Influences of Flow Channel on Electrochemical Characteristics of Polymer Electrolyte Fuel Cells Humidified with NaCl Contained H2O
Abstract
:1. Introduction
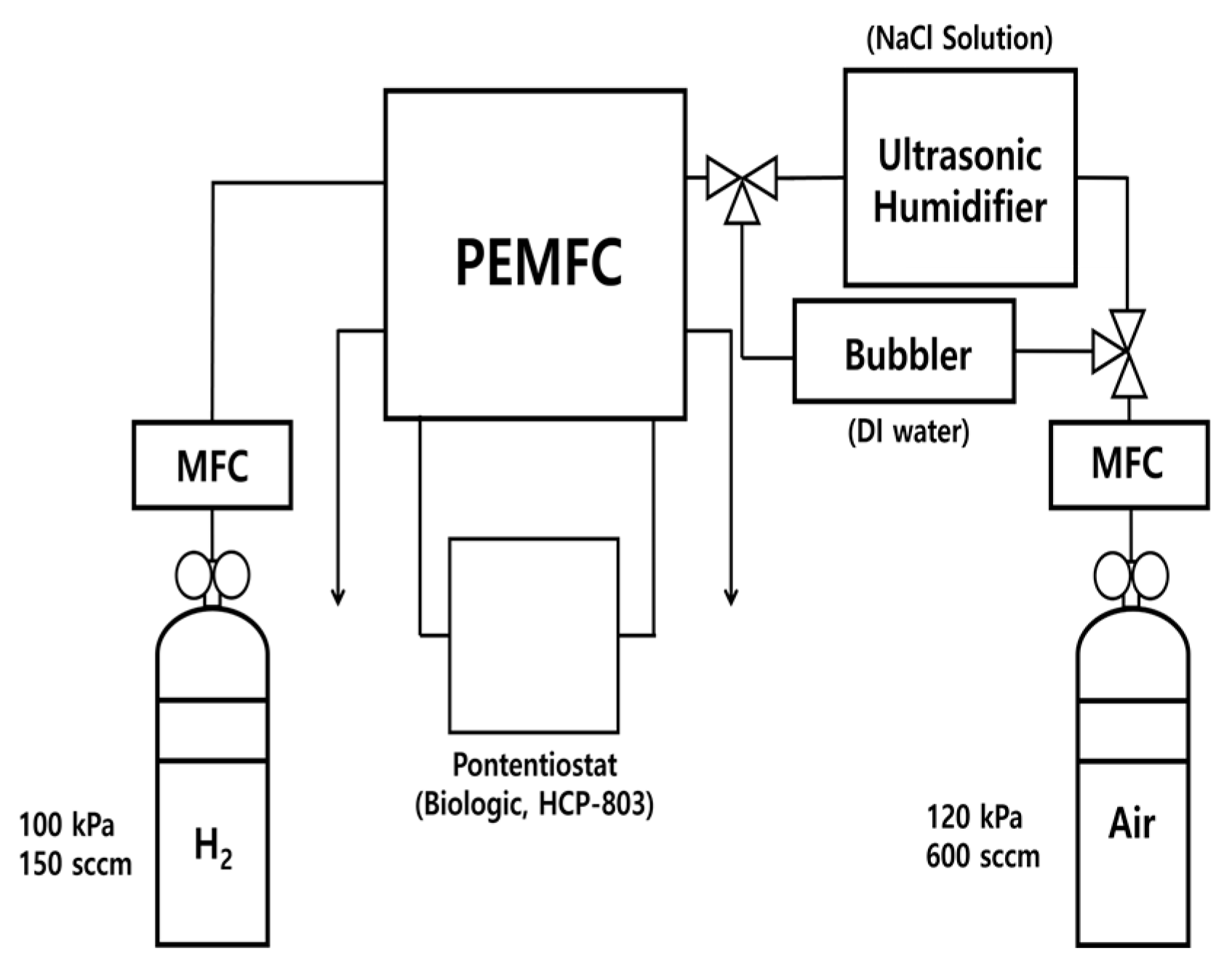
2. Experiments
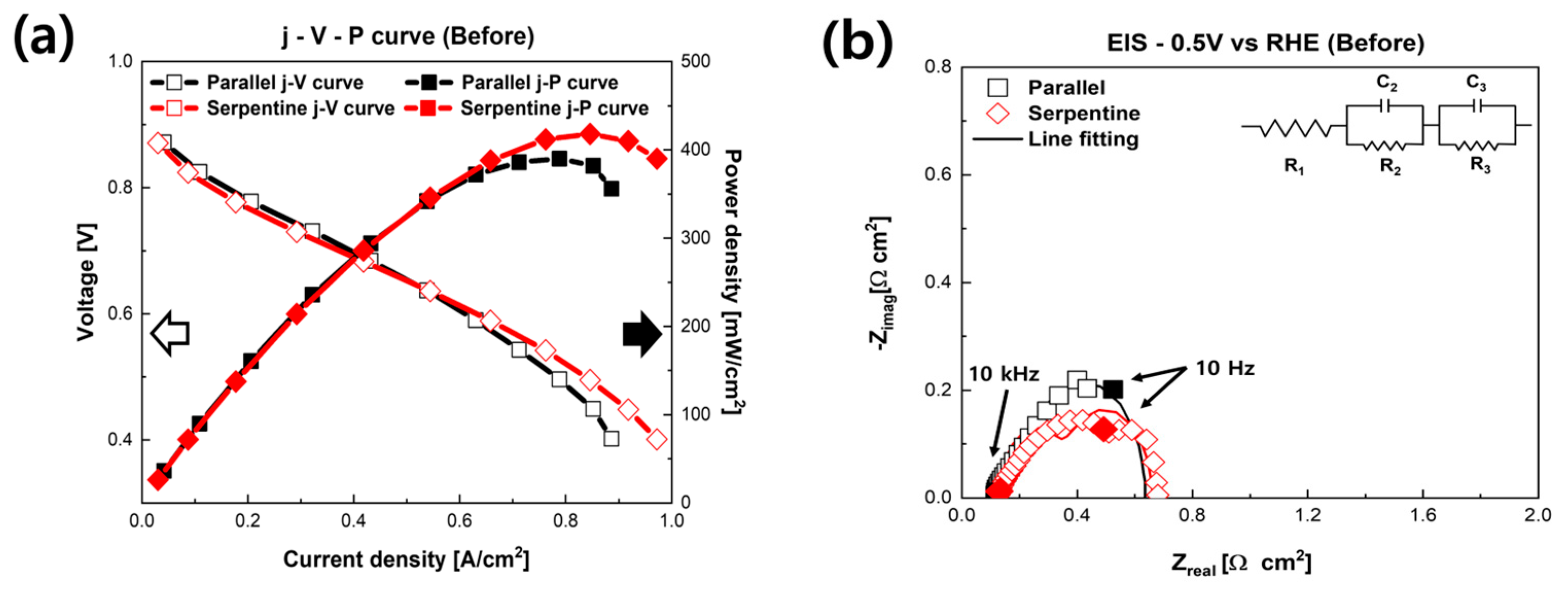
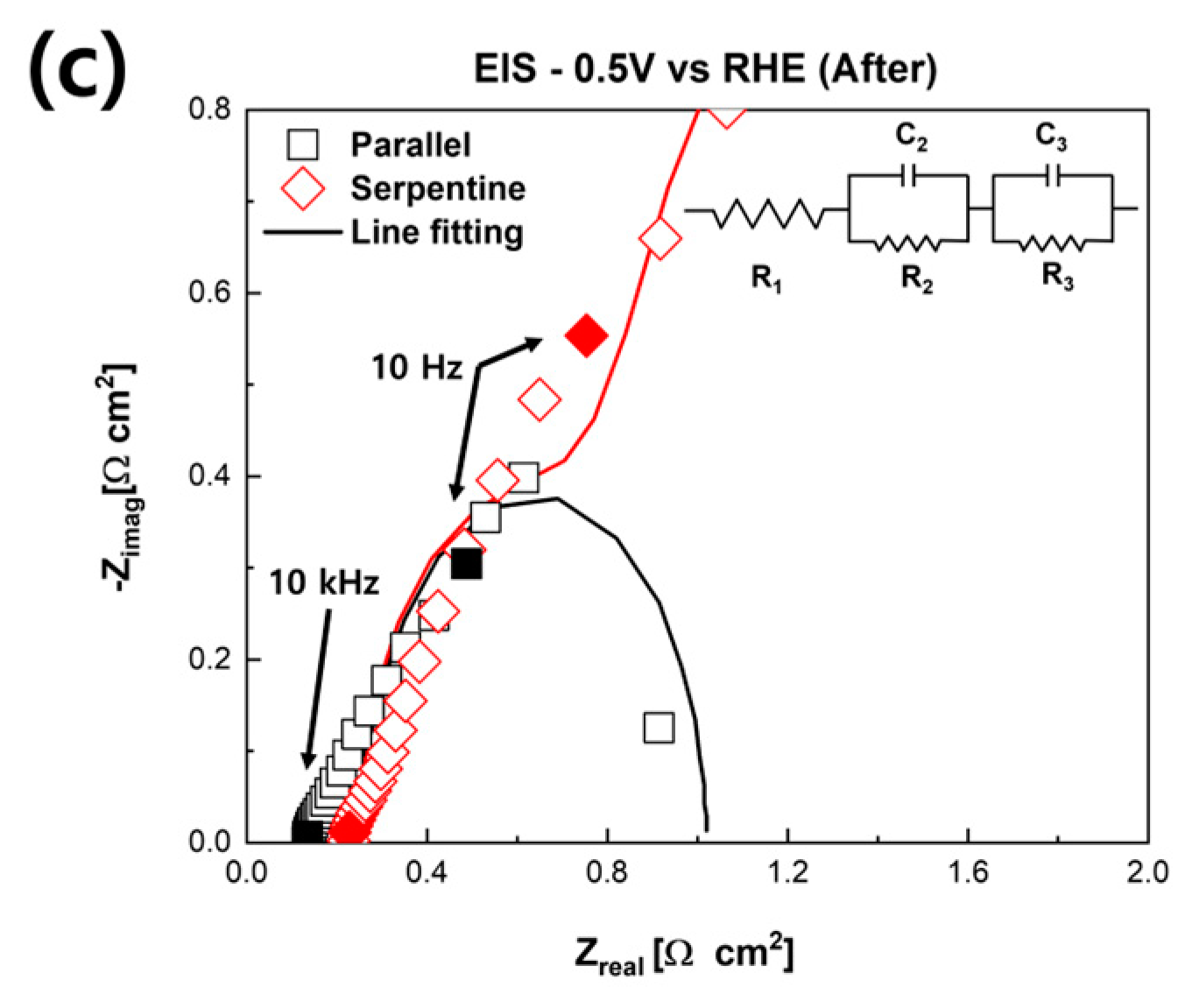
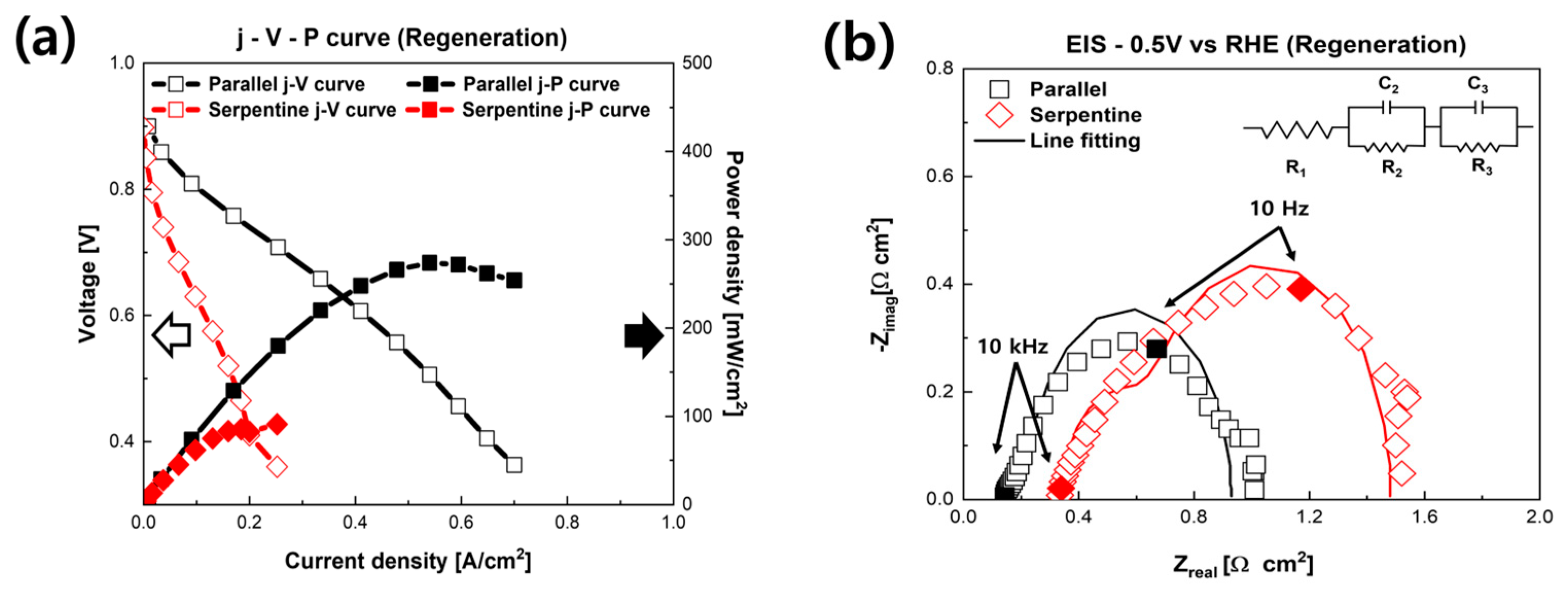
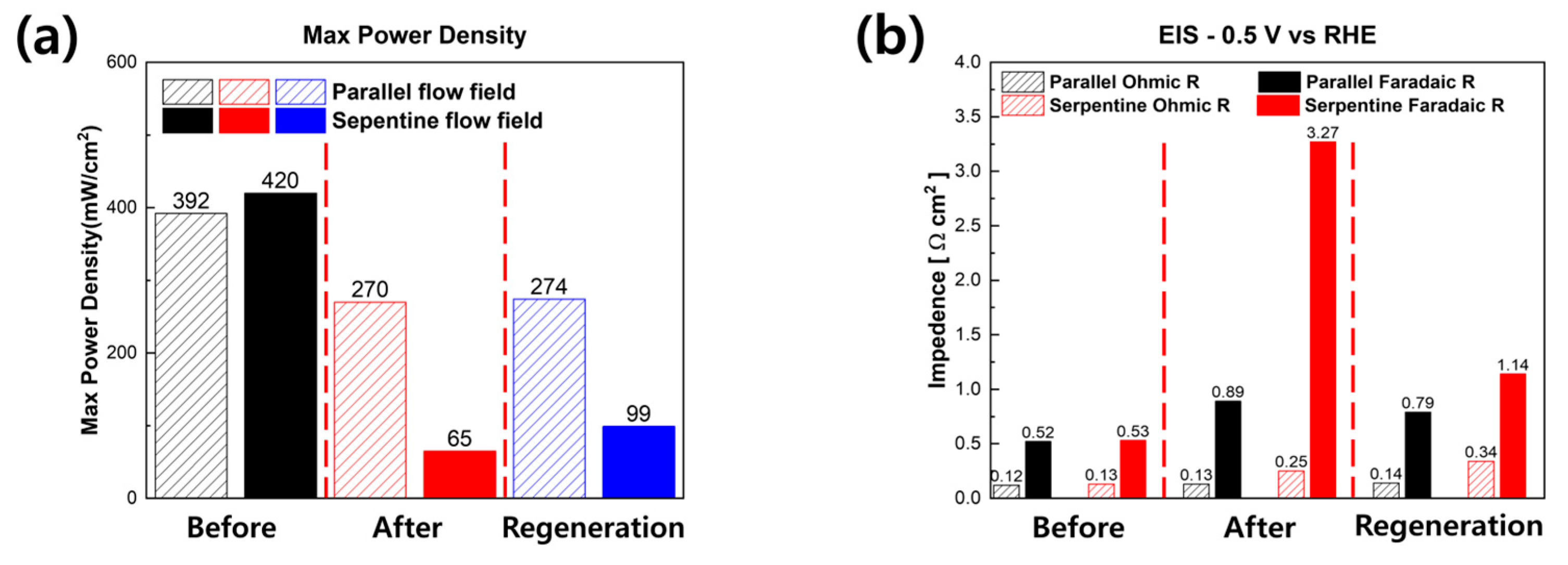
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Costamagna, P.; Srinivasan, S. Quantum jumps in the PEMFC science and technology from the 1960s to the year 2000. J. Power Sources 2001, 102, 242–252. [Google Scholar] [CrossRef]
- Kirubakaran, A.; Jain, S.; Nema, R.K. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Patil, A.S.; Dubois, T.G.; Sifer, N.; Bostic, E.; Gardner, K.; Quah, M.; Bolton, C. Portable fuel cell systems for America’s army: Technology transition to the field. J. Power Sources 2004, 136, 220–225. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, W.; Pan, M.; Wan, Z. Experimental investigation on the dynamic performance of a hybrid PEM fuel cell/battery system for lightweight electric vehicle application. Appl. Energy 2011, 88, 68–76. [Google Scholar] [CrossRef]
- Shih, N.-C.; Weng, B.-J.; Lee, J.-Y.; Hsiao, Y.-C. Development of a small fuel cell underwater vehicle. Int. J. Hydrogen Energy 2013, 38, 11138–11143. [Google Scholar] [CrossRef]
- Pan, Z.F.; An, L.; Wen, C.Y. Recent advances in fuel cells- based propulsion systems for unmanned aerial vehicles. Appl. Energy 2019, 240, 473–485. [Google Scholar] [CrossRef]
