Technoeconomic Assessment of Biopolymer Production from Crustacean Waste with the UK as a Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Crab Shell Waste
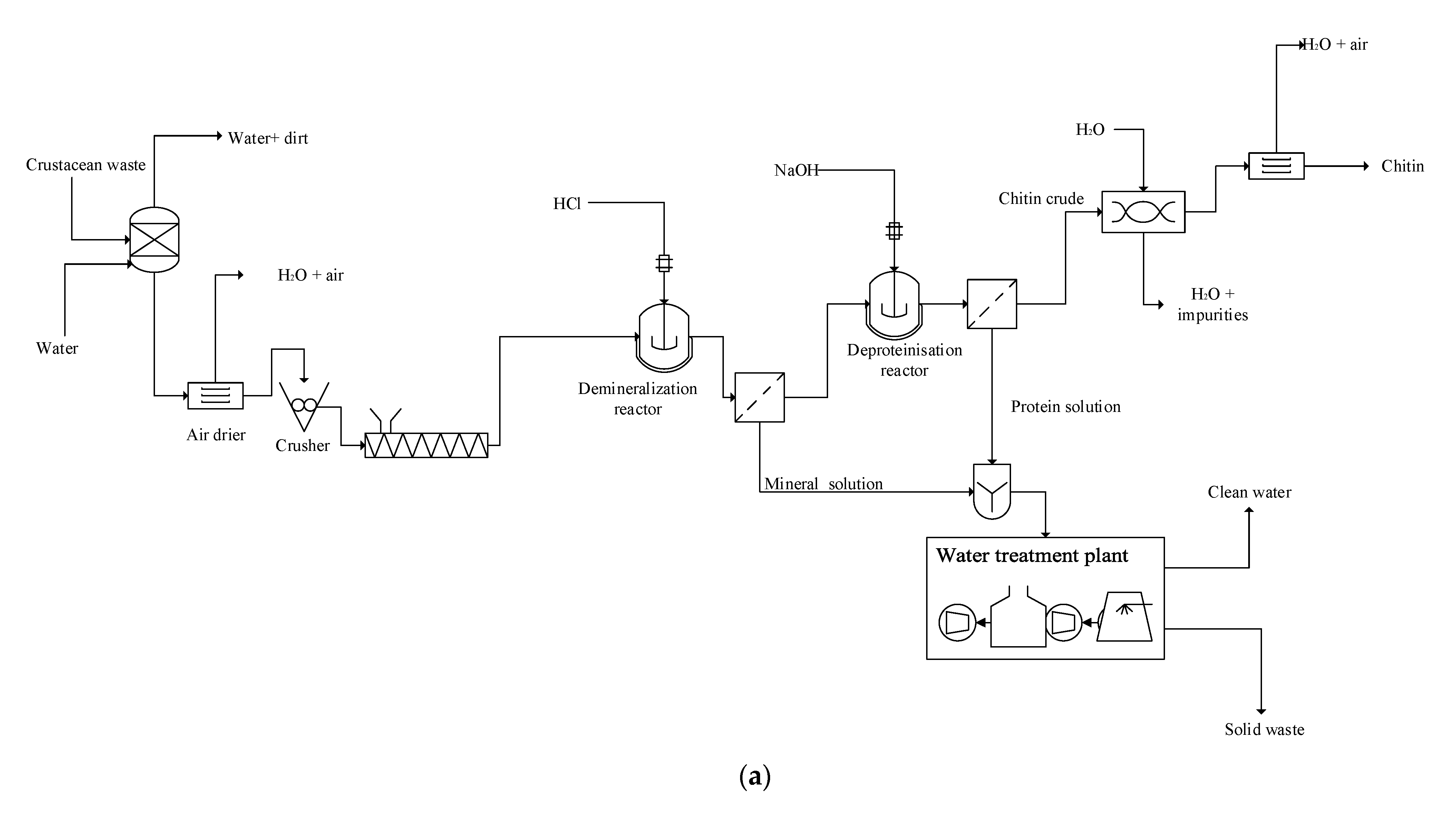
2.2. Chemical Method for Chitin Production
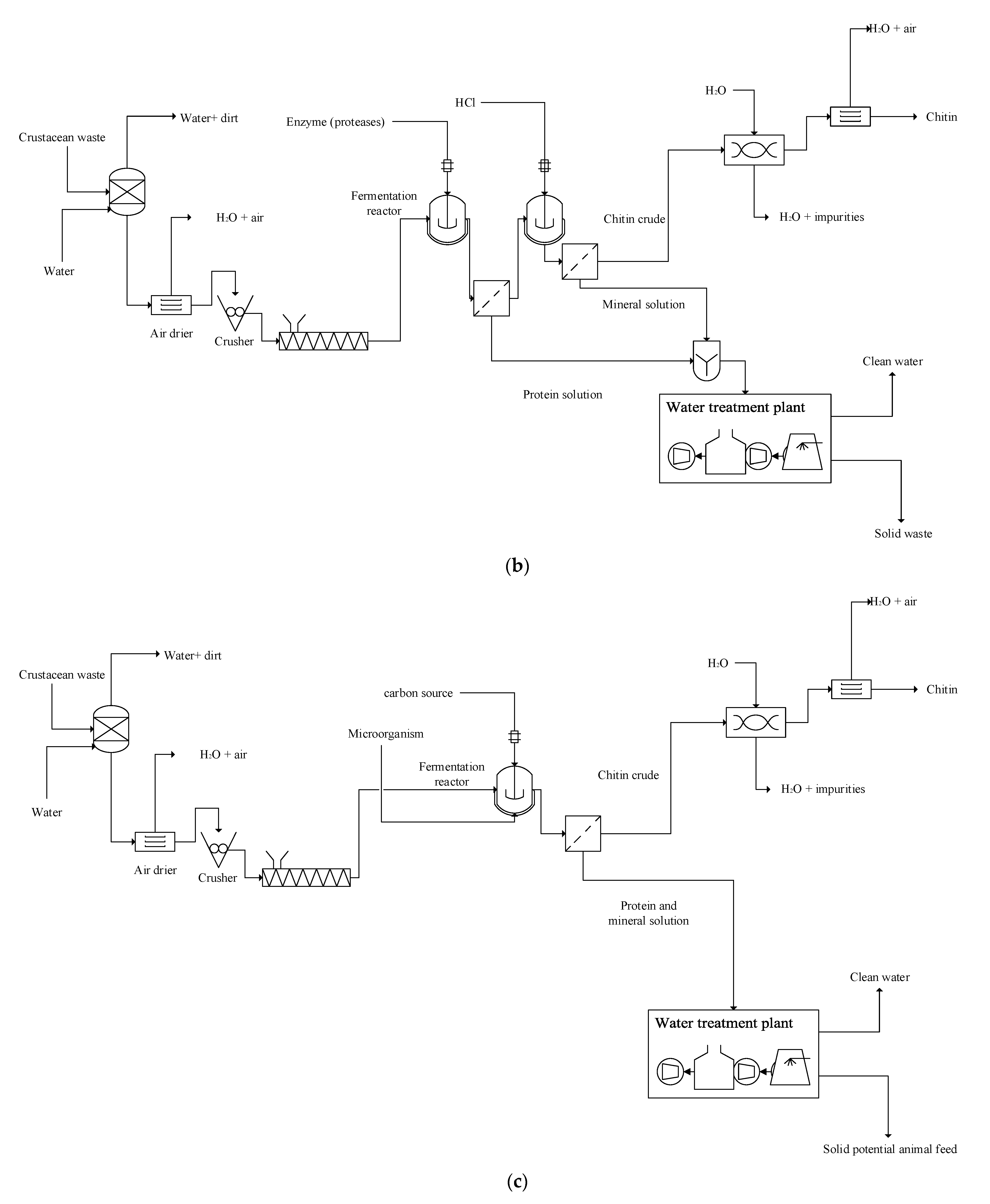
2.3. Enzymatic–Chemical Approach for Chitin Production
2.4. Microbial Approach for Chitin Production
2.5. Process Design, Modelling, and Simulation
2.6. Economic Performance Assessment
2.7. Cost–Benefit Analysis of the Alternative Chitin Production Pathways
3. Results
3.1. Sample Characterization and Chitin Extraction
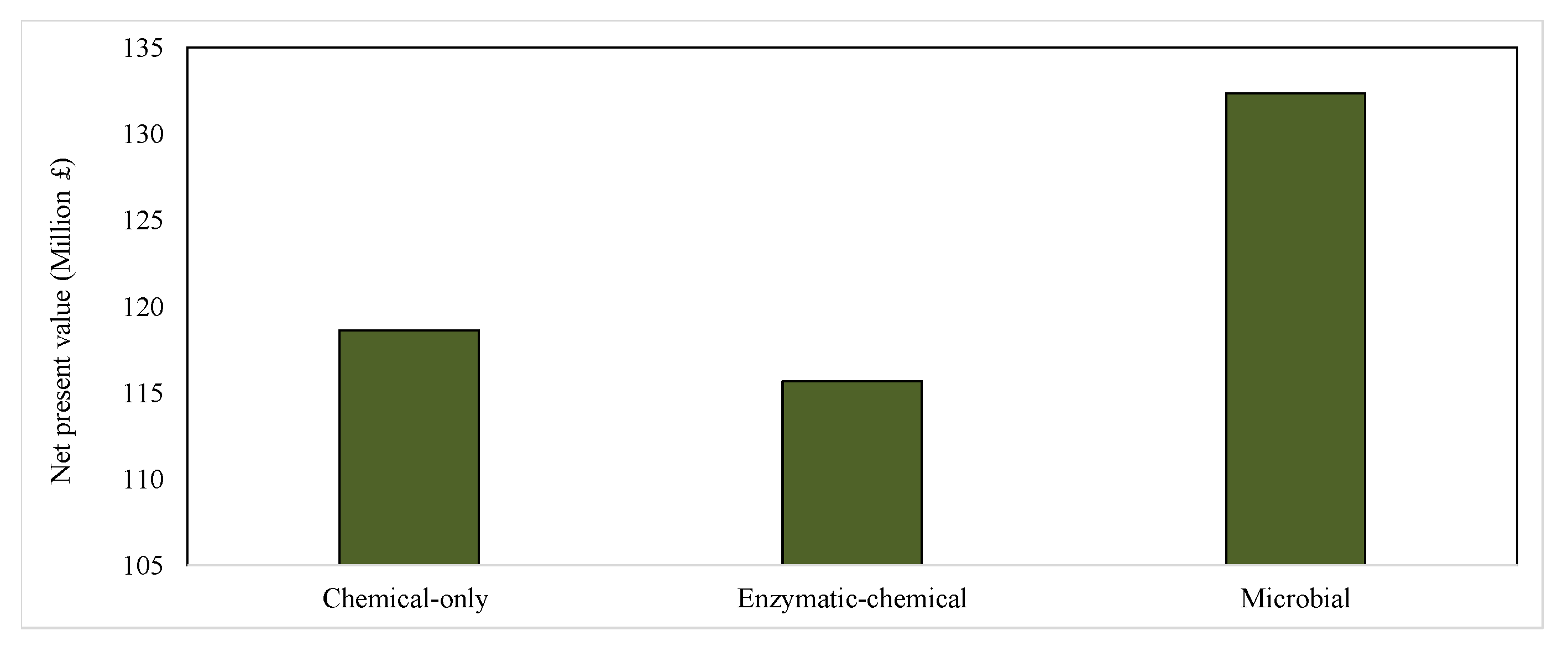
3.2. Scaled-Up Chitin Production from Crab Shells: Comparative Technical and Economic Assessments
4. Discussion
4.1. Characterization of Sample
4.2. Chitin Yields via Alternative Pathways
4.3. Simulation-Based Analysis of Alternative Chitin Production Pathways
4.4. Benefits of Exploring Technological Improvements to the Microbial Pathway
5. Conclusions and Future Research
- ○
