When the Trawl Ban Is a Good Option: Opportunities to Restore Fish Biomass and Size Structure in a Mediterranean Fisheries Restricted Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design and Data Collection
2.2.1. Biomass
2.2.2. Size
- Lm, median length;
- L95, the 95% percentile of each LFD;
- L2/3, the percentage of individuals larger than 2/3 of the maximum length recorded in the samples.
2.3. Data Analysis
2.3.1. Biomass
- Total trawlable assemblage
- Target species
- Commercial categories
2.3.2. Size
3. Results
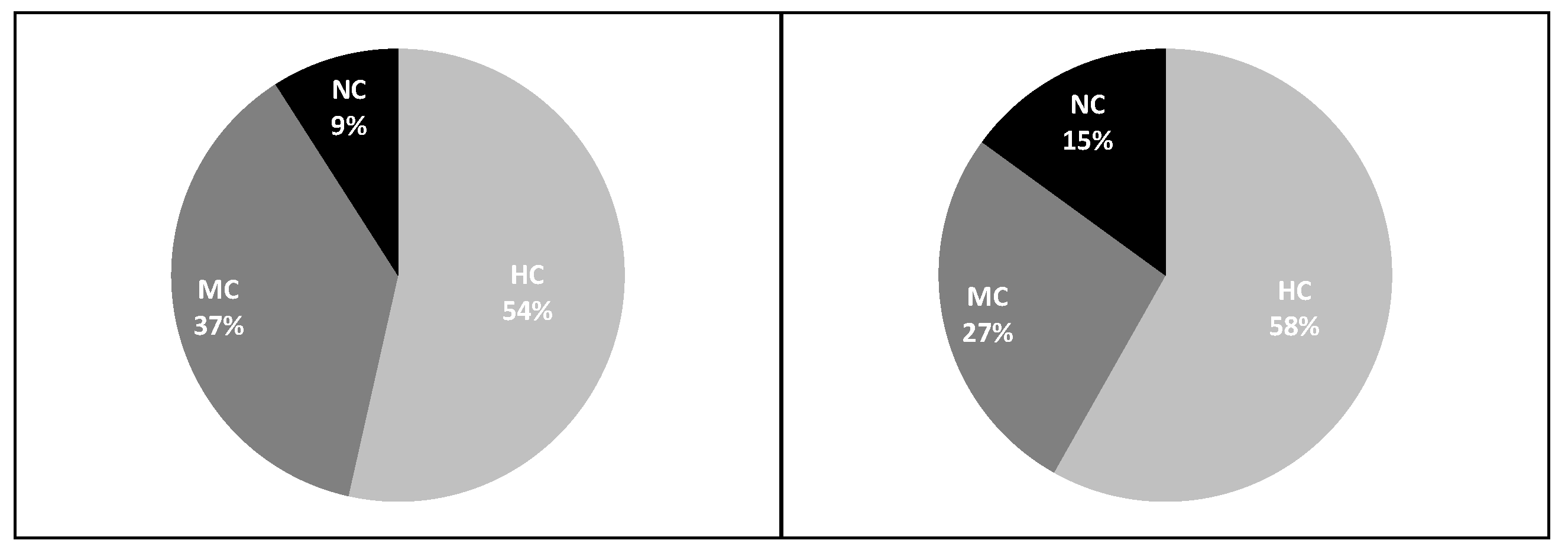
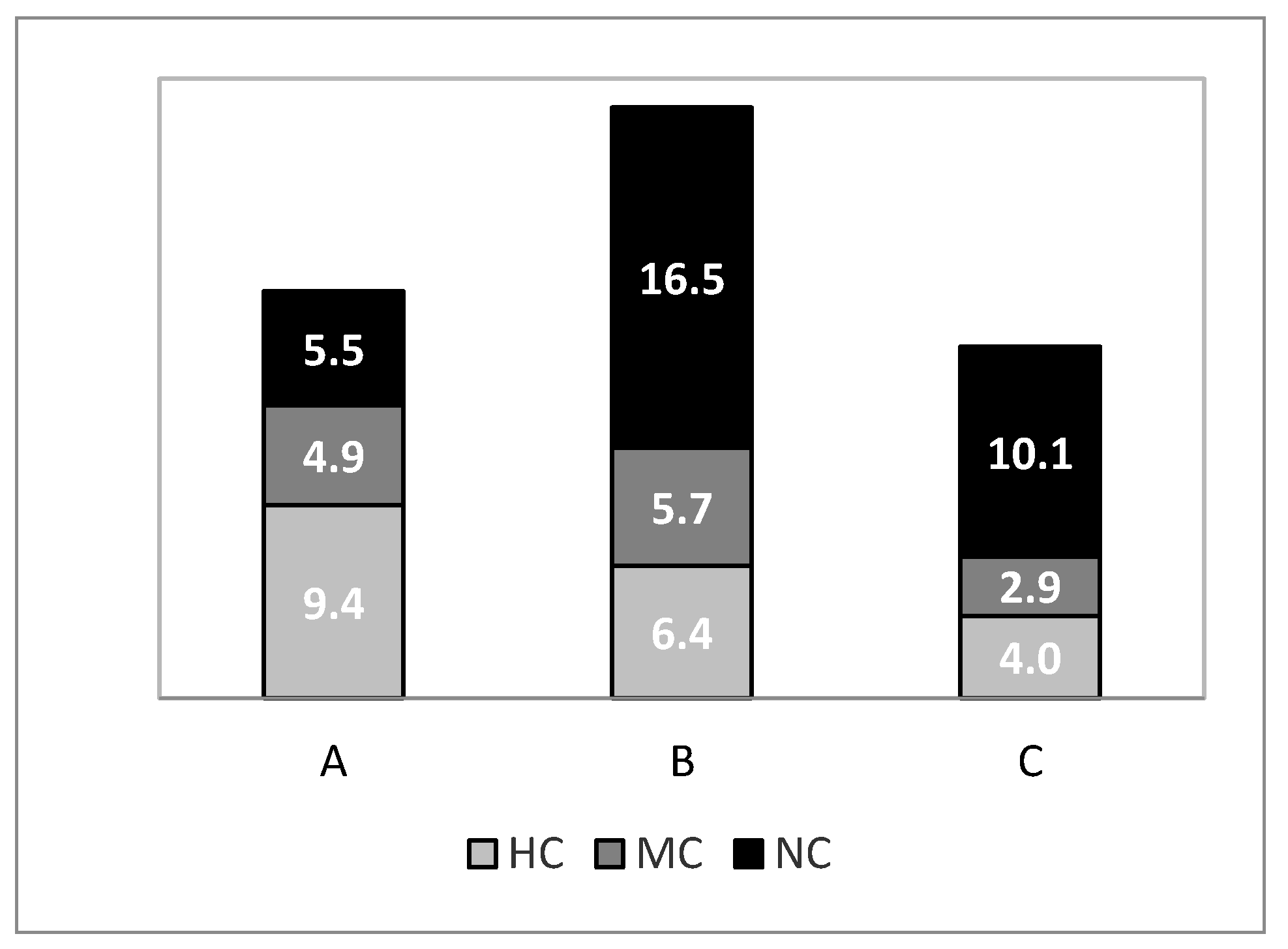
3.1. Biomass
- Total trawlable assemblage
- Target species
- Commercial categories in GCAST
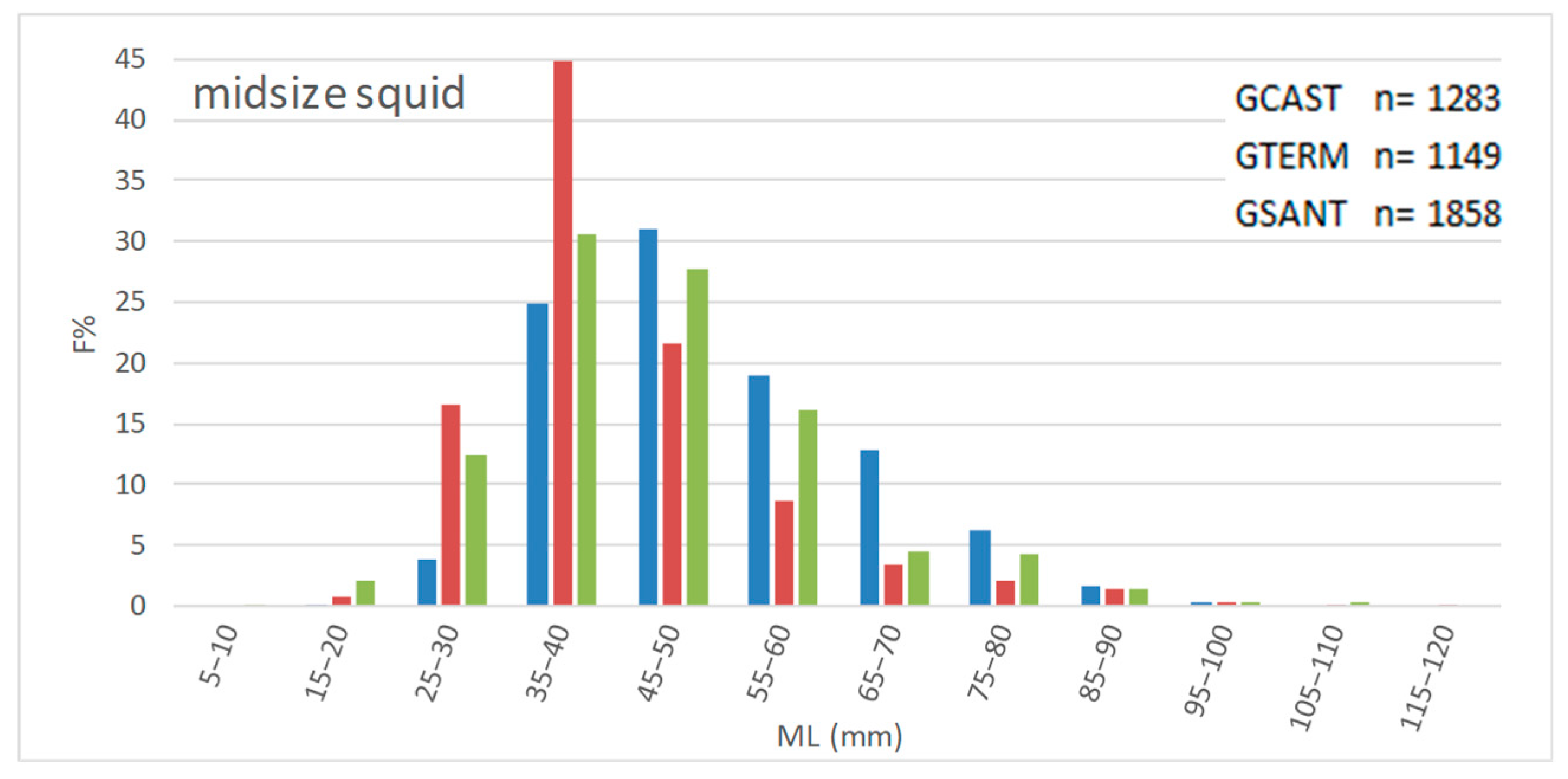
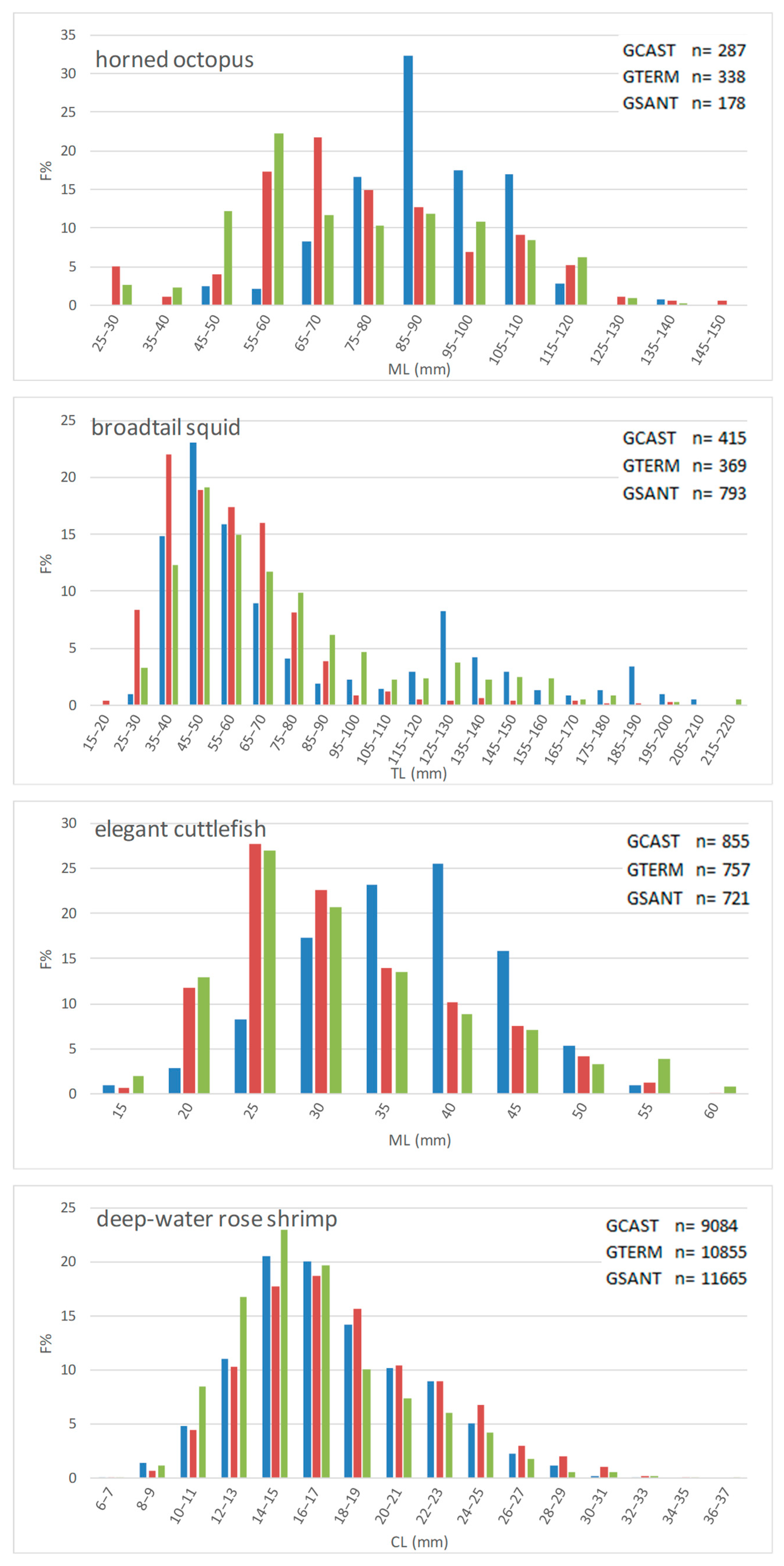
3.2. Size
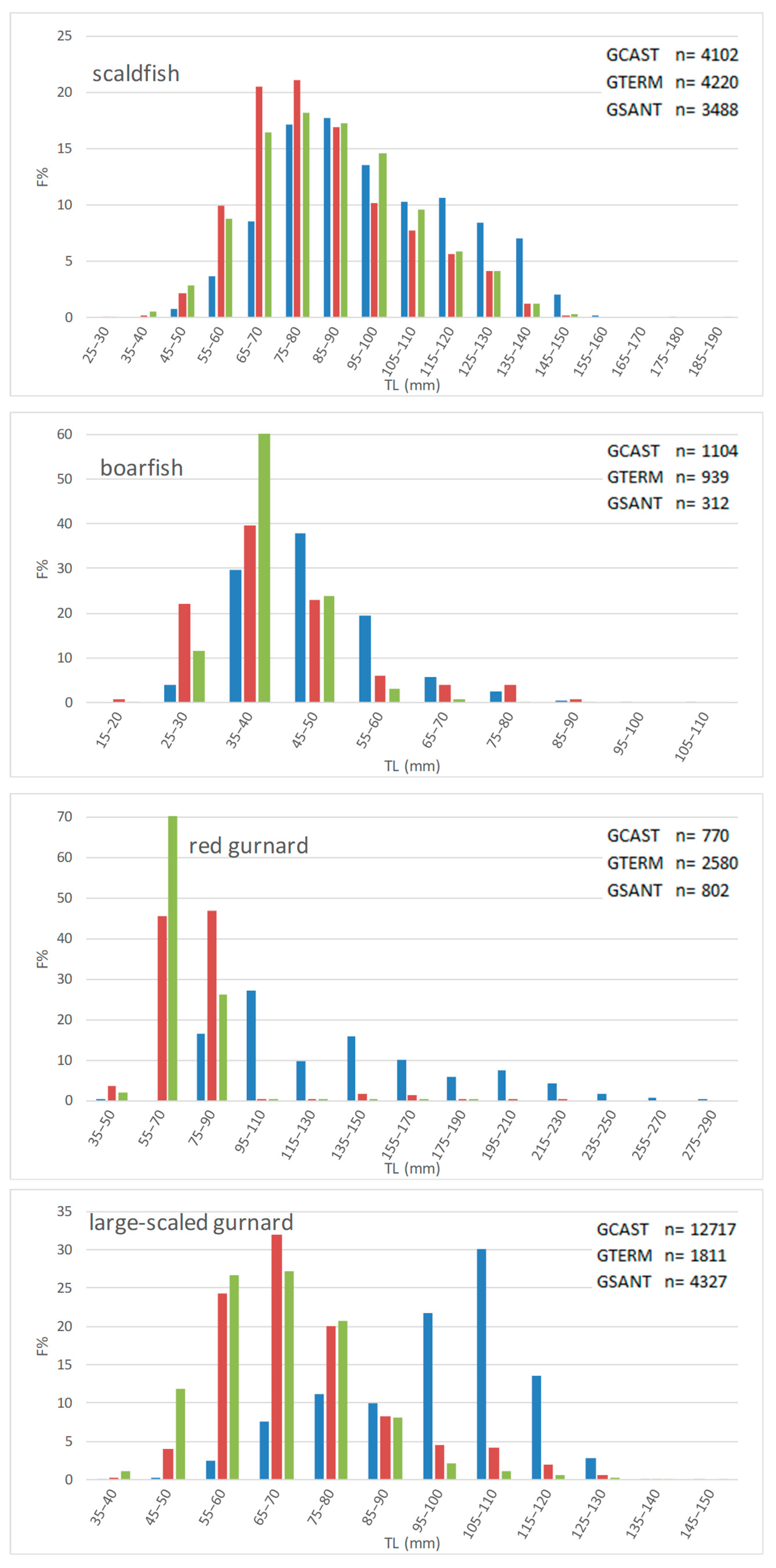
- Length frequency distributions
- Length-based indices
4. Discussion
4.1. Biomass
- Total trawlable assemblage
- Target species
- Commercial categories in the GCAST
4.2. Size
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botsford, L.W.; Castilla, J.C.; Peterson, C.H. The management of fisheries and marine ecosystems. Science 1997, 277, 509–515. [Google Scholar] [CrossRef]
- Colloca, F.; Cardinale, M.; Maynou, F.; Giannoulaki, M.; Scarcella, G.; Jenko, K.; Bellido, J.M.; Fiorentino, F. Rebuilding Mediterranean fisheries: A new paradigm for ecological sustainability. Fish Fish. 2013, 14, 80–109. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Yamauchi, A.; Nakazawa, T.; Wang, W.F. Fishing effects on age and spatial structures undermine population stability of fishes. Aquat. Sci. 2010, 72, 165–178. [Google Scholar] [CrossRef]