- Keim, M.; Kallo, J.; Friedrich, K.A.; Werner, C.; Saballus, M.; Gores, F. Multifunctional fuel cell system in an aircraft environment: An investigation focusing on fuel tank inerting and water generation. Aerosp. Sci. Technol. 2013, 29, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhao, D.; Li, W.; Wang, Z.; Huang, Y.; You, Y.; Becker, S. Current technologies and challenges of applying fuel cell hybrid propulsion systems in unmanned aerial vehicles. Prog. Aerosp. Sci. 2020, 116, 100620. [Google Scholar] [CrossRef]
- Javed, S.M.; Ahmad, Z.; Ahmed, S.; Iqbal, S.; Naqvi, I.J.; Usman, M.; Ashiq, M.N.; Elnaggar, A.Y.; El-Bahy, Z.M. Highly dispersed active sites of Ni nanoparticles onto hierarchical reduced graphene oxide architecture towards efficient water oxidation. Fuel 2022, 312, 122926. [Google Scholar] [CrossRef]
- Ahmad, Z.; Maqbool, A.; Hussain, M.A.; Pashameah, R.A.; Shahzadi, A.; Nazar, N.; Iqbal, S.; Alanazi, A.K.; Ashiq, M.N.; Abo-Dief, H.M. One-pot solvothermal synthesis of highly catalytic Janus transition metal phosphides (TMPs) for high per-formance OER. Fuel 2023, 331, 125913. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Bender, G.; Bethune, K.; Rocheleau, R. A segmented cell approach for studying the effects of serpentine flow field parameters on PEMFC current distribution. Electrochim. Acta 2013, 88, 571–579. [Google Scholar] [CrossRef]
