.Y.; Salvador, M.J.; Wisniewski, A.; Simionatto, E.L. Chemical composition and antimicrobial activity of essential oils of Eugenia chlorophylla (Myrtaceae). J. Essent. Oil Res. 2008, 20, 75–78. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Mayanti, T.; Tjokronegoro, R.; Supratman, U.; Mukhtar, M.R.; Awang, K.; Hadi, A.H.A. Antifeedant triterpenoids from the seeds and bark of Lansium domesticum cv Kokossan (Meliaceae). Molecules 2011, 16, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, F.M.; Meilanie, S.R.; Zulfikar; Farabi, K.; Herlina, T.; Al Anshori, J.; Mayanti, T. Kokosanolide d: A New tetranortriterpenoid from fruit peels of Lansium domesticum Corr. cv kokossan. Molbank 2021, 2021, M1232. [Google Scholar] [CrossRef]
- Purwani, S.; Nahar, J.; Mayanti, T. Molecular docking on kokosanolide a and c for anticancer activity against human breast cancer Cell MCF-7. J. Kim. Valensi 2021, 7, 52–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).