- Explore the use of a combination of microbial strains characterized by multi-functionalities: For instance, microbes capable of producing high concentrations of organic acids, such as P. acidilactici [69], and microbes equipped with excellent protein valorization properties, such as L. helveticus [70], may be combined. When such microbes are combined, their synergizing effects are optimized to facilitate enhanced demineralization and deproteinization.
- ○
- Explore options for enhancing both the yield and purity of the chitin product: This will be achieved via an experimental design incorporating the controlled variation of the process variables governing the microbe-based extraction process. Thus, process variables such as pH, the nitrogen source (i.e., ammonium sulfate), the carbon/carbohydrate source (i.e., sucrose), the solid–solvent ratio, etc., will be considered. In this scenario, the responses of the ‘yield’ and ‘purity’ of the chitin will be employed as sufficient objective functions that must be optimized. Having optimized the chitin yield and purity, we will seek to model and investigate the kinetics and multiphase fluid mechanics as a precursor to developing a chitin production kinetic model as a crucial input for future scale-up executions.
- ○
- Explore the use of non-conventional carbon sources: According to the circularity paradigm, alternative sugars or carbohydrate-dense waste streams (e.g., apple pomace) must be explored as renewable and cheap carbon sources. This is because the carbon source was determined to constitute the most impactful cost component in the microbial chitin production process since it accounts for ~41% of the total operating cost. It must be emphasized that the exploration of alternative carbon sources in the proposed project is of timely importance to the UK due to the persistent concerns previously raised regarding the inadequacies of current efforts towards achieving the zero-emission target. Indeed, using such waste streams may also promote synergies between different sectors of the food industry.