- Spernjak, D.; Prasad, A.K.; Advani, S.G. In situ comparison of water content and dynamics in parallel, single-serpentine, and interdigitated flow fields of polymer electrolyte membrane fuel cells. J. Power Sources 2010, 195, 3553–3568. [Google Scholar] [CrossRef]
- Jiao, K.; Park, J.; Li, X. Experimental investigations on liquid water removal from the gas diffusion layer by reactant flow in a PEM fuel cell. Appl. Energy 2010, 87, 2770–2777. [Google Scholar] [CrossRef]
- Le, A.D.; Zhou, B. A general model of proton exchange membrane fuel cell. J. Power Sources 2008, 182, 197–222. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, R.; Técher, L.; Cui, X. Experimental study of variable operating parameters effects on overall PEMFC performance and spatial performance distribution. Energy 2016, 115, 550–560. [Google Scholar] [CrossRef]
- Yoshida, T.; Kojima, K. Toyota MIRAI Fuel Cell Vehicle and Progress Toward a Future Hydrogen Society. Interface Mag. 2015, 24, 45–49. [Google Scholar] [CrossRef]
- Bi, H.T.; Sauriol, P.; Stumper, J. Two-phase flow distributors for fuel cell flow channels. Particuology 2010, 8, 582–587. [Google Scholar] [CrossRef]
- Boddu, R.; Marupakula, U.K.; Summers, B.; Majumdar, P. Development of bipolar plates with different flow channel configurations for fuel cells. J. Power Sources 2009, 189, 1092. [Google Scholar] [CrossRef]
- Lim, B.H.; Majlan, E.H.; Daud, W.R.W.; Husaini, T.; Rosli, M.I. Effects of flow field design on water management and reactant distribution in PEMFC: A review. Ionics 2016, 22, 301–316. [Google Scholar] [CrossRef]
