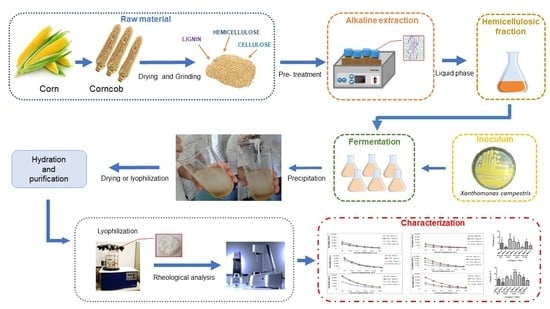
Corncob as Carbon Source in the Production of Xanthan Gum in Different Strains Xanthomonas sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Pre-Treatment for Fractionation
2.3. Quantification of Composition of the Corncob and Liquid Fractions Obtained after Alkaline Pre-Treatment
2.4. XG Production
2.5. XG Recovery
- P = Production
- m = final mass
- v = supernatant volume
2.6. Analysis of the Apparent Viscosity in the Aqueous Solutions of XG
2.7. Characterization of XG by Fourier-Transform Infrared Spectroscopy (FTIR)
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Corn Cob and Hemicellulosic Fraction (Liquid Phase)
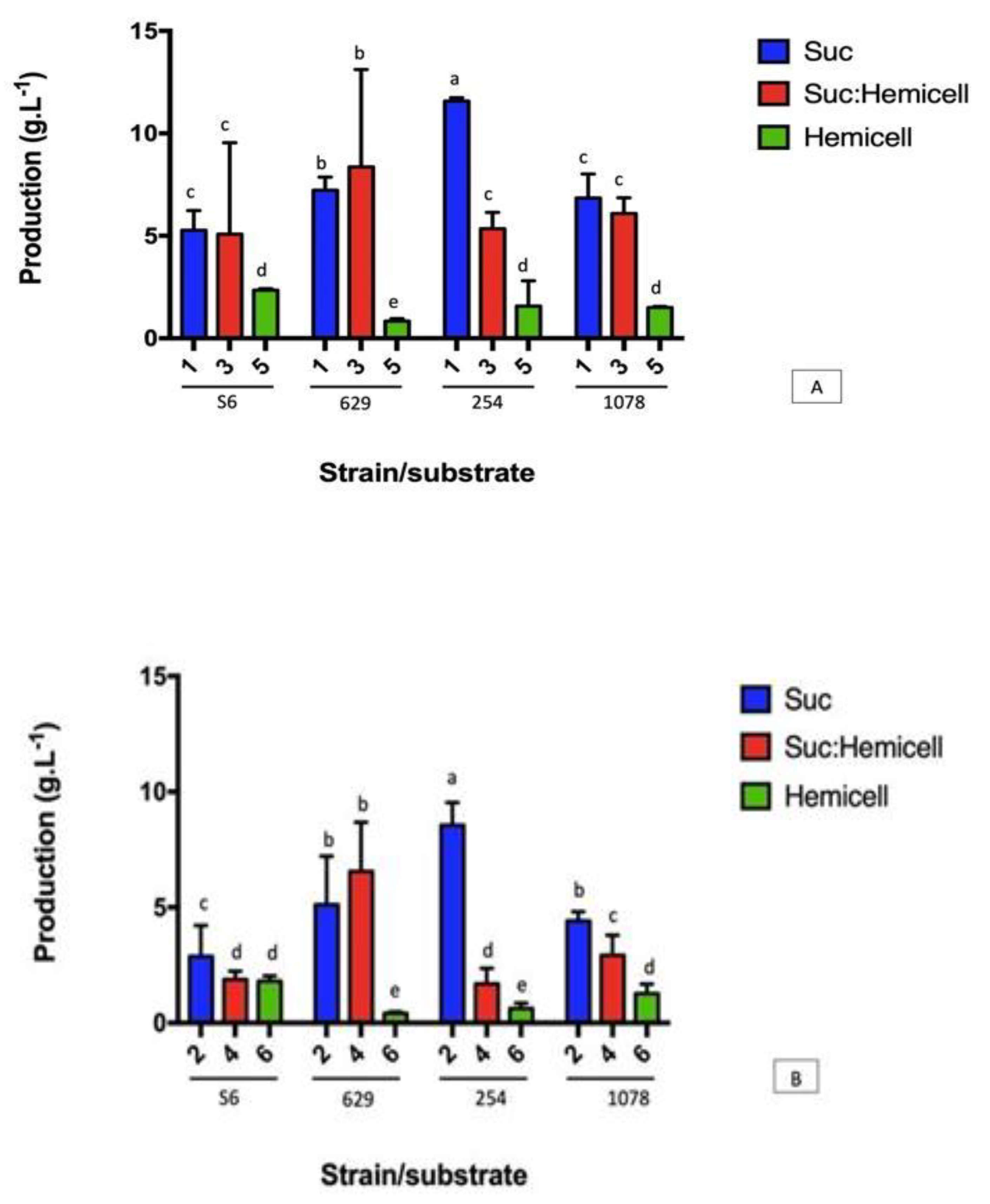
3.2. Yields of XG Using the Different Carbon Sources and the Different Strains of Xanthomonas campestris (629, S6, 254 e 1078)
3.3. Apparent Viscosity in XG Using Different Sources of Carbon and Different Strains of Xanthomonas sp. (629, S6, 254, and 1078)
3.4. Characterization of XG by Characterization of XG by Fourier-Transform Infrared Spectroscopy (FTIR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.U.; Yoon, K.R. Xanthan Gum-Derived Materials for Applications in Environment and Eco-Friendly Materials: A Review. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulations Title 21 Food and Drugs i—Food and Drug Administration of Health and Human Services b—Food for Human Consumption. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172 (accessed on 4 January 2023).