- ○
- Explore the potential of recycling the microbe-containing exit stream: The potential of recycling the microbes for use in the fermentation broth will further reduce the cost of inputs required in a scaled-up system, thus improving the overall economic viability.
- ○
- Explore the potential of producing valuable by-products: Notably, future studies should explore the viability of employing the protein-containing residual stream from microbial–chitin production as a cheap and sustainable animal feed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dave, U.; Somanader, E.; Baharlouei, P.; Pham, L.; Rahman, M.A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pai, A.R.; Pasquini, D.; Shao-Yuan, L.; Khalil, A.H.P.S.; Thomas, S. Chapter—State of Art, New Challenges, and Opportunities. In Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–24. [Google Scholar]
- Zhang, J.; Feng, M.; Lu, X.; Shi, C.; Li, X.; Xin, J.; Yue, G.; Zhang, S. Base-free preparation of low molecular weight chitin from crab shell. Carbohydr. Polym. 2018, 190, 148–155. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, R.; Zhu, Y.; Yang, P.; Lin, Z.; Wang, Z.; Cong, W. Efficient extraction of chitin from crustacean waste via a novel ternary natural deep eutectic solvents. Carbohydr. Polym. 2022, 286, 119281. [Google Scholar] [CrossRef]
- Cohen, E. Chapter 43—Chitin. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 156–157. [Google Scholar]
- Chen, X.; Yang, H.; Yan, N. Shell Biorefinery: Dream or Reality? Chemistry 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- Kaya, M.; Lelešius, E.; Nagrockaitė, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PloS ONE 2015, 10, e0115531. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, J.; Pei, Y.; Zheng, X.; Tang, K. Recent Progress in Preparation and Application of Nano-Chitin Materials. Energy Environ. Mater. 2020, 3, 492–515. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A.J. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Makino, A.; Ohmae, M.; Kobayashi, S. Synthesis of fluorinated chitin derivatives via enzymatic polymerization. Macromol. Biosci. 2006, 6, 862–872. [Google Scholar] [CrossRef]
- Pestov, A.; Bratskaya, S. Chitosan and Its Derivatives as Highly Efficient Polymer Ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [Green Version]
- Kudzin, M.H.; Giełdowska, M.; Krata, A.A.; Sulak, E.; Urbaniak, P.; Drabowicz, J. Phosphorylation of chitosan (chitin) surface with PCl3. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 625–629. [Google Scholar] [CrossRef]
- Kurita, K.; Yoshino, H.; Nishimura, S.-I.; Ishii, S.; Mori, T.; Nishiyama, Y. Mercapto-chitins: A new type of supports for effective immobilization of acid phosphatase. Carbohydr. Polym. 1997, 32, 171–175. [Google Scholar] [CrossRef]
- Yamamoto, C.; Hayashi, T.; Okamoto, Y. High-performance liquid chromatographic enantioseparation using chitin carbamate derivatives as chiral stationary phases. J. Chromatogr. A 2003, 1021, 83–91. [Google Scholar] [CrossRef]
- Seafish. Crustacea Processing Waste Management Data; Seafish Edinburgh: Edinburgh, UK, 2022; Available online: https://www.seafish.org/document/?id=A8C385D7-0CDD-4074-A1F0-72A41474EE64/ (accessed on 9 September 2022).
- Venugopal, V. Valorization of Seafood Processing Discards: Bioconversion and Bio-Refinery Approaches. Front. Sustain. Food Syst. 2021, 5, 611835. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels—A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608. [Google Scholar] [CrossRef]
- Kim, Y.; Park, R.-D. Progress in bioextraction processes of chitin from crustacean biowastes. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 545–554. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Hong, S.; Lian, H.; Mei, C.; Lee, J.; Wu, Q.; Hubbe, M.A.; Li, M.-C. Recent advances in extraction and processing of chitin using deep eutectic solvents. Chem. Eng. J. 2022, 446, 136953. [Google Scholar] [CrossRef]
- Okoro, O.V.; Jafari, H.; Hobbi, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Enhanced keratin extraction from wool waste using a deep eutectic solvent. Chem. Pap. 2022, 76, 2637–2648. [Google Scholar] [CrossRef]
- Al-Tamimi, M.; Azure, J.D.-C.; Ramanathan, R. Corporate Reporting on Food Waste by UK Seafood Companies: Literature Review and an Assessment of Current Practices. Sustainability 2023, 15, 1213. [Google Scholar] [CrossRef]
- ASTM D3175-11; Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2017-98; Standard Test Method of Accelerated Laboratory Test of Natural Decay Resistance of Woods, Decay, Evaluation, Laboratory, Natural, Resistance and Subjected to Termite Bioassay According to No-choice Test Procedure Based upon AWPA E1-97 (AWPA, 1). American Society for Testing and Materials: West Conshohocken, PA, USA, 1998.
