Impact of Integrating Annual and Perennial Legumes under Coffea arabica on Sloping Land
Abstract
:1. Introduction
2. Materials and Methods
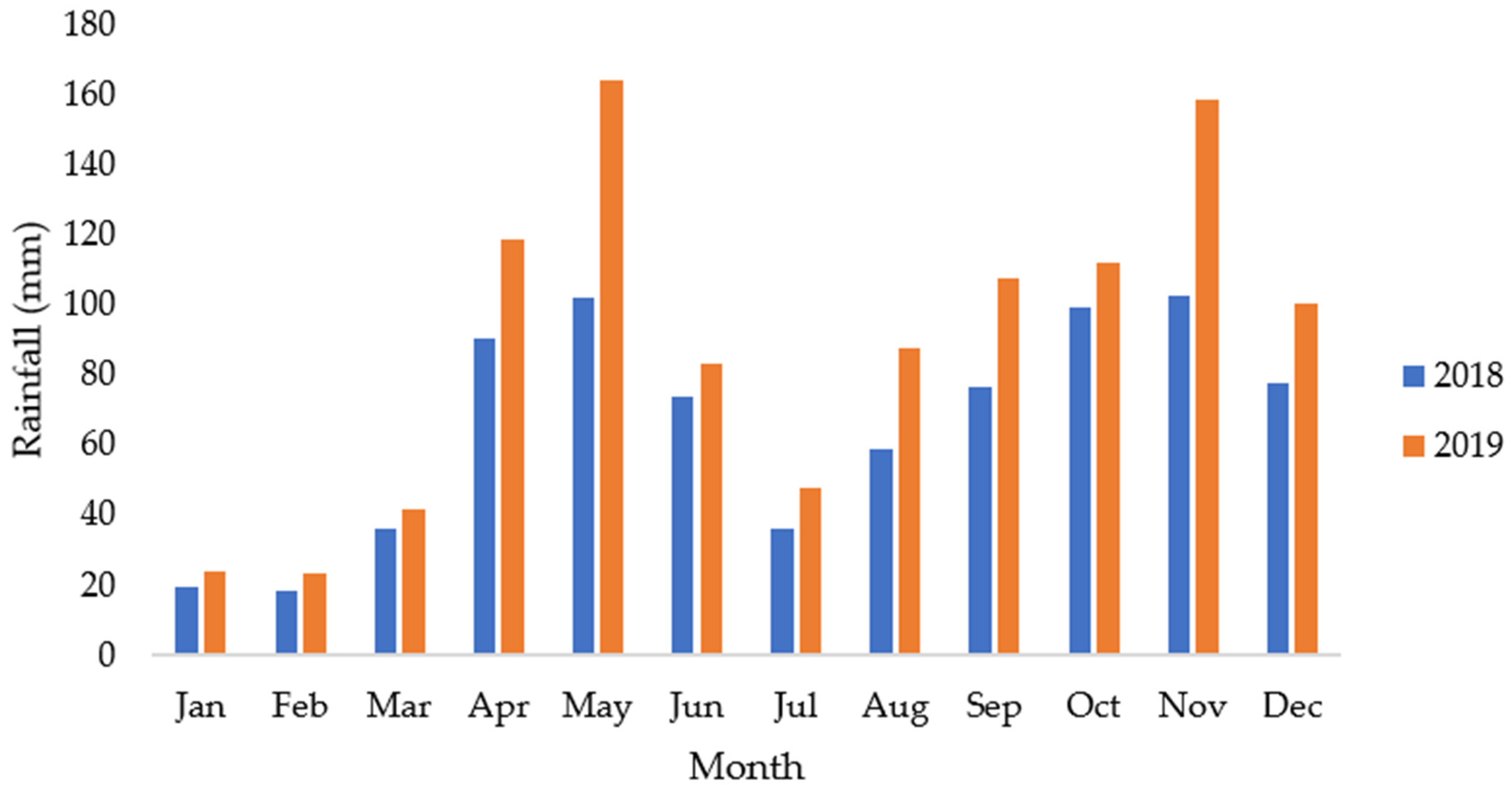
2.1. Description of the Study Area
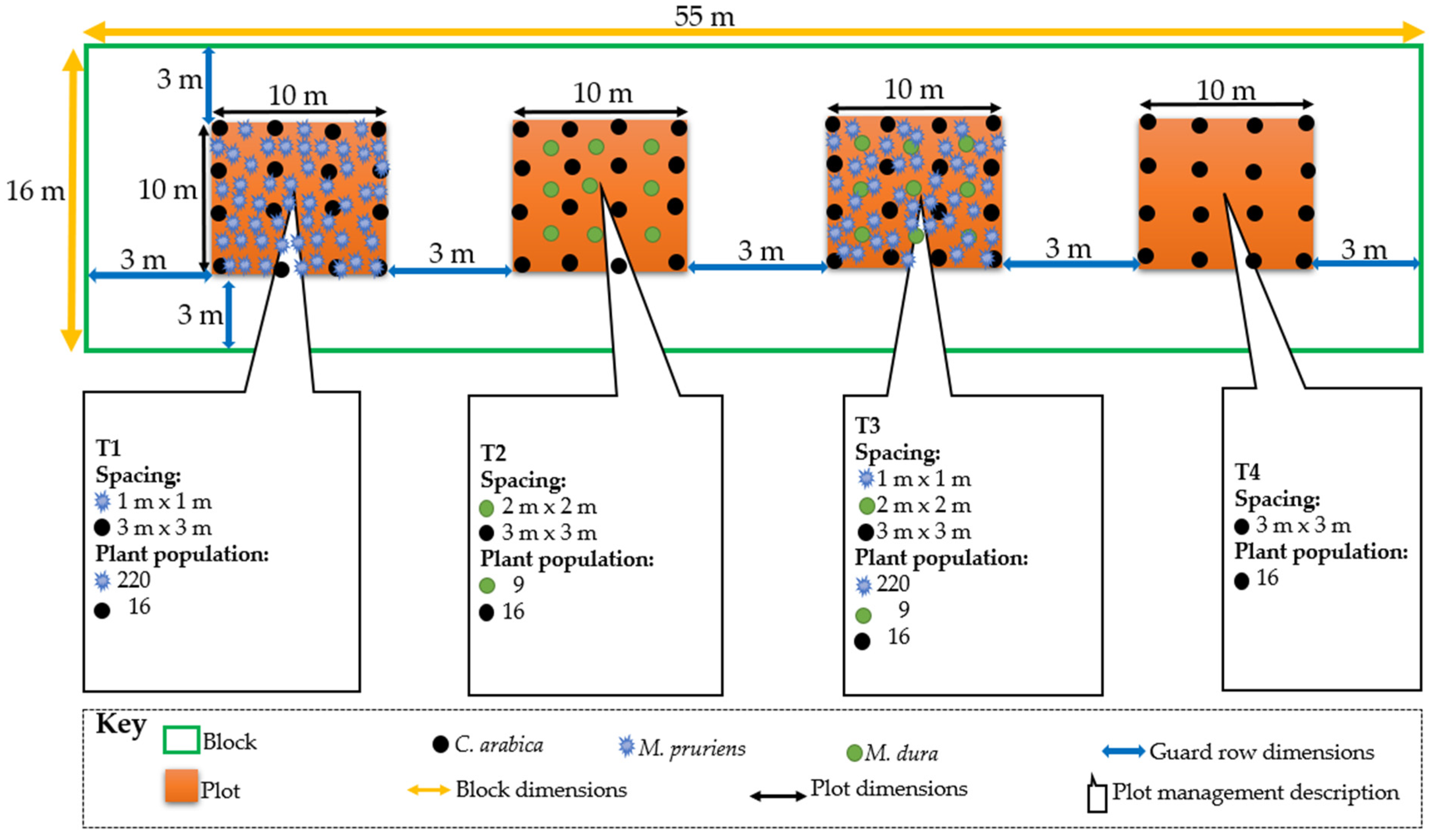
2.2. Experimental Design and Establishment
2.3. Data Collection
2.3.1. Above-Ground Biomass
2.3.2. Soil Sampling and Testing
2.3.3. Coffee Yield
2.3.4. Profitability
2.4. Statistical Analysis
3. Results
3.1. Above-Ground Biomass
3.2. Soil Macronutrients
3.3. Coffee Yield
3.4. Profitability
4. Discussion
4.1. Above-Ground Biomass
4.2. Available Soil Macronutrients
4.3. Coffee Yield
4.4. Profitability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Etienne, H. Somatic Embryogenesis Protocol: Coffee (Coffea arabica L. and C. canephora P.). In Protocol for Somatic Embryogenesis in Woody Plants; Jain, S.M., Gupta, P.K., Eds.; Forestry Sciences; Springer-Verlag: Berlin/Heidelberg, Germany, 2005; Volume 77, pp. 167–179. ISBN 978-1-4020-2984-4. [Google Scholar]
- Ovalle-Rivera, O.; Läderach, P.; Bunn, C.; Obersteiner, M.; Schroth, G. Projected Shifts in Coffea arabica Suitability among Major Global Producing Regions Due to Climate Change. PLoS ONE 2015, 10, e0124155. [Google Scholar] [CrossRef] [Green Version]
- Labrière, N.; Locatelli, B.; Laumonier, Y.; Freycon, V.; Bernoux, M. Soil erosion in the humid tropics: A systematic quantitative review. Agric. Ecosyst. Environ. 2015, 203, 127–139. [Google Scholar] [CrossRef]