- Misran, E.; Hassan, N.S.M.; Daud, W.R.W.; Majlan, E.H.; Rosli, M.I. Water transport characteristics of a PEM fuel cell at various operating pressures and temperatures. Int. J. Hydrogen Energy 2013, 38, 9408. [Google Scholar] [CrossRef]
- Kim, D.K.; Koh, J.S.; Kim, M.S.; Song, H.H. Experimental and computational study on the dynamic interaction be-tween load variation and back pressure control in a polymer electrolyte membrane fuel cell for automotive application. Int. J. Hydrogen Energy 2015, 40, 12381. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Juarez-Robles, D.; Wang, K.; Hernandez-Guerrero, A. Experimental Study and Comparison of Various De-signs of Gas Flow Fields to PEM Fuel Cells and Cell Stack Performance. Front. Energy Res. 2014, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Sando, Y. Research and Development of Fuel Cell Vehicles at Honda. ECS Trans. 2009, 25, 211–224. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Fuel Cell Systems Analysed, Fuel Cell Systems Explained; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 369–389. [Google Scholar] [CrossRef]
- Le, A.D.; Zhou, B. Fundamental understanding of liquid water effects on the performance of a PEMFC with serpentine-parallel channels. Electrochim. Acta 2009, 54, 2137–2154. [Google Scholar] [CrossRef]