- Hobbi, P.; Okoro, O.V.; Delporte, C.; Alimoradi, H.; Podstawczyk, D.; Nie, L.; Bernaerts, K.V.; Shavandi, A. Kinetic modelling of the solid–liquid extraction process of polyphenolic compounds from apple pomace: Influence of solvent composition and temperature. Bioresour. Bioprocess. 2021, 8, 114. [Google Scholar] [CrossRef]
- ASTM D6175-20; Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2020.
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Lynch, J.M.; Barbano, D.M. Kjeldahl Nitrogen Analysis as a Reference Method for Protein Determination in Dairy Products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Method of Analysis. In Association of Analytical Chemists, 19th ed.; AOAC International: Washington, DC, USA, 2012; pp. 121–130. [Google Scholar]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef]
- Deng, J.-J.; Mao, H.-H.; Fang, W.; Li, Z.-Q.; Shi, D.; Li, Z.-W.; Zhou, T.; Luo, X.-C. Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J. Clean. Prod. 2020, 271, 122655. [Google Scholar] [CrossRef]
- Hongkulsup, C.; Khutoryanskiy, V.V.; Niranjan, K. Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei). J. Chem. Technol. Biotechnol. 2016, 91, 1250–1256. [Google Scholar] [CrossRef]
- Castro, R.; Guerrero-Legarreta, I.; Bórquez, R. Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 2018, 20, e00287. [Google Scholar] [CrossRef]
- Alshekhli, O.; Foo, D.C.Y.; Hii, C.L.; Law, C.L. Process simulation and debottlenecking for an industrial cocoa manufacturing process. Food Bioprod. Process. 2011, 89, 528–536. [Google Scholar] [CrossRef]
- Okoro, O.V.; Nie, L.; Podstawczyk, D.; Shavandi, A. Technoeconomic and Environmental Assessment of Alternative Biorefineries for Bioenergy and Polyphenolic Production from Pomace Biomass. BioEnergy Res. 2022, 15, 1–15. [Google Scholar] [CrossRef]
- Okoro, O.V.; Shavandi, A. An assessment of the utilization of waste apple slurry in bio-succinic acid and bioenergy production. Int. J. Environ. Sci. Technol. 2021, 19, 1323–1334. [Google Scholar] [CrossRef]
- Xia, Y. An Experimental Research of Biomass Crushing System. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Suzhou, China, 22–24 March 2017; p. 012006. [Google Scholar]
- Gómez-Ríos, D.; Barrera-Zapata, R.; Ríos-Estepa, R. Comparison of process technologies for chitosan production from shrimp shell waste: A techno-economic approach using Aspen Plus®. Food Bioprod. Process. 2017, 103, 49–57. [Google Scholar] [CrossRef]
- Chemical-Engineering. CE Plant Cost Index. 2021. Available online: https://www.chemengonline.com/ (accessed on 9 September 2022).
- Alibaba. Reactor Cost Alibaba. 2022. Available online: https://www.alibaba.com/product-detail/High-Pressure-Reactor-250ml-Lab-Mini_1600454993140.html?spm=a2700.galleryofferlist.normal_offer.d_title.3c48cb12E2OZyw&s=p (accessed on 12 July 2022).
- Bartels, J.R. A Feasibility Study of Implementing an Ammonia Economy; Iowa State University: Ames, IA, USA, 2008. [Google Scholar]
- Holmgren, K. Investment Cost Estimates for Gasification-Based Biofuel Production Systems; Swedish Environmental Research Institute: Stockholm, Sweden, 2015. [Google Scholar]
- GOV.UK. Guidance: Rates and Allowances for Corporation Tax; Crown Copyright. 2022. Available online: https://www.gov.uk/government/publications/rates-and-allowances-corporation-tax/rates-and-allowances-corporation-tax (accessed on 15 June 2022).
- Towler, G.; Sinnott, R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Okoro, O.V.; Sun, Z.; Birch, J. Techno-Economic Assessment of a Scaled-Up Meat Waste Biorefinery System: A Simulation Study. Materials 2019, 12, 1030. [Google Scholar] [CrossRef] [Green Version]
- BBC. Where Does Recycling and Rubbish from the UK Go? 2021. Available online: https://www.bbc.com/news/science-environment-49827945 (accessed on 6 September 2022).