- Wang, N.; Jassogne, L.; van Asten, P.J.A.; Mukasa, D.; Wanyama, I.; Kagezi, G.; Giller, K.E. Evaluating coffee yield gaps and important biotic, abiotic, and management factors limiting coffee production in Uganda. Eur. J. Agron. 2015, 63, 1–11. [Google Scholar] [CrossRef]
- Workman, D. Coffee Exports by Country. Available online: https://www.worldstopexports.com/coffee-exports-country/ (accessed on 8 January 2023).
- Wisevoter. Coffee Consumption by Country; Wisevoter: Washington, DC, USA, 2023. [Google Scholar]
- Clay, J. World Agriculture and the Environment: A Commodity-by-Commodity Guide to Impacts and Practices; Island Press: Washington, DC, USA, 2004; ISBN 978-1-55963-370-3. [Google Scholar]
- MAAIF. National Coffee Policy; Ministry of Agriculture Animal Industry and Fisheries (MAIF): Entebbe, Uganda, 2013. [Google Scholar]
- UCDA. Uganda National Coffee Strategy 2040 Plan for 2015/16–2019/2; UCDA: Kampala, Uganda, 2015. [Google Scholar]
- UCDA. Daily Coffee Market Prices; Uganda Coffe Development Authority: Kampala, Uganda, 2023. [Google Scholar]
- Mukiibi, J.K. Agriculture in Uganda, 2nd ed.; Fountain Publishers: Kampala, Uganda, 2001. [Google Scholar]
- Geromel, C.; Ferreira, L.P.; Davrieux, F.; Guyot, B.; Ribeyre, F.; Brígida dos Santos Scholz, M.; Protasio Pereira, L.F.; Vaast, P.; Pot, D.; Leroy, T.; et al. Effects of shade on the development and sugar metabolism of coffee (Coffea arabica L.) fruits. Plant Physiol. Biochem. 