- Wang, X.-D.; Duan, Y.-Y.; Yan, W.-M. Novel serpentine-baffle flow field design for proton exchange membrane fuel cells. J. Power Sources 2007, 173, 210–221. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, D.; Kim, G.H.; Kwon, O.; Cha, H.; Choi, H.; Yoo, H.; Park, T. Mass diffusion characteristics on perfor-mance of polymer electrolyte membrane fuel cells with serpentine channels of different width. Int. J. Heat Mass Transf. 2022, 183, 122106. [Google Scholar] [CrossRef]
- Shimpalee, S.; Vanzee, J. Numerical studies on rib & channel dimension of flow-field on PEMFC performance. Int. J. Hydrogen Energy 2007, 32, 842–856. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Hemminger, J.C. Physical Chemistry of Airborne Sea Salt Particles and Their Components. J. Phys. Chem. A 2000, 104, 11463–11477. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Qian, W.; Yu, Y.; Yuan, X.Z.; Wang, H.; Jiang, M.; Wessel, S.; Cheng, T.T.H. Impacts of operating conditions on the effects of chloride contamination on PEM fuel cell performance and durability. J. Power Sources 2012, 218, 375–382. [Google Scholar] [CrossRef]
- Mikkola, M.S.; Rockward, T.; Uribe, F.A.; Pivovar, B.S. The Effect of NaCl in the Cathode Air Stream on PEMFC Performance. Fuel Cells 2007, 7, 153–158. [Google Scholar] [CrossRef]
- Ali, S.T.; Li, Q.; Pan, C.; Jensen, J.O.; Nielsen, L.P.; Møller, P. Effect of chloride impurities on the performance and durability of polybenzimidazole-based high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 1628–1636. [Google Scholar] [CrossRef]