- Jennings, S.; Reynolds, J.D. Body size, exploitation and conservation of marine organisms. In Body Size: The Structure and Function of Aquatic Ecosystems; Hildrew, A.G., Raffaelli, D.G., Edmonds-Brown, R., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 266–285. [Google Scholar]
- Kaiser, M.J.; Jennings, S. Ecosystem Effects of Fishing. In Handbook of Fish Biology and Fisheries; Hart, P.J.B., Reynolds, J.D., Eds.; Blackwell Science Ltd.: Malden, MA, USA, 2008; pp. 342–366. [Google Scholar]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.; Gaines, S.D.; Costello, C. Marine reserves solve an important bycatch problem in fisheries. Proc. Natl. Acad. Sci. USA 2017, 114, 8927–8934. [Google Scholar] [CrossRef] [PubMed]
- Melnychuk, M.C.; Kurota, H.; Mace, P.M.; Pons, M.; Minto, C.; Osio, G.C.; Jensen, O.P.; de Moor, C.L.; Parma, A.M.; Little, L.R.; et al. Identifying management actions that promote sustainable fisheries. Nat. Sustain. 2021, 4, 440–449. [Google Scholar] [CrossRef]
- Rice, J.C. Every which way but up: The sad story of Atlantic groundfish, featuring Northern cod and North Sea cod. Bull. Mar. Sci. 2006, 78, 429–465. [Google Scholar]
- Coll, M.; Cury, P.; Azzurro, E.; Bariche, M.; Bayadas, G.; Bellido, J.M.; Chaboud, C.; Claudet, J.; El-Sayed, A.; Gascuel, D.; et al. The scientific strategy needed to promote a regional ecosystem-based approach to fisheries in the Mediterranean and Black Seas. Rev. Fish Biol. Fish. 2013, 23, 415–434. [Google Scholar] [CrossRef]
- Stelzenmüller, V.; Breen, P.; Stamford, T.; Thomsen, F.; Badalamenti, F.; Borja, A.; Buhl-Mortensen, L.; Carlstom, J.; D’Anna, G.; Dankers, N.; et al. Monitoring and evaluation of spatially managed areas: A generic framework for implementation of ecosystem based marine management and its application. Mar. Policy 2013, 37, 149–164. [Google Scholar] [CrossRef]