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Use of Xanthan Gum for Whole Cell Immobilization and Its Impact in Bioremediation—a Review. Bioresour. Technol. 2022, 351, 126918. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.A.; Azzaz, H.H.; Abo-Elkhair, A.G. Production of Xanthan Gum from Nontraditional Substrates with Perspective of the Unique Properties and Wide Industrial Applications. JSMC Microbiol. 2019, pp. 1–6. Available online: https://www.jsmcentral.org/Microbiology/jsmcm619628.php (accessed on 25 January 2023).
- Singh, J.; Dhaliwal, A.S. Water Retention and Controlled Release of KCl by Using Microwave-Assisted Green Synthesis of Xanthan Gum-Cl-Poly (Acrylic Acid)/AgNPs Hydrogel Nanocomposite. Polym. Bull. 2020, 77, 4867–4893. [Google Scholar] [CrossRef]
- Kayra, N.; Aytekin, A.Ö. Synthesis of Cellulose-Based Hydrogels: Preparation, Formation, Mixture, and Modification BT—Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 407–434. ISBN 978-3-319-77830-3. [Google Scholar]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Characterization of Xanthan Gum Produced from Glycerol by a Mutant Strain Xanthomonas Campestris CCTCC M2015714. Carbohydr. Polym. 2017, 157, 521–526. [Google Scholar] [CrossRef]
- Maize Starch Price in US—2023 Prices and Charts. Available online: https://www.selinawamucii.com/insights/prices/united-states-of-america/maize-starch/ (accessed on 4 January 2023).