2008, 46, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Bosselmann, A.S.; Dons, K.; Oberthur, T.; Olsen, C.S.; Ræbild, A.; Usma, H. The influence of shade trees on coffee quality in small holder coffee agroforestry systems in Southern Colombia. Agric. Ecosyst. Environ. 2009, 129, 253–260. [Google Scholar] [CrossRef]
- Briggs, L.; Twomlow, S.J. Organic material flows within a smallholder highland farming system of South West Uganda. Agric. Ecosyst. Environ. 2002, 89, 191–212. [Google Scholar] [CrossRef] [Green Version]
- Asten, P.V.; Wanyama, I.; Mukasa, D.; Nansamba, R.; Kisaakye, J.; Sserubiri, I.; Bongers, G.; Jassogne, L. Mapping and Evaluating Improved Intercrop and Soil Management Options for Ugandan Coffee Farmers; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2012. [Google Scholar] [CrossRef]
- Jacobs, L.; Dewitte, O.; Poesen, J.; Delvaux, D.; Thiery, W.; Kervyn, M. The Rwenzori Mountains, a landslide-prone region? J. Int. Consort. Landslides 2016, 13, 519–536. [Google Scholar] [CrossRef] [Green Version]
- Muhumuza, M.; Byarugaba, D. Impact of land use on the ecology of uncultivated plant species in the Rwenzori mountain range, mid western Uganda. Afr. J. Ecol. 2009, 47, 614–621. [Google Scholar] [CrossRef]
- Jassogne, L.; Läderach, P.; van Asten, P. The Impact of Climate Change on Coffee in Uganda: Lessons from a Case Study in the Rwenzori Mountains; Oxfam: Oxford, UK, 2013. [Google Scholar]
- UBOS. The National Population and Housing Census 2014—Main Report. Available online: http://www.ubos.org/2014-census/census-2014-final-results/ (accessed on 4 July 2017).