- Yan, W.-M.; Chu, H.-S.; Liu, Y.-L.; Chen, F.; Jang, J.-H. Effects of chlorides on the performance of proton exchange mem-brane fuel cells. Int. J. Hydrogen Energy 2011, 36, 5435–5441. [Google Scholar] [CrossRef]
- Veleva, L.; Farro, W. Influence of seawater and its aerosols on copper patina composition. Appl. Surf. Sci. 2012, 258, 10072–10076. [Google Scholar] [CrossRef]
- Carton, J.G.; Olabi, A.G. Design of experiment study of the parameters that affect performance of three flow plate con-figurations of a proton exchange membrane fuel cell. Energy 2010, 35, 2796–2806. [Google Scholar] [CrossRef] [Green Version]
- Dhahad, H.A.; Alawee, W.H.; Hassan, A.K. Experimental study of the effect of flow field design to PEM fuel cells performance. Renew. Energy Focus 2019, 30, 71–77. [Google Scholar] [CrossRef]
- Mojica, F.; Rahman, M.A.; Mora, J.M.; Ocon, J.D.; Chuang, P.Y.A. Experimental Study of Three Channel Designs with Model Comparison in a PEM Fuel Cell. Fuel Cells 2020, 20, 547–557. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Karthikeyan, P.; Muthukumar, M.; Kannan, V.M.; Thanarajan, K.; Maiyalagan, T.; Hong, C.W.; Jothi, V.R.; Yi, S.C. Adoption of novel porous inserts in the flow channel of pem fuel cell for the mitigation of cathodic flooding. Int. J. Hydrogen Energy 2020, 45, 7863–7872. [Google Scholar] [CrossRef]
- Heidary, H.; Kermani, M.J.; Advani, S.G.; Prasad, A.K. Experimental investigation of in-line and staggered blockages in parallel flowfield channels of PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 6885–6893. [Google Scholar] [CrossRef]