- Neufeld, D. The Carbon Footprint of the Food Supply Chain. 2020. Available online: https://www.visualcapitalist.com/visualising-the-greenhouse-gas-impact-of-each-food/ (accessed on 9 September 2022).
- Clifford, C. Here’s What You Need to Know about ‘the Social Cost of Greenhouse Gases’—A Key Climate Metric; CNBC. 2021. Available online: https://www.cnbc.com/2021/03/09/heres-everything-you-need-to-know-about-the-social-cost-of-greenhouse-gases.html#:~:text=There%20are%20estimates%20for%20the,according%20to%20the%20SCC%20measure (accessed on 15 June 2022).
- Asunción Lage-Yusty, M.; Vilasoa-Martínez, M.; Álvarez-Pérez, S.; López-Hernández, J. Chemical composition of snow crab shells (Chionoecetes opilio) Composición química del caparazón del cangrejo de las nieves (Chionoecetes opilio). CyTA-J. Food 2011, 9, 265–270. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Jakab, E.; Domán, A.; Bokrossy, P.; Bertóti, I.; Madarász, J.; László, K. Thermal degradation of crab shell biomass, a nitrogen-containing carbon precursor. J. Therm. Anal. Calorim. 2020, 142, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Wardiatno, Y.; Riyanto, B.; Iskandar, N.A.; Kleinertz, S.; Funch, P.; Kurniawan, F. A New Marine Biomaterial: The Shell of Mangrove Horseshoe Crabs, Carcinoscorpius rotundicauda (Latreille, 1802) Emphasizing Its Physico-Chemical Characteristics. Front. Mar. Sci. 2021, 8, 612016. [Google Scholar] [CrossRef]
- Pathak, N.; Shakila, R.; Jeyasekaran, G.; Shalini, R.; Ulaganathan, A.; Patel, A.; Kumar, U.; Hema, A.; Mayilvahnan, R.; Malini, A. Variation in the Nutritional Composition of Soft and Hard Blue Swimming Crabs (Portunus pelagicus) Having Good Export Potential. J. Aquat. Food Prod. Technol. 2021, 30, 706–719. [Google Scholar] [CrossRef]
- Cira, L.A.; Huerta, S.; Hall, G.M.; Shirai, K. Pilot scale lactic acid fermentation of shrimp wastes for chitin recovery. Process Biochem. 2002, 37, 1359–1366. [Google Scholar] [CrossRef]
- Rao, M.S.; Muñoz, J.; Stevens, W.F. Critical factors in chitin production by fermentation of shrimp biowaste. Appl. Microbiol. Biotechnol. 2000, 54, 808–813. [Google Scholar] [CrossRef]
- AACE. AACE Classification System; AACE International Recommended Practice No. 18R-97. 2005. Available online: http://www.costengineering.eu/Downloads/articles/AACE_CLASSIFICATION_SYSTEM.pdf (accessed on 17 June 2022).
- VMR. Global Chitin Market Size By Derivative Type, By End-User, By Geographic Scope And Forecast; Verified Market. 2021. Available online: https://www.verifiedmarketresearch.com (accessed on 17 June 2022).
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Jardine, A.; Sayed, S.J.P.; Chemistry, A. Valorisation of chitinous biomass for antimicrobial applications. Pure Appl. Chem. 2018, 90, 293–304. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Topmarketsreports. Chitin Market with Amid New Technological Factors; Topmarketsreports. 2021. Available online: https://topmarketsreports.com/chitin-market-with-amid-new-technological-factors-no-of-pages-124/24401/07/15/17/2022/ (accessed on 9 August 2022).