- Ssebunya, B.R.; Schmid, E.; van Asten, P.; Schader, C.; Altenbuchner, C.; Stolze, M. Stakeholder engagement in prioritizing sustainability assessment themes for smallholder coffee production in Uganda. Renew. Agric. Food Syst. 2017, 32, 428–445. [Google Scholar] [CrossRef]
- Karamage, F.; Zhang, C.; Liu, T.; Maganda, A.; Isabwe, A. Soil Erosion Risk Assessment in Uganda. Forests 2017, 8, 52. [Google Scholar] [CrossRef]
- Grüter, R.; Trachsel, T.; Laube, P.; Jaisli, I. Expected global suitability of coffee, cashew and avocado due to climate change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef]
- Scopel, E.; Triomphe, B.; Affholder, F.; Da Silva, F.A.M.; Corbeels, M.; Xavier, J.H.V.; Lahmar, R.; Recous, S.; Bernoux, M.; Blanchart, E.; et al. Conservation agriculture cropping systems in temperate and tropical conditions, performances and impacts. A review. Agron. Sustain. Dev. 2013, 33, 113–130. [Google Scholar] [CrossRef]
- Berry, L.; Olson, J.M.; Campbell, D. Assessing the Extent, Cost and Impact of Land Degradation at the National Level: Findings and Lessons Learned from Seven Pilot Case Studies; World Bank: Washington, DC, USA, 2003. [Google Scholar]
- Van Der Vossen, H.A.M. A critical analysis of the agronomic and economic sustainability of organic coffee production. Ex. Agric. 2005, 41, 449–473. [Google Scholar] [CrossRef] [Green Version]
- Shiferaw, B.A.; Okello, J.; Reddy, R.V. Adoption and adaptation of natural resource management innovations in smallholder agriculture: Reflections on key lessons and best practices. Environ. Dev. Sustain. 2009, 11, 601–619. [Google Scholar] [CrossRef] [Green Version]
- Muhamud, N.W. Socio-Economic Factors Assessment Affecting the Adoption of Soil Conservation Technologies on Rwenzori Mountain. IJG 2015, 47, 26. [Google Scholar] [CrossRef]
- Tibasiima, T.K.; Ekyaligonza, D.M.; Bwambale, B. Can Agroecology Provide a Panacea for Sustaining the Adoption of Soil Erosion Control Measures? A Case of Smallholder Coffea arabica Production in the Rwenzori Mountain Region, Uganda. Sustainability 2022, 14, 13461. [Google Scholar] [CrossRef]
- Banadda, N. Gaps, barriers and bottlenecks to sustainable land management (SLM) adoption in Uganda. Afr. J. Agric. Res. 2010, 5, 3571–3580. [Google Scholar]
- Haggblade, S.; Tembo, G.; Donovan, C. Household level financial incentives to adoption of conservation agricultural technologies in Africa. Res. Agric. Appl. Econ. 2004, 30, 54466. [Google Scholar]
- Seitz, S.; Goebes, P.; Puerta, V.L.; Pereira, E.I.P.; Wittwer, R.; Six, J.; van der Heijden, M.G.A.; Scholten, T. Conservation tillage and organic farming reduce soil erosion. Agron. Sustain. Dev. 2019, 39, 4. [Google Scholar] [CrossRef] [Green Version]
- Tessaro, A.A.; Pereira, M.A.; Calegari, A.; Onofre, S.B.; Ralisch, R. Soybean Yields and Biomass Production of Winter Cover Crops in the Southwest of Parana—Brazil. JSD 2019, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Calegari, A.; Tiecher, T.; Wutke, E.B.; Canalli, L.B.D.S.; Bunch, R.; Rheinheimer, D.D.S. The role and management of soil mulch and cover crops in Conservation Agriculture systems. In Advances in Conservation Agriculture; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; ISBN 978-0-429-26872-4. [Google Scholar]