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in Xanthan Gum Production, Modifications and Its Applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- da Silva, J.A.; Cardoso, L.G.; de Jesus Assis, D.; Gomes, G.V.P.; Oliveira, M.B.P.P.; de Souza, C.O.; Druzian, J.I. Xanthan Gum Production by Xanthomonas Campestris Pv. Campestris IBSBF 1866 and 1867 from Lignocellulosic Agroindustrial Wastes. Appl. Biochem. Biotechnol. 2018, 186, 750–763. [Google Scholar] [CrossRef]
- Gunasekar, V.; Reshma, K.R.; Treesa, G.; Gowdhaman, D.; Ponnusami, V. Xanthan from Sulphuric Acid Treated Tapioca Pulp: Influence of Acid Concentration on Xanthan Fermentation. Carbohydr. Polym. 2014, 102, 669–673. [Google Scholar] [CrossRef]
- Prajapati, J.; Panchal, R.; Patel, D.; Goswami, D. Production and Characterization of Xanthan Gum by Xanthomonas Campestris Using Sugarcane Bagasse as Sole Carbon Source. In Biotechnology and Biological Sciences; CRC Press: Boca Raton, FL, USA, 2019; pp. 363–367. [Google Scholar] [CrossRef]
- Mohsin, A.; Zhang, K.; Hu, J.; Salim-ur-Rehman; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized Biosynthesis of Xanthan via Effective Valorization of Orange Peels Using Response Surface Methodology: A Kinetic Model Approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar] [CrossRef]
- Li, P.; Zeng, Y.; Xie, Y.; Li, X.; Kang, Y.; Wang, Y.; Xie, T.; Zhang, Y. Effect of Pretreatment on the Enzymatic Hydrolysis of Kitchen Waste for Xanthan Production. Bioresour. Technol. 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Demirci, A.S.; Arici, M.; Gumus, T. Xanthan Gum Production from Hydrolyzed Rice Bran as a Carbon Source by Xanthomonas spp. Korean J. Microbiol. Biotechnol. 2012, 40, 356–363. [Google Scholar] [CrossRef]
- Ozdal, M.; Kurbanoglu, E.B. Valorisation of Chicken Feathers for Xanthan Gum Production Using Xanthomonas Campestris MO-03. J. Genet. Eng. Biotechnol. 2018, 16, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Soltaninejad, A.; Jazini, M.; Karimi, K. Biorefinery for Efficient Xanthan Gum, Ethanol, and Biogas Production from Potato Crop Residues. Biomass Bioenergy 2022, 158, 106354. [Google Scholar] [CrossRef]
- Rončević, Z.; Grahovac, J.; Dodić, S.; Vučurović, D.; Dodić, J. Utilisation of Winery Wastewater for Xanthan Production in Stirred Tank Bioreactor: Bioprocess Modelling and Optimisation. Food Bioprod. Process. 2019, 117, 113–125. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Karlen, D.L.; Kovar, J.L.; Birrell, S.J. Corn Stover Nutrient Removal Estimates for Central Iowa, USA. Sustainability 2015, 7, 8621–8634. [Google Scholar] [CrossRef] [Green Version]
- Nunes, H.M.A.R.; Vieira, I.M.M.; Santos, B.L.P.; Silva, D.P.; Ruzene, D.S. Biosurfactants Produced from Corncob: A Bibliometric Perspective of a Renewable and Promising Substrate. Prep. Biochem. Biotechnol. 2022, 52, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.L.; Cunha, J.T.; Romaní, A.; Domingues, L. Xylitol Production from Lignocellulosic Whole Slurry Corn Cob by Engineered Industrial Saccharomyces Cerevisiae PE-2. Bioresour. Technol. 2018, 267, 481–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzene, D.S.; Silva, D.P.; Vicente, A.A.; Teixeira, J.A.; Pessoa De Amorim, M.T.; Gonçalves, A.R. Cellulosic Films Obtained from the Treatment of Sugarcane Bagasse Fibers with N-Methylmorpholine-N-Oxide (NMMO). Appl. Biochem. Biotechnol. 2009, 154, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Intratec. Sodium Hydroxide Production Costs | Q1 2022; Intratec: Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.-Y. Lignin Plays a Negative Role in the Biochemical Process for Producing Lignocellulosic Biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Rottava, I.; Batesini, G.; Silva, M.F.; Lerin, L.; de Oliveira, D.; Padilha, F.F.; Toniazzo, G.; Mossi, A.; Cansian, R.L.; Di Luccio, M.; et al. Xanthan Gum Production and Rheological Behavior Using Different Strains of Xanthomonas sp. Carbohydr. Polym. 2009, 77, 65–71. [Google Scholar] [CrossRef]