- Goñi, R.; Badalamenti, F.; Tupper, M. Effects of marine protected areas on local fisheries: Evidence from empirical studies. In Marine Protected Areas—A Multidisciplinary Approach; Claudet, J., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 72–98. [Google Scholar]
- Pérez-Ruzafa, A.; Martin, E.; Marcos, C.; Zamarro, J.M.; Stobart, B.; Harmelin Vivien, M.; Polti, S.; Planes, S.; Garcia Charton, J.A.; Gonzalez-Wanguemert, M. Modelling spatial and temporal scales for spill-over and biomass exportation from MPAs and their potential for fisheries enhancement. J. Nat. Conserv. 2008, 16, 234–255. [Google Scholar] [CrossRef]
- Sala, E.; Mayorga, J.; Bradley, D.; Cabral, R.B.; Atwood, T.B.; Auber, A.; Cheung, W.; Costello, C.; Ferretti, F.; Friedlander, A.M.; et al. Protecting the global ocean for biodiversity, food and climate. Nature 2021, 592, 7854. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Heinemann, D.; Evans, N. The Role of Marine Reserves as Fisheries Management Tools. A Review of Concepts, Evidence and International Experience; Bureau of Rural Sciences, Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2001; p. 192. [Google Scholar]
- Hixon, M.A.; Johnson, D.W.; Sogard, S.M. BOFFFFs: On the importance of conserving old-growth age structure in fishery populations. ICES J. Mar. Sci. 2014, 71, 2171–2185. [Google Scholar] [CrossRef]
- Baskett, M.L.; Barnett, L.A.K. The ecological and evolutionary consequences of marine reserves. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 49–73. [Google Scholar] [CrossRef]
- Apostolaki, P.; Milner-Gulland, E.J.; McAllister, M.K.; Kirkwood, G.P. Modelling the effects of establishing a marine reserve for mobile fish species. Can. J. Fish. Aquat. Sci. 2002, 59, 405–415. [Google Scholar] [CrossRef]