- Nasir Ahmad, N.S.B.; Mustafa, F.B.; Muhammad Yusoff, S.Y.; Didams, G. A systematic review of soil erosion control practices on the agricultural land in Asia. Int. Soil Water Conserv. Res. 2020, 8, 103–115. [Google Scholar] [CrossRef]
- Kocira, A.; Staniak, M.; Tomaszewska, M.; Kornas, R.; Cymerman, J.; Panasiewicz, K.; Lipińska, H. Legume Cover Crops as One of the Elements of Strategic Weed Management and Soil Quality Improvement. A Review. Agriculture 2020, 10, 394. [Google Scholar] [CrossRef]
- Bekeko, Z. Effect of Maize Stover Application as Soil Mulch on Yield of Arabica coffee (Coffee arabica L., Rubiaceae) at Western Hararghe Zone, Eastern Ethiopia. SAR 2013, 2, 15. [Google Scholar] [CrossRef]
- Wezel, A.; Rath, T. Resource conservation strategies in agro-ecosystems of semi-arid West Africa. J. Arid Environ. 2002, 51, 383–400. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K. Agronomy and Physiology of Tropical Cover Crops. J. Plant Nutr. 2007, 30, 1287–1339. [Google Scholar] [CrossRef]
- Praharaj, S.; Maitra, S. Importance of Legumes in Agricultural Production System: An Overview. Agro Econ. 2020, 7, 69–71. [Google Scholar]
- Tully, K.L.; McAskill, C. Promoting soil health in organically managed systems: A review. Org. Agric. 2020, 10, 339–358. [Google Scholar] [CrossRef]
- Diacono, M.; Trinchera, A.; Montemurro, F. An Overview on Agroecology and Organic Agriculture Strategies for Sustainable Crop Production. Agronomy 2021, 11, 223. [Google Scholar] [CrossRef]
- Vasconcelos, M.W.; Grusak, M.A.; Pinto, E.; Gomes, A.; Ferreira, H.; Balázs, B.; Centofanti, T.; Ntatsi, G.; Savvas, D.; Karkanis, A.; et al. The Biology of Legumes and Their Agronomic, Economic, and Social Impact. In The Plant Family Fabaceae; Hasanuzzaman, M., Araújo, S., Gill, S.S., Eds.; Springer: Singapore, 2020; pp. 3–25. ISBN 9789811547515. [Google Scholar]
- Nzeyimana, I.; Hartemink, A.E.; Ritsema, C.; Mbonigaba, J.J.M.; Geissen, V. Mulching effects on soil nutrient levels and yield in coffee farming systems in Rwanda. Soil Use Manag. 2020, 36, 58–70. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Vaast, P.; Pagella, T.; Sinclair, F. Local Knowledge about Ecosystem Services Provided by Trees in Coffee Agroforestry Practices in Northwest Vietnam. Land 2020, 9, 486. [Google Scholar] [CrossRef]
- Meza, K.; Vanek, S.J.; Sueldo, Y.; Olivera, E.; Ccanto, R.; Scurrah, M.; Fonte, S.J. Grass–Legume Mixtures Show Potential to Increase Above- and Belowground Biomass Production for Andean Forage-Based Fallows. Agronomy 2022, 12, 142. [Google Scholar] [CrossRef]
- Ekyaligonza, D.M.; Kahigwa, T.T.; Dietrich, P.; Akoraebirungi, B.; Kagorora, J.P.; Friedel, J.K.; Melcher, A.; Freyer, B. Biomass contribution and nutrient recycling of organic matter management practices in tropical smallholder annual farming systems. Acta Agric. Scand. Sect. B Soil Plant Sci. 2022, 72, 945–956. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Osei, B.A.; Bonsu, M. Regenerative capacity and factors influencing the management of Mucuna pruriens var. utilis live-mulch. Ghana J. Agric. Sci. 2008, 40, 51–58. [Google Scholar] [CrossRef]