- da Silva Haas, I.C.; Toaldo, I.M.; Burin, V.M.; Bordignon-Luiz, M.T. Extraction Optimization for Polyphenolic Profiling and Bioactive Enrichment of Extractives of Non-Pomace Residue from Grape Processing. Ind. Crops Prod. 2018, 112, 593–601. [Google Scholar] [CrossRef]
- Krishna Leela, J.; Sharma, G. Studies on Xanthan Production from Xanthomonas Campestris. Bioprocess Eng. 2000, 23, 687–689. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan Gum: Production, Recovery, and Properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef] [PubMed]
- Bilanovic, D.; Chang, F.H.; Isobaev, P.; Welle, P. Lactic Acid and Xanthan Fermentations on an Alternative Potato Residues Media—Carbon Source Costs. Biomass Bioenergy 2011, 35, 2683–2689. [Google Scholar] [CrossRef]
- López, M.J.; Moreno, J.; Ramos-Cormenzana, A. Xanthomonas Campestris Strain Selection for Xanthan Production from Olive Mill Wastewaters. Water Res. 2001, 35, 1828–1830. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in Bacterial Exopolysaccharides: From Production to Biotechnological Applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Trindade, R.A.; Munhoz, A.P.; Burkert, C.A.V. Impact of a Carbon Source and Stress Conditions on Some Properties of Xanthan Gum Produced by Xanthomonas Campestris Pv. Mangiferaeindicae. Biocatal. Agric. Biotechnol. 2018, 15, 167–172. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; González López, Ó. Water-Soluble Polymers in Agriculture: Xanthan Gum as Eco-Friendly Alternative to Synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 Analytical Procedure—Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral Valorization of Vine Pruning Residue by Sequential Autohydrolysis Stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Menezes, D.B.; Brazil, O.A.V.; Romanholo-Ferreira, L.F.; de Lourdes, T.M.; Polizeli, M.; Ruzene, D.S.; Silva, D.P.; Costa, L.P.; Hernández-Macedo, M.L. Prospecting Fungal Ligninases Using Corncob Lignocellulosic Fractions. Cellulose 2017, 24, 4355–4365. [Google Scholar] [CrossRef]
- Baptista, S.L.; Carvalho, L.C.; Romaní, A.; Domingues, L. Development of a Sustainable Bioprocess Based on Green Technologies for Xylitol Production from Corn Cob. Ind. Crops Prod. 2020, 156, 112867. [Google Scholar] [CrossRef]
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Velidandi, A.; Sharma, M.; Kuhad, R.C.; Tabatabaei, M.; Aghbashlo, M.; Baadhe, R.R.; Gupta, V.K. Corncob-Based Biorefinery: A Comprehensive Review of Pretreatment Methodologies, and Biorefinery Platforms. J. Energy Inst. 2022, 101, 290–308. [Google Scholar] [CrossRef]
- Jesus, M.; Romaní, A.; Mata, F.; Domingues, L. Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites Following the Concept of Biorefinery: A Review. Polymers 2022, 14, 1640. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K.; Serri, N.A.; Yusoff, S.B.; Shahrin, N.A.M.; Seng, K.Y.; Bakar, M.H.A.; Keong, L.C. Sustainable Biorefinery Concept for Industrial Bioprocessing. Biorefinery Prod. Technol. Chem. Energy 2020, 1, 15–53. [Google Scholar] [CrossRef]