- Russo, T.; D’Andrea, L.; Franceschini, S.; Accadia, P.; Cucco, A.; Garofalo, G.; Gristine, M.; Parisi, A.; Quattrocchi, G.; Sabatella, R.F.; et al. Simulating the Effects of Alternative Management Measures of Trawl Fisheries in the Central Mediterranean Sea: Application of a Multi-Species Bio-economic Modeling Approach. Front. Mar. Sci. 2019, 6, 542. [Google Scholar] [CrossRef]
- Sladek Nowlis, J.; Roberts, C.M. Fisheries benefits and optimal design of marine reserves. Fish. Bull. 1999, 97, 604–616. [Google Scholar]
- Barnes, B.; Sidhu, H. The impact of marine closed areas on fishing yield under a variety of management strategies and stock depletion levels. Ecol. Model. 2013, 269, 113–125. [Google Scholar] [CrossRef]
- Yamazaki, S.; Grafton, Q.R.; Kompas, T.; Jennings, S. Biomass management targets and the conservation and economic benefits of marine reserves. Fish Fisher. 2014, 15, 196–208. [Google Scholar] [CrossRef]
- Fernández-Chacón, A.; Moland, E.; Heiberg Espeland, S.; Moland Olsen, E. Demographic effects of full vs. partial protection from harvesting: Inference from an empirical before–after control-impact study on Atlantic cod. J. Appl. Ecol. 2015, 52, 1206–1215. [Google Scholar] [CrossRef]
- Murawski, S.A.; Wigley, S.E.; Fogarty, M.J.; Rago, P.J.; Mountain, D.G. Effort distribution and catch patterns adjacent to temperate MPAs. ICES J. Mar. Sci. 2005, 62, 1150–1167. [Google Scholar] [CrossRef]
- Gubbay, S. Marine Protected Areas in the Context of Marine Spatial Planning: Discussing the Links; WWF: Godalming, UK, 2004; p. 22. Available online: http://assets.wwf.org.uk/downloads/mpas_marinespacialplanning.pdf (accessed on 11 July 2022).