- Reportlinker. Global Chitin and Chitosan Derivatives Industry; Global Industry Analysts: Lyon, France, 2020. [Google Scholar]
- Thomas, S.K.; Parameswaranpillai, J.; Krishnasamy, S.; Begum, P.M.S.; Nandi, D.; Siengchin, S.; George, J.J.; Hameed, N.; Salim, N.V.; Sienkiewicz, N. A comprehensive review on cellulose, chitin, and starch as fillers in natural rubber biocomposites. Carbohydr. Polym. Technol. Appl. 2021, 2, 100095. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, M.M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [Green Version]
- Elieh Ali Komi, D.; Sharma, L.; Dela Cruz, C.S. Chitin and Its Effects on Inflammatory and Immune Responses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Holland, R.; Crow, V.; Curry, B. Lactic Acid Bacteria|Pediococcus spp. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 149–152. [Google Scholar]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: The proteolytic system. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; NREL Technical Report: NREL/TP-5100-47764; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Zyl, v.M. Valorisation of Bio-aromatics from Pulp Mill Residues and Commercial Forest Species in South Africa; Stellenbosch University: Stellenbosch, South Africa, 2017. [Google Scholar]
- TDT. Fourth Quarter 2007 Guide to Labor Burden; Texas Department of Transportation: Austin, TX, USA, 2007. [Google Scholar]
- Macedo, J.V.C.; Ranke, F.F.d.B.; Escaramboni, B.; Campioni, T.S.; Núñez, E.G.F.; Neto, P.d.O. Cost-effective lactic acid production by fermentation of agro-industrial residues. Biocatal. Agric. Biotechnol. 2020, 27, 101706. [Google Scholar] [CrossRef]





| Characterization | Value |
|---|---|
| Moisture content (% w/w, wet CW basis) | 70.92 |
| Lipid content (% w/w, dry CW basis) | 3.00 |
| Carbohydrate content (% w/w, dry CW basis) | 34.00 |
| Protein content (% w/w, dry CW basis) | 31.55 |
| Ash content (% w/w, dry CW basis) | 31.45 |
| Volatiles (% w/w, dry CW basis) | 66.65 |
| Fixed carbon (% w/w, dry CW basis) | 1.90 |
| Carbon content (% w/w, dry CW basis) | 44.53 |
| Hydrogen content (% w/w, dry CW basis) | 5.75 |
| Nitrogen content (% w/w, dry CW basis) | 5.53 |
| Oxygen content (% w/w, dry CW basis) | 42.02 |
| Sulphur content (% w/w, dry CW basis) | 2.19 |
| Approach | DD (wt.%) | DP (wt.%) | Yield of Crude Chitin (wt.%) |
|---|---|---|---|
| Chemical-only | 87.75 | 95.80 | 22.70 |
| Enzymatic–chemical | 79.50 | 50.23 | 40.20 |
| Microbial | 61.84 | 56.41 | 71.36 |
| Stream Name | Scenario (a) | Scenario (b) | Scenario (c) |
|---|---|---|---|
| Experimental crude chitin yield (kg/kg dry CW) | 0.227 | 0.402 | 0.714 |
| Simulated crude chitin yield (kg/kg dry CW) | 0.215 | 0.441 | 0.750 |
| Solid waste for treatment (kg/kg dry CW) | 36.72 | 0.643 | 0 |
| External cooling utility (MW) | 4.043 | 0.044 | 0.240 |
| External heating requirement (MW) | 3.123 | 0.057 | 2.730 |
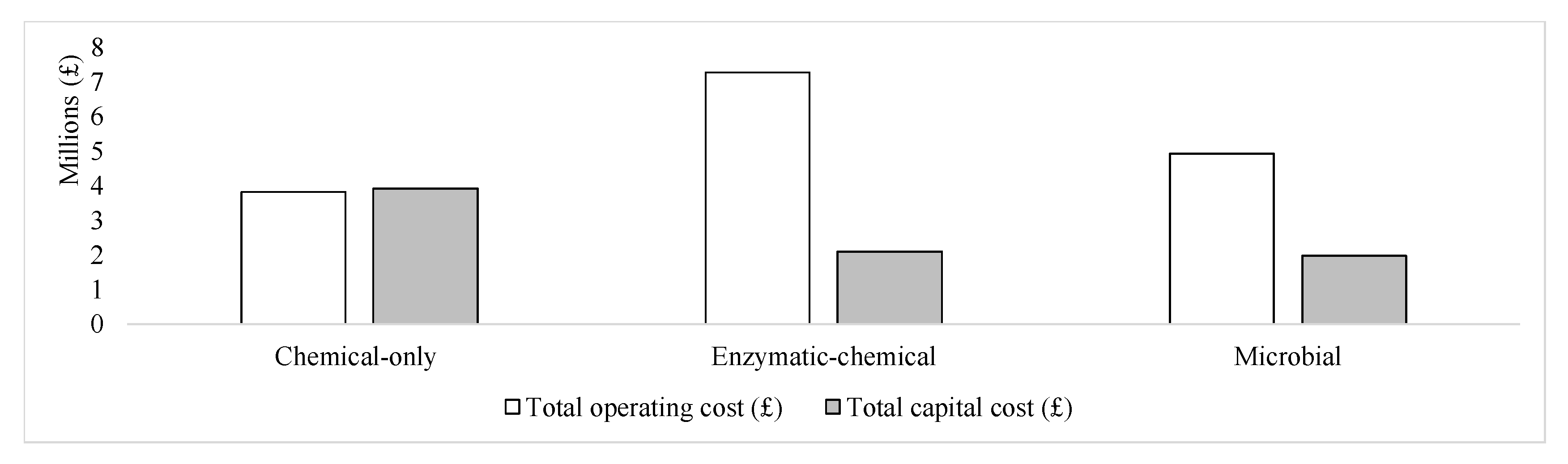
| Cost Components | Chemical-Only | Enzymatic–Chemical | Microbial |
|---|---|---|---|
| Total equipment purchase cost (GBP) | 882,725.71 | 544,137.25 | 441,247.61 |
| Total equipment installation cost (GBP) | 1,978,969.99 | 1,062,009.77 | 998,935.49 |
| Warehouse cost (GBP) | 79,158.80 | 42,480.39 | 39,957.42 |
| Home office and construction fee (GBP) | 465,057.95 | 249,572.30 | 234,749.84 |