- Luporini, S.; Bretas, R.E.S. Caracterização Reológica Da Goma Xantana: Influência de Íons Metálicos Univalente e Trivalente e Temperatura Em Experimentos Dinâmicos. Polímeros 2011, 21, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Brandão, L.V.; Assis, D.J.; López, J.A.; Espiridião, M.C.A.; Echevarria, E.M.; Druzian, J.I. Bioconversion from Crude Glycerin by Xanthomonas Campestris 2103: Xanthan Production and Characterization. Brazilian J. Chem. Eng. 2013, 30, 737–746. [Google Scholar] [CrossRef]
- Cheng, R.; Lin, L.; Zhang, Y. Hydrogen Peroxide (H2O2) Supply Significantly Improves Xanthan Gum Production Mediated by Xanthomonas Campestris in Vitro. J. Ind. Microbiol. Biotechnol. 2012, 39, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.; Vieira, P.A.; Resende, M.M.; Ribeiro, E.J.; Cardoso, V.L. Application of a Model Using the Phenomenological Approach for Prediction of Growth and Xanthan Gum Production with Sugar Cane Broth in a Batch Process. LWT -Food Sci. Technol. 2010, 43, 498–506. [Google Scholar] [CrossRef]
- Mabrouk, M.E.M.; El-Ahwany, A.M.D.; Beliah, M.M.B.; Sabry, S.A. Xanthan Production by a Novel Mutant Strain of Xanthomonas Campestris: Application of Statistical Design for Optimization of Process Parameters. Life Sci. J. 2013, 10, 1660–1667. [Google Scholar]
- Carmona, J.A.; Ramírez, P.; Calero, N.; Muñoz, J. Large Amplitude Oscillatory Shear of Xanthan Gum Solutions. Effect of Sodium Chloride (NaCl) Concentration. J. Food Eng. 2014, 126, 165–172. [Google Scholar] [CrossRef]
- Coppini, L.Z.; Waitzberg, D.L.; Campos, F.G.; Habr-Gama, A. Indicações e Usos de Suplementos Nutricionais Orais. In Fibras Alimentares e Ácidos Graxos de Cadeia Curta; Atheneu: São Paulo, Brazil, 2009; pp. 149–168. ISBN 978-85-388-0045-3. [Google Scholar]
- Sworn, G. Xanthan Gum. In Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2021; pp. 833–853. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective Variables on Production and Structure of Xanthan Gum and Its Food Applications: A Review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Bachmann, R.T.; Johnson, A.C.; Edyvean, R.G.J. Biotechnology in the Petroleum Industry: An Overview. Int. Biodeterior. Biodegrad. 2014, 86, 225–237. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Md Yuha, Y.B.; Tay, W.H.; Ofei, T.N.; Lal, B.; Mukhtar, H. Experimental and Modelling of the Impact of Quaternary Ammonium Salts/Ionic Liquid on the Rheological and Hydrate Inhibition Properties of Xanthan Gum Water-Based Muds for Drilling Gas Hydrate-Bearing Rocks. J. Pet. Sci. Eng. 2019, 183, 106468. [Google Scholar] [CrossRef]
- Pervaiz, F.; Mushtaq, R.; Noreen, S. Formulation and Optimization of Terbinafine HCl Loaded Chitosan/Xanthan Gum Nanoparticles Containing Gel: Ex-Vivo Permeation and in-Vivo Antifungal Studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102935. [Google Scholar] [CrossRef]
- Vázquez, E.; Piguillem, S.; Rubio, S.; Díaz, J.; Baldoni, H.; Vega, E.; Masuelli, M. Structural Analysis of Xanthan GUM-FE (III) Capsules. Acad. J. Chem. 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Li, Z.X.; Chen, J.Y.; Wu, Y.; Huang, Z.Y.; Wu, S.T.; Chen, Y.; Gao, J.; Hu, Y.; Huang, C. Effect of Downstream Processing on the Structure and Rheological Properties of Xanthan Gum Generated by Fermentation of Melaleuca Alternifolia Residue Hydrolysate. Food Hydrocoll. 2022, 132, 107838. [Google Scholar] [CrossRef]
- Saleh, H.M.; Annuar, M.S.M.; Simarani, K. Ultrasound Degradation of Xanthan Polymer in Aqueous Solution: Its Scission Mechanism and the Effect of NaCl Incorporation. Ultrason. Sonochem. 2017, 39, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Said, M.; Haq, B.; Al Shehri, D.; Rahman, M.M.; Muhammed, N.S.; Mahmoud, M. Modification of Xanthan Gum for a High-Temperature and High-Salinity Reservoir. Polymers 2021, 13, 4212. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.; De Oliveira Petkowicz, C.L.; De Morais, S.A.L.; Terrones, M.G.H.; De Resende, M.M.; De Frana, F.P.; Cardoso, V.L. Characterization of Xanthan Gum Produced from Sugar Cane Broth. Carbohydr. Polym. 2011, 86, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, L.; Subramanian, S. Surface Chemical Properties and Selective Flocculation Studies on Alumina and Silica Suspensions in the Presence of Xanthan Gum. Miner. Eng. 2016, 98, 213–222. [Google Scholar] [CrossRef]