- Fraschetti, S.; Pipitone, C.; Mazaris, A.D.; Rilov, G.; Badalamenti, F.; Bevilacqua, S.; Claudet, J.; Caric, H.; Dahl, K.; D’Anna, G.; et al. Light and shade in marine conservation across European and contiguous seas. Front. Mar. Sci. 2018, 5, 420. [Google Scholar] [CrossRef]
- Agardy, T. Justified ambivalence about MPA effectiveness. ICES J. Mar. Sci. 2018, 75, 1183–1185. [Google Scholar] [CrossRef]
- Sciberras, M.; Jenkins, S.R.; Mant, R.; Kaiser, M.J.; Hawkins, S.J.; Pullin, A.S. Evaluating the relative conservation value of fully and partially protected marine areas. Fish Fisher. 2015, 16, 58–77. [Google Scholar] [CrossRef]
- Bailey, C. Lessons from Indonesia’s 1980 trawler ban. Mar. Policy 1997, 21, 225–235. [Google Scholar] [CrossRef]
- Pipitone, C.; Badalamenti, F.; Vega Fernández, T.; D’Anna, G. Spatial Management of Fisheries in the Mediterranean Sea: Problematic Issues and a Few Success Stories. In Marine Managed Areas and Fisheries; Johnson, M.L., Sandell, J., Lesser, M., Eds.; Advances in Marine Biology; Academic Press: Oxford, UK, 2014; Volume 69, pp. 371–402. [Google Scholar]
- Pastoors, M.A.; Rijnsdorp, A.D.; van Beek, F.A. Effects of a partially closed area in the North Sea (“plaice box”) on stock development of plaice. ICES J. Mar. Sci. 2000, 57, 1014–1022. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; p. 206. [Google Scholar]
- Guidetti, P.; Bussotti, S.; Pizzolante, F.; Ciccolella, A. Assessing the potential of an artisanal fishing co-management in the Marine Protected Area of Torre Guaceto (southern Adriatic Sea, SE Italy). Fish. Res. 2010, 101, 180–187. [Google Scholar] [CrossRef]
- Whitmarsh, D.; James, C.; Pickering, H.; Pipitone, C.; Badalamenti, F.; D’Anna, G. Economic effects of fisheries exclusion zones: A Sicilian case study. Mar. Res. Econ. 2002, 17, 239–250. [Google Scholar] [CrossRef]
- Whitmarsh, D.; Pipitone, C.; Badalamenti, F.; D’Anna, G. The economic sustainability of artisanal fisheries: The case of the trawl ban in the Gulf of Castellammare, NW Sicily. Mar. Policy 2003, 27, 489–497. [Google Scholar] [CrossRef]
- Pipitone, C.; Badalamenti, F.; D’Anna, G.; Patti, B. Fish biomass increase after a four-year trawl ban in the Gulf of Castellammare (NW Sicily, Mediterranean Sea). Fish. Res. 2000, 48, 23–30. [Google Scholar] [CrossRef]
- Sweeting, C.J.; Badalamenti, F.; D’Anna, G.; Pipitone, C.; Polunin, N.V.C. Steeper biomass spectra of demersal fish communities after trawler exclusion in Sicily. ICES J. Mar. Sci. 2009, 66, 195–202. [Google Scholar] [CrossRef]
- Badalamenti, F.; Sweeting, C.J.; Polunin, N.V.C.; Pinnegar, J.; D’Anna, G.; Pipitone, C. Limited trophodynamics effects of trawling on three Mediterranean fishes. Mar. Biol. 2008, 154, 765–773. [Google Scholar] [CrossRef]
- Fanelli, E.; Badalamenti, F.; D’Anna, G.; Pipitone, C. Diet and trophic level of scaldfish Arnoglossus laterna in the southern Tyrrhenian Sea (western Mediterranean): Contrasting trawled versus untrawled areas. J. Mar. Biol. Ass. UK 2009, 89, 817–828. [Google Scholar] [CrossRef]
- Fanelli, E.; Badalamenti, F.; D’Anna, G.; Pipitone, C.; Romano, C. Trophodynamic effects of trawling on the feeding ecology of pandora, Pagellus erythrinus, off the northern Sicily coast (Mediterranean Sea). Mar. Freshw. Res. 2010, 61, 408–417. [Google Scholar] [CrossRef]
- Sinopoli, M.; Fanelli, E.; D’Anna, G.; Badalamenti, F.; Pipitone, C. Assessing the effects of a trawling ban on diet and trophic level of hake, Merluccius merluccius, in the southern Tyrrhenian Sea. Sci. Mar. 2012, 76, 677–690. [Google Scholar] [CrossRef]
- Giacalone, V.M.; D’Anna, G.; Badalamenti, F.; Pipitone, C. Weight-length relationships and condition factor trends for thirty-eight fish species in trawled and untrawled areas off the coast of northern Sicily (central Mediterranean Sea). J. Appl. Ichtyol. 2010, 26, 954–957. [Google Scholar] [CrossRef]
- Sieli, G.; Badalucco, C.; Di Stefano, G.; Rizzo, P.; D’Anna, G.; Fiorentino, F. Biology of red mullet, Mullus barbatus (L. 1758), in the Gulf of Castellammare (NW Sicily, Mediterranean Sea) subject to a trawling ban. J. Appl. Ichtyol. 2011, 27, 1218–1225. [Google Scholar] [CrossRef]
- Sinopoli, M.; Pipitone, C.; Badalamenti, F.; D’Anna, G.; Fiorentino, F.; Gristina, M.; Lauria, V.; Rizzo, P.; Milisenda, G. Effects of a trawling ban on the growth of young-of-the-year European hake, Merluccius merluccius in a Mediterranean fishing exclusion zone. Reg. Stud. Mar. Sci. 2022, 50, 102151. [Google Scholar] [CrossRef]
- Fiorentino, F.; Badalamenti, F.; D’Anna, G.; Garofalo, G.; Gianguzza, P.; Gristina, M.; Pipitone, C.; Rizzo, P.; Fortibuoni, T. Changes in spawning-stock structure and recruitment pattern of red mullet, Mullus barbatus, after a trawl ban in the Gulf of Castellammare (central Mediterranean Sea). ICES J. Mar. Sci. 2008, 65, 1175–1183. [Google Scholar] [CrossRef]
- Fanelli, E.; Cartes, J.E.; Badalamenti, F.; D’Anna, G.; Pipitone, C.; Azzurro, E.; Rumolo, P.; Sprovieri, M. Meso-scale variability of coastal suprabenthic communities in the southern Tyrrhenian Sea (western Mediterranean). Est. Coast. Mar. Sci. 2011, 91, 351–360. [Google Scholar] [CrossRef]
- Romano, C.; Fanelli, E.; D’Anna, G.; Pipitone, C.; Vizzini, S.; Mazzola, A.; Badalamenti, F. Spatial variability of soft-bottom macrobenthic communities in northern Sicily (Western Mediterranean): Contrasting trawled vs. untrawled areas. Mar. Environ. Res. 2016, 122, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Relini, G.; Bertrand, J.; Zamboni, A. (Eds.) Synthesis of the Knowledge on Bottom Fishery Resources in Central Mediterranean (Italy and Corsica); SIBM: Genova, Italy, 1999; Volume 6, (Suppl. 1), p. 868. [Google Scholar]
- Maiorano, P.; Sabatella, R.F.; Marzocchi, B.M. (Eds.) Annuario Sullo Stato delle Risorse e Sulle Strutture Produttive dei Mari Italiani; CNR, CoNISMa, COISPA, CIBM, NISEA, Consorzio Rete Mare: Ancona, Italy, 2019; p. 432. [Google Scholar]
- Sparre, R.; Venema, S.C. Introduction to tropical fish stock assessment. Part 1. Manual. FAO Fish. Tech. Pap. 1998, 306, 407. [Google Scholar]
- Fiorentini, L.; Cosimi, G.; Sala, A.; Palumbo, V. Characteristic and performance of the fishing gears used for demersal stock assessment in Italy. Biol. Mar. Medit. 1994, 1, 115–134. [Google Scholar]
- Dimarchopoulou, D.; Dogrammatzi, A.; Karachle, P.K.; Tsikliras, A.C. Spatial fishing restrictions benefit demersal stocks in the northeastern Mediterranean Sea. Sci. Rep. 2018, 8, 5967. [Google Scholar] [CrossRef] [PubMed]
- Probst, W.N.; Stelzenmüller, V.; Fock, H.O. Using cross-correlations to assess the relationship between time-lagged pressure and state indicators: An exemplary analysis of North Sea fish population indicators. ICES J. Mar. Sci. 2012, 69, 670–681. [Google Scholar] [CrossRef]
- Trenkel, V.M.; Rochet, M.J. Combining time trends in multiple metrics for identifying persistent changes in population processes or environmental stressors. J. Appl. Ecol. 2010, 47, 751–758. [Google Scholar] [CrossRef]
- Shin, Y.J.; Rochet, M.-J.; Jennings, S.; Field, J.G.; Gislason, H. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef]
- Green, R.H. Sampling Design and Statistical Methods for Environmental Biologists; John Wiley and Sons: Hoboken, NJ, USA, 1979; p. 272. [Google Scholar]
- Schwarz, C.J. Analysis of BACI experiments. In Course Notes for Beginning and Intermediate Statistics; Simon Fraser University: Burnaby, BC, Canada, 2014; Available online: https://www.coursehero.com/file/14392359/R-part013/ (accessed on 4 November 2019).