- Lee, P.H.; Hwang, S.S. Performance Characteristics of a PEM Fuel Cell with Parallel Flow Channels at Different Cath-ode Relative Humidity Levels. Sensors 2009, 11, 9104–9121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Canut, J.-M.; Abouatallah, R.M.; Harrington, D.A. Detection of Membrane Drying, Fuel Cell Flooding, and Anode Catalyst Poisoning on PEMFC Stacks by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2006, 153, A857. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.; Kwon, O.; Yoo, H.; Kim, H.; Cha, H.; Park, T. Observation of flooding-induced performance enhancement in PEMFCs. Int. J. Hydrogen Energy 2022, 47, 6259–6268. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.J.; Cho, G.Y. Influences of Flow Channel on Electrochemical Characteristics of Polymer Electrolyte Fuel Cells Humidified with NaCl Contained H2O. Sustainability 2023, 15, 2415. https://doi.org/10.3390/su15032415
Yoo HJ, Cho GY. Influences of Flow Channel on Electrochemical Characteristics of Polymer Electrolyte Fuel Cells Humidified with NaCl Contained H2O. Sustainability. 2023; 15(3):2415. https://doi.org/10.3390/su15032415
Chicago/Turabian StyleYoo, Ho Jun, and Gu Young Cho. 2023. "Influences of Flow Channel on Electrochemical Characteristics of Polymer Electrolyte Fuel Cells Humidified with NaCl Contained H2O" Sustainability 15, no. 3: 2415. https://doi.org/10.3390/su15032415