- Lawall Werneck Cerqueira, A.F.; Protta Neiva, G.; Fernandes, M.F.; Leira Mota Conegundes, J.; Stephani, R.; Cappa de Oliveira, L.F.; da Costa Ludwig, Z.M.; de Carvalho dos Anjos, V.; Pinto Vilela, F.M.; Scio, E.; et al. Influence of the Xanthan Gum as a Crosslinking Agent on the Physicochemical Properties of Chitosan Microparticles Containing Green Coffee Extract. Biocatal. Agric. Biotechnol. 2020, 29, 101782. [Google Scholar] [CrossRef]

| Fermentation Medium | Saccharose (%, w/w) | Hemicellulosic Fraction 1 (%, w/w) | Supplementation with Salts 2 |
|---|---|---|---|
| Medium 1 | 5.0 | 0 | yes |
| Medium 2 | 5.0 | 0 | no |
| Medium 3 | 1.25 | 3.75 | yes |
| Medium 4 | 1.25 | 3.75 | no |
| Medium 5 | 0 | 5.0 | yes |
| Medium 6 | 0 | 5.0 | no |
| Chemical Composition (%, w/w) | Corncob In Natura | Hemicellulosic Fraction |
|---|---|---|
| Cellulose | 26.3 ± 1.1 | 10.5 ± 0.9 |
| Hydroxymethylfurfural | 0.23 ± 0.11 | 0.18 ± 0.11 |
| Hemicelluloses | 25.2 ± 1.7 | 48.8 ± 1.3 |
| Furfural | 1.8 ± 1.1 | 0.6 ± 0.11 |
| Total Lignin | 34.9 ± 1.2 | - |
| Soluble Lignin | 11.8 ± 1.9 | 14.3 ± 1.1 |
| Klason Lignin | 23.1 ± 1.3 | - |
| Total Composition | 88.4 ± 2.2 | 74.4 ± 1.9 |
| Strain | Medium (Carbon Source) 1 | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| S6 | 5.26 ± 1.14 bcA | 2.88 ± 2.34 cB | 5.08 ± 5.15 bA | 1.88 ± 0.36 cC | 2.34 ± 0.08 aB | 1.81 ± 0.22 aC |
| 629 | 7.23 ± 0.61 bB | 5.02 ± 3.10 cC | 8.37 ± 5.75 aA | 6.56 ± 4.12 bBC | 0.84 ± 0.11 bC | 0.42 ± 0.067 cD |
| 254 | 11.58 ± 0.16 aA | 8.56 ± 1.18 bB | 5.35 ± 0.93 bC | 1.69 ± 0.67 cD | 1.57 ± 1.23 bD | 0.63 ± 1.23 cD |
| 1078 | 6.84 ± 1.01 bA | 4.42 ± 0.39 cBC | 6.09 ± 0.76 bAB | 2.93 ± 0.87 cCD | 1.50 ± 0.05 bD | 1.27 ± 0.421 bD |
| Strain | Medium (Carbon Source) 1 | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| S6 | 4869 ± 9 aC | 2543 ± 11 bE | 6599 ± 8 cB | 4779 ± 6 bC | 8790 ± 11 aA | 3899 ± 12 cD |
| 629 | 3292 ± 13 dD | 1106 ± 4 cE | 9298 ± 31 aA | 7687 ± 13 aC | 8710 ± 25 abB | 8488 ± 8 aB |
| 254 | 3711 ± 13 cD | 3468 ±15 aE | 9278 ± 13 aA | 2870 ± 23 cF | 8209 ± 20 bB | 6572 ± 21 bC |
| 1078 | 3711 ± 15 bC | 3468 ± 13 bE | 9278 ± 11 bA | 2870 ± 15 dF | 8209 ± 8 cB | 3750 ± 14 cD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, M.; Mata, F.; Batista, R.A.; Ruzene, D.S.; Albuquerque-Júnior, R.; Cardoso, J.C.; Vaz-Velho, M.; Pires, P.; Padilha, F.F.; Silva, D.P. Corncob as Carbon Source in the Production of Xanthan Gum in Different Strains Xanthomonas sp. Sustainability 2023, 15, 2287. https://doi.org/10.3390/su15032287
Jesus M, Mata F, Batista RA, Ruzene DS, Albuquerque-Júnior R, Cardoso JC, Vaz-Velho M, Pires P, Padilha FF, Silva DP. Corncob as Carbon Source in the Production of Xanthan Gum in Different Strains Xanthomonas sp. Sustainability. 2023; 15(3):2287. https://doi.org/10.3390/su15032287
Chicago/Turabian StyleJesus, Meirielly, Fernando Mata, Rejane A. Batista, Denise S. Ruzene, Ricardo Albuquerque-Júnior, Juliana C. Cardoso, Manuela Vaz-Velho, Preciosa Pires, Francine F. Padilha, and Daniel P. Silva. 2023. "Corncob as Carbon Source in the Production of Xanthan Gum in Different Strains Xanthomonas sp." Sustainability 15, no. 3: 2287. https://doi.org/10.3390/su15032287