- Anderson, M.J.; ter Braak, C.J.F. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; p. 192. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2022. [Google Scholar]
- Sandrini-Neto, A.L.; Camargo, M.G. GAD: General ANOVA Designs; Centro de Estudos do Mar da Universidade Federal do Parana: Pontal do Parana, Brazil, 2022. [Google Scholar]
- Francis, R.C.; Hixon, M.A.; Clarke, M.E.; Murawski, S.A.; Ralston, S. Ten commandments for ecosystem-based fisheries scientists. Fisheries 2007, 32, 217–233. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B.; et al. Ecosystem-Based Fishery Management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef]
- Jorgensen, C.; Enberg, K.; Dunlop, E.S.; Arlinghaus, R.; Boukal, D.S.; Brander, K.; Ernande, B.; Gardmark, A.; Johnston, F.; Matsumura, S.; et al. Managing evolving fish stocks. Science 2007, 318, 1247–1248. [Google Scholar] [CrossRef]
- Agardy, T.; Notarbartolo di Sciara, G.; Christie, P. Mind the gap: Addressing the shortcomings of marine protected areas through large scale marine spatial planning. Mar. Policy 2011, 35, 226–232. [Google Scholar] [CrossRef]
- Frank, K.T.; Shackell, N.L.; Simon, J.E. An evaluation of the Emerald/Western Bank juvenile haddock closed area. ICES J. Mar. Sci. 2000, 57, 1023–1034. [Google Scholar] [CrossRef]
- Mak, Y.K.Y.; Tao, L.S.R.; Ho, V.C.M.; Dudgeon, D.; Cheung, W.W.L.; Leung, K.M.Y. Initial recovery of demersal fish communities in coastal waters of Hong Kong, South China, following a trawl ban. Rev. Fish Biol. Fish. 2021, 31, 989–1007. [Google Scholar] [CrossRef]
- Mullowney, D.R.J.; Morris, C.J.; Dawe, E.G.; Skanes, K.R. Impacts of a bottom trawling exclusion zone on Snow Crab abundance and fish harvester behavior in the Labrador Sea, Canada. Mar. Policy 2012, 36, 567–575. [Google Scholar] [CrossRef]
- Pranovi, F.; Monti, M.A.; Caccin, A.; Brigolin, D.; Zucchetta, M. Permanent trawl fishery closures in the Mediterranean Sea: An effective management strategy? Mar. Policy 2015, 60, 272–279. [Google Scholar] [CrossRef]
- Sherwood, G.D.; Grabowski, J.H. A comparison of cod life-history parameters inside and outside of four year-round groundfish closed areas in New England, USA. ICES J. Mar. Sci. 2016, 73, 316–328. [Google Scholar] [CrossRef]
- Tao, L.S.R.; Mak, Y.K.Y.; Ho, V.C.M.; Sham, R.C.T.; Hui, T.T.Y.; Lau, D.C.P.; Leung, K.M.Y. Improvements of Population Fitness and Trophic Status of a Benthic Predatory Fish Following a Trawling Ban. Front. Mar. Sci. 2021, 8, 835. [Google Scholar] [CrossRef]
- Auster, P.J.; Shackell, N.L. Fishery reserves. In Northwest Atlantic Groundfish: Perspectives on a Fishery Collapse; Boreman, J.G., Nakashima, B.S., Wilson, J.A., Kendall, R.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 1997; pp. 159–166. [Google Scholar]
- Badalamenti, F.; Pipitone, C.; Fiorentino, F.; D’Anna, G. The trawling ban in Hong Kong’s inshore waters—A round of applause and a plea to learn from others’ mistakes. Mar. Poll. Bull. 2012, 64, 1513–1514. [Google Scholar] [CrossRef]
- Lauck, T.; Clark, C.W.; Mangel, M.; Munro, G.R. Implementing the precautionary principle in fisheries management through marine reserves. Ecol. Appl. 1998, 8, 72–78. [Google Scholar] [CrossRef]
- Mesnildrey, L.; Gascuel, D.; Le Pape, O. Integrating Marine Protected Areas in fisheries management systems: Some criteria for ecological efficiency. Aquat. Living Resour. 2013, 26, 159–170. [Google Scholar] [CrossRef]
- Polunin, N.V.C. Marine Protected Areas, Fish and Fisheries. In Handbook of Fish Biology and Fisheries; Hart, P.J.B., Reynolds, J.D., Eds.; Blackwell Science Ltd.: Malden, MA, USA, 2008; pp. 293–318. [Google Scholar]
- D’Arienzo, V.; Di Salvia, B. (Eds.) Pesci, Barche, Pescatori Nell’area Mediterranea dal Medioevo All’età Contemporanea; Franco Angeli: Milano, Italy, 2010; p. 638. [Google Scholar]
- Arculeo, M.; D’Anna, G.; Riggio, S. Valutazione delle risorse demersali nell’ area compresa fra Capo Gallo e Capo San Vito (Sicilia nord-occidentale): Risultati delle campagne condotte nel 1985. Atti Semin. Pesca Acquac. Roma C.N.R. E Min. Mar. Merc. 1988, 3, 1413–1451. [Google Scholar]
- Florin, A.B.; Bergstrom, U.; Ustups, D.; Lundstrom, K.; Jonsson, P.R. Effects of a large northern European no-take zone on flatfish populations. J. Fish Biol. 2013, 83, 939–962. [Google Scholar] [CrossRef]
- Jaworski, A.; Solmundsson, J.; Ragnarsson, S.A. The effect of area closures on the demersal fish community off the east coast of Iceland. ICES J. Mar. Sci. 2006, 63, 897–911. [Google Scholar] [CrossRef]
- Kincaid, K.; Rose, G. Effects of closing bottom trawling on fisheries, biodiversity, and fishing communities in a boreal marine ecosystem: The Hawke Box off Labrador, Canada. Can. J. Fish. Aquat. Sci. 2017, 74, 1490–1502. [Google Scholar] [CrossRef]