| Project contingency (GBP) | 232,528.97 | 124,786.15 | 117,374.92 |
| Other costs (start-up, permits) (GBP) | 232,528.97 | 124,786.15 | 117,374.92 |
| Fixed capital investment (GBP) | 3,720,463.59 | 1,996,578.36 | 1,877,998.73 |
| Working capital cost (GBP) | 186,023.18 | 99,828.92 | 93,899.94 |
| Total capital investment (GBP) | 3,906,486.77 | 2,096,407.28 | 1,971,898.66 |
| Labor cost (GBP) | 913,196.16 | 913,196.16 | 913,196.16 |
| Labor burden cost (GBP) | 821,876.54 | 821,876.54 | 821,876.54 |
| Maintenance cost (GBP) | 26,481.77 | 16,324.12 | 13,237.43 |
| Property insurance (GBP) | 26,043.25 | 13,976.05 | 13,145.99 |
| Total variable cost (GBP) | 2,032,663.29 | 5,506,565.00 | 3,160,360.71 |
| Fixed operating cost (GBP) | 1,787,597.72 | 1,765,372.87 | 1,761,456.12 |
| Total operating cost (GBP) | 3,820,261.01 | 7,271,937.87 | 4,921,816.83 |
| Process | Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|---|
| Chemical-only | The chitin produced is characterized by a high level of purity. Additionally, this is a mature technology implying that significant information regarding its application is available in the literature. | This approach has a substantial environmental footprint. There may also be health and safety concerns due to the use of mineral acids for demineralization operations. | Not applicable | Countries with weaker environmental regulations than the UK may ignore the need for waste water treatment. This would lead to lower costs and thus a more competitive chitin product overall. |
| Enzymatic–chemical | The chitin produced is characterized by a moderate level of purity. | Moderate environmental footprint. There may also be health and safety concerns due to the use of mineral acids for demineralization operations. | Further work could explore the use of organic acids for demineralization processes. | Countries with weaker environmental regulations than the UK may ignore the need for waste water treatment. This would lead to lower costs and thus a more competitive chitin product overall. |
| Microbial | Minimal environmental footprint. This process is also the most economically viable overall, thus making it sustainable. | The chitin produced is characterized by a low level of purity. Additionally, microbial systems are time consuming and difficult to control. | Further work could explore approaches to further improve the chitin purity. The opportunity for microbe re-use could also be investigated. | The complexity of the process and the need for specialist skills could limit acceptance of this process. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoro, O.V.; Nie, L.; Gunduz, O.; Ulag, S.; Hamidi, M.; Shavandi, A. Technoeconomic Assessment of Biopolymer Production from Crustacean Waste with the UK as a Case Study. Sustainability 2023, 15, 2280. https://doi.org/10.3390/su15032280
Okoro OV, Nie L, Gunduz O, Ulag S, Hamidi M, Shavandi A. Technoeconomic Assessment of Biopolymer Production from Crustacean Waste with the UK as a Case Study. Sustainability. 2023; 15(3):2280. https://doi.org/10.3390/su15032280
Chicago/Turabian StyleOkoro, Oseweuba Valentine, Lei Nie, Oguzhan Gunduz, Songul Ulag, Masoud Hamidi, and Amin Shavandi. 2023. "Technoeconomic Assessment of Biopolymer Production from Crustacean Waste with the UK as a Case Study" Sustainability 15, no. 3: 2280. https://doi.org/10.3390/su15032280