- Murawski, S.A. Rebuilding depleted fish stocks: The good, the bad, and, mostly, the ugly. ICES J. Mar. Sci. 2010, 67, 1830–1840. [Google Scholar] [CrossRef]
- Tuset, V.M.; Farré, M.; Fernandez-Arcaya, U.; Balcells, M.; Lombarte, A.; Recasens, L. Effects of a fishing closure area on the structure and diversity of a continental shelf fish assemblage in the NW Mediterranean Sea. Reg. Stud. Mar. Sci. 2021, 43, 101700. [Google Scholar] [CrossRef]
- Agnetta, D.; Badalamenti, F.; D’Anna, G.; Sinopoli, M.; Andaloro, F.; Vizzini, S.; Pipitone, C. Sizing up the role of predators on an overpopulation of Mullus barbatus in Mediterranean no-trawl areas. Fish. Res. 2019, 213, 196–203. [Google Scholar] [CrossRef]
- Sala-Coromina, J.; Garcia, J.A.; Martin, P.; Fernandez-Arcaya, U.; Recasens, L. European hake (Merluccius merluccius, Linnaeus 1758) spillover analysis using VMS and landings data in a no-take zone in the northern Catalan coast (NW Mediterranean). Fish. Res. 2021, 237, 105870. [Google Scholar] [CrossRef]
- Jennings, S.; Greenstreet, S.P.R.; Reynolds, J.D. Structural change in an exploited fish community: A consequence of differential fishing effects on species with contrasting life histories. J. Anim. Ecol. 1999, 68, 617–627. [Google Scholar] [CrossRef]
- Quetglas, A.; Rueda, L.; Alvarez Berastegui, D.; Guijarro, B.; Massuti, E. Contrasting Responses to Harvesting and Environmental Drivers of Fast and Slow Life History Species. PLoS ONE 2016, 11, e0148770. [Google Scholar] [CrossRef]
- Caddy, J.F.; Rodhouse, P.G. Cephalopod and groundfish landings: Evidence for ecological change in global fisheries? Rev. Fish Biol. Fish. 1998, 8, 431–444. [Google Scholar] [CrossRef]
- Sobrino, I.; Silva, C.; Sbrana, M.; Kapiris, K. A review of the biology and fisheries of the deep water rose shrimp, Parapenaeus longirostris, in European Atlantic and Mediterranean waters (Decapoda, Dendrobranchiata, Penaeidae). Crustaceana 2005, 78, 1153–1184. [Google Scholar] [CrossRef]
- Rochet, M.J.; Trenkel, V.M. Which community indicators can measure the impact of fishing? A review and proposals. Can. J. Fish. Aquat. Sci. 2003, 60, 86–99. [Google Scholar] [CrossRef]
- Stefanoni, S.; D’Anna, G.; Pipitone, C.; Badalamenti, F. Analisi economica delle politiche di gestione della pesca nel Golfo di Castellammare. In La Valutazione delle Risorse Ambientali. Approcci Multidisciplinari al Golfo di Castellammare; Pipitone, V., Cognata, A., Eds.; Franco Angeli: Milano, Italy, 2008; pp. 178–201. [Google Scholar]
- Jennings, S.; Blanchard, J.L. Fish abundance with no fishing: Predictions based on macroecological theory. J. Animal Ecol. 2004, 73, 632–642. [Google Scholar] [CrossRef]
- Edwards, C.T.T.; Plaganyi, E.E. Protecting old fish through spatial management: Is there a benefit for sustainable exploitation? J. Appl. Ecol. 2011, 48, 853–863. [Google Scholar] [CrossRef]
- Gårdmark, A.; Jonzén, N.; Mangel, M. Density-dependent body growth reduces the potential of marine reserves to enhance yields. J. Appl. Ecol. 2006, 43, 61–69. [Google Scholar] [CrossRef]
- Vega Fernández, T.; Zenone, A. Socio-economic characterization of the small-scale fishery in and around the trawl-exclusion area of the Gulf of Castellammare. In Proceedings of the Regional Conference on Building a Future for Sustainable Small-Scale Fisheries in the Mediterranean and the Black Sea, Algiers, Algeria, 7–9 March 2016; Srour, A., Carlson, A., Nastasi, A., Carmignac, C., Bourdenet, D., Pierraccini, J., Sessa, M., Ferri, N., Eds.; Fisheries and Aquaculture Proceedings. FAO: Rome, Italy, 2018; pp. 74–90. [Google Scholar]



| Group | Species | Common Name | Biomass/Size | Ecology/Biology |
|---|---|---|---|---|
| Cephalopod | Alloteuthis media | midsize squid | S | BePel/SL |
| Cephalopod | Eledone cirrhosa | horned octopus | B/S | Be/SL |
| Cephalopod | Illex coindetii | broadtail squid | S | BePel/SL |
| Cephalopod | Octopus vulgaris | common octopus | B | Be/SL |
| Cephalopod | Sepia elegans | elegant cuttlefish | S | Be/SL |
| Cephalopod | Sepia officinalis | common cuttlefish | B | Be/SL |
| Crustacean | Parapenaeus longirostris | deep-water rose shrimp | B/S | BePel/SL |
| Fish | Arnoglossus laterna | scaldfish | S | Be/ML |
| Fish | Capros aper | boarfish | S | BePel/ML |
| Fish | Chelidonichthys cuculus | red gurnard | S | Be/ML |
| Fish | Diplodus annularis | annular seabream | B | BePel/ML |
| Fish | Lepidotrigla cavillone | large-scaled gurnard | S | Be/ML |
| Fish | Lophius budegassa | anglerfish | B | Be/LL |
| Fish | Merluccius merluccius | hake | B/S | BePel/LL |
| Fish | Mullus barbatus | red mullet | B/S | BePel/ML |
| Fish | Pagellus acarne | axillary seabream | B | BePel/ML |
| Fish | Pagellus erythrinus | pandora | B/S | BePel/ML |
| Fish | Phycis blennoides | greater forkbeard | S | Be/LL |
| Fish | Spicara flexuosum | picarel | S | BePel/ML |
| Strata | Replicates | |
|---|---|---|
| Horned octopus | B-C | 22 |
| Common octopus | A-B-C | 32 |
| Common cuttlefish | A-B | 20 |
| Deep-water rose shrimp | C | 12 |
| Annular seabream | A-B | 20 |
| Anglerfish | A-B-C | 32 |
| Hake | A-B-C | 32 |
| Red mullet | A-B | 20 |
| Axillary seabream | A-B-C | 32 |
| Pandora | A-B | 20 |
| Before-1990 | s.d. | After-1990 | s.d. | |
|---|---|---|---|---|
| GCAST | 203.1 | ±54.12 | 1265.6 | ±866.81 |
| GTERM | 108.7 | ±43.15 | 130.7 | ±49.39 |
| GSANT | 139.9 | ±53.59 | 132.0 | ±90.06 |
| GCAST | GTERM | GSANT | ||||
|---|---|---|---|---|---|---|
| Before-1990 | After-1990 | Before-1990 | After-1990 | Before-1990 | After-1990 | |
| Horned octopus | 10.1 | 17.9 | 11.2 | 18.7 | 11.7 | 9.1 |
| ±12.27 | ±24.66 | ±15.24 | ±14.43 | ±12.35 | ±9.36 | |
| Common octopus | 6.2 | 24.4 | 22.4 | 2.9 | 27.4 | 5.0 |
| ±15.14 | ±33.23 | ±24.87 | ±12.25 | ±27.11 | ±16.13 | |
| Common cuttlefish | 1.7 | 12.3 | 4.7 | 1.8 | 5.3 | 5.0 |
| ±2.86 | ±1.53 | ±7.52 | ±3.00 | ±5.00 | ±6.40 | |
| Deep-water rose shrimp | 4.5 | 25.0 | 27.7 | 24.7 | 13.3 | 18.4 |
| ±7.27 | ±16.04 | ±27.70 | ±15.07 | ±13.53 | ±10.01 | |
| Annular seabream | 14.3 | 143.7 | 4.4 | 10.3 | 3.3 | 2.6 |
| ±20.95 | ±218.66 | ±4.35 | ±30.33 | ±4.05 | ±4.83 | |
| Anglerfish | 5.6 | 34.3 | 6.7 | 3.4 | 12.6 | 2.8 |
| ±8.73 | ±40.86 | ±8.25 | ±5.26 | ±11.78 | ±5.48 | |
| Hake | 45.4 | 138.8 | 15.4 | 21.8 | 17.7 | 23.9 |
| ±52.41 | ±98.36 | ±20.82 | ±23.36 | ±17.01 | ±16.18 | |
| Red mullet | 47.1 | 313.4 | 18.9 | 5.9 | 31.6 | 22.8 |
| ±77.16 | ±425.13 | ±24.72 | ±10.29 | ±45.84 | ±69.10 | |
| Axillary seabream | 15.6 | 86.3 | 8.7 | 0.6 | 7.0 | 0.5 |
| ±51.43 | ±157.26 | ±15.07 | ±1.90 | ±12.46 | ±1.25 | |
| Pandora | 11.6 | 128.2 | 1.6 | 9.9 | 7.3 | 18.0 |
| ±12.26 | ±130.51 | ±3.24 | ±10.69 | ±7.65 | ±15.48 | |
| Before-1990 | After-1990 | |||||
|---|---|---|---|---|---|---|
| HC | MC | NC | HC | MC | NC | |
| A | 145.1 | 101.2 | 22.5 | 1356.8 | 491.5 | 124.0 |
| ±90.4 | ±143.5 | ±16.1 | ±1088.7 | ±521.7 | ±99.6 | |
| B | 124.9 | 84.0 | 20.6 | 802.2 | 478.8 | 339.8 |
| ±81.7 | ±60.0 | ±15.7 | ±592.5 | ±249.7 | ±163.0 | |
| C | 95.5 | 70.2 | 18.8 | 382.4 | 201.3 | 191.0 |
| ±49.6 | ±65.8 | ±20.1 | ±290.3 | ±116.3 | ±101.5 | |
| Lm (mm) | L95 (mm) | L2/3 (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GCAST | GTERM | GSANT | GCAST | GTERM | GSANT | GCAST | GTERM | GSANT | |
| midsize squid | 52 | 47 | 42 | 77 | 78 | 67 | 15 | 6 | 2 |
| horned octopus | 92 | 72 | 77 | 111 | 117 | 121 | 38 | 27 | 19 |
| broadtail squid | 62 | 68 | 58 | 183 | 153 | 91 | 11 | 5 | 2 |
| elegant cuttlefish | 38 | 32 | 32 | 51 | 53 | 51 | 47 | 15 | 13 |
| deep-water rose shrimp | 16 | 16 | 18 | 111 | 117 | 121 | 38 | 27 | 19 |
| scaldfish | 97 | 88 | 82 | 142 | 127 | 127 | 24 | 6 | 24 |
| boarfish | 48 | 42 | 42 | 72 | 51 | 74 | 3 | 2 | 11 |
| red gurnard | 132 | 72 | 77 | 223 | 81 | 90 | 16 | 1 | 2 |
| large-scaled gurnard | 102 | 68 | 72 | 121 | 92 | 108 | 61 | 3 | 11 |
| hake | 127 | 87 | 87 | 262 | 192 | 203 | 7 | 0 | 0 |
| red mullet | 123 | 122 | 117 | 172 | 166 | 172 | 4 | 11 | 15 |
| pandora | 168 | 117 | 142 | 268 | 211 | 212 | 6 | 21 | 16 |
| greater forkbeard | 127 | 108 | 103 | 232 | 172 | 171 | 9 | 10 | 15 |
| picarel | 128 | 122 | 122 | 172 | 177 | 178 | 39 | 22 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipitone, C.; Agnetta, D.; Zenone, A.; Giacalone, V.M.; Badalamenti, F.; Fiorentino, F.; Rinelli, P.; Sinopoli, M.; Vega Fernández, T.; D’Anna, G. When the Trawl Ban Is a Good Option: Opportunities to Restore Fish Biomass and Size Structure in a Mediterranean Fisheries Restricted Area. Sustainability 2023, 15, 2425. https://doi.org/10.3390/su15032425
Pipitone C, Agnetta D, Zenone A, Giacalone VM, Badalamenti F, Fiorentino F, Rinelli P, Sinopoli M, Vega Fernández T, D’Anna G. When the Trawl Ban Is a Good Option: Opportunities to Restore Fish Biomass and Size Structure in a Mediterranean Fisheries Restricted Area. Sustainability. 2023; 15(3):2425. https://doi.org/10.3390/su15032425
Chicago/Turabian StylePipitone, Carlo, Davide Agnetta, Arturo Zenone, Vincenzo Maximiliano Giacalone, Fabio Badalamenti, Fabio Fiorentino, Paola Rinelli, Mauro Sinopoli, Tomás Vega Fernández, and Giovanni D’Anna. 2023. "When the Trawl Ban Is a Good Option: Opportunities to Restore Fish Biomass and Size Structure in a Mediterranean Fisheries Restricted Area" Sustainability 15, no. 3: 2425. https://doi.org/10.3390/su15032425
APA StylePipitone, C., Agnetta, D., Zenone, A., Giacalone, V. M., Badalamenti, F., Fiorentino, F., Rinelli, P., Sinopoli, M., Vega Fernández, T., & D’Anna, G. (2023). When the Trawl Ban Is a Good Option: Opportunities to Restore Fish Biomass and Size Structure in a Mediterranean Fisheries Restricted Area. Sustainability, 15(3), 2425. https://doi.org/10.3390/su15032425