- Muoni, T.; Öborn, I.; Mhlanga, B.; Okeyo, I.; Mutemi, M.; Duncan, A. The Role of Mucuna pruriens in Smallholder Farming Systems of Eastern and Southern Africa: A Review. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 485–498. ISBN 978-981-329-782-1. [Google Scholar]
- Kaizzi, C.K.; Ssali, H.; Vlek, P.L.G. Differential use and benefits of Velvet bean (Mucuna pruriens var. utilis) and N fertilizers in maize production in contrasting agro-ecological zones of E. Uganda. Agric. Syst. 2006, 88, 44–60. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Agro-ecological approaches to enhance resilience. Farming Matters 2012, 28, 14–17. [Google Scholar]
- Hauser, S.; Nolte, C. Biomass production and N fixation of five Mucuna pruriens varieties and their effect on maize yields in the forest zone of Cameroon. J. Plant Nutr. Soil Sci. 2002, 165, 101–109. [Google Scholar] [CrossRef]
- Masikati, P.; Manschadi, A.; van Rooyen, A.; Hargreaves, J. Maize–mucuna rotation: An alternative technology to improve water productivity in smallholder farming systems. Agric. Syst. 2014, 123, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Carsky, R.J.; Becker, M.; Hauser, S. Mucuna Cover Crop Fallow Systems: Potential and limitations. In SSSA Special Publications; Tian, G., Ishida, F., Keatinge, D., Carsky, R., Wendt, J., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 2015; pp. 111–135. ISBN 978-0-89118-952-7. [Google Scholar]
- Barthès, B.; Azontonde, A.; Blanchart, E.; Girardin, C.; Villenave, C.; Lesaint, S.; Oliver, R.; Feller, C. Effect of a legume cover crop (Mucuna pruriens var. utilis) on soil carbon in an Ultisol under maize cultivation in southern Benin. Soil Use Manag. 2006, 20, 231–239. [Google Scholar] [CrossRef]
- Satriawan, H.; Harahap, E.M.; Karim, A. Effectiveness of Soil Conservation to Erosion Control on Several Land Use Types. Agric. (Polnohospodárstvo) 2015, 61, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Satriawan, H.; Fuady, Z.; Mayani, N. Soil Conservation by Vegetative Systems in Oil Palm Cultivation. PJSS 2017, 49, 223. [Google Scholar] [CrossRef] [Green Version]
- Mureithi, J.G.; Gachene, C.K.K.; Ojiem, J. The Role of Green Manure Legumes in Smallholder Farming Systems in Kenya: The Legume Research Network Project. Trop. Subtrop. Agroecosystems 2003, 1, 57–70. [Google Scholar]
- Pender, J.; Jagger, P.; Nkonya, E.; Sserunkuuma, D. Development Pathways and Land Management in Uganda: Causes and Implications. Res. Agric. Appl. Econ. 2002, 131, 16124. [Google Scholar]
- Makhaye, N.; Aremu, A.O.; Gruz, J.; Magadlela, A. Effects of soil nutrients and microbe symbiosis on the nutrient assimilation rates, growth carbon cost and phytochemicals in Mucuna pruriens (L.) DC. Acta Physiol. Plant 2021, 43, 153. [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Byakagaba, P. Soil organic carbon stocks under coffee agroforestry systems and coffee monoculture in Uganda. Agric. Ecosyst. Environ. 2016, 216, 188–193. [Google Scholar] [CrossRef]
- Lin, B.B. Agroforestry management as an adaptive strategy against potential microclimate extremes in coffee agriculture. Agric. For. Meteorol. 2007, 144, 85–94. [Google Scholar] [CrossRef]
- Vaast, P.; Harmand, J.-M.; Rapidel, B.; Jagoret, P.; Deheuvels, O. Coffee and Cocoa Production in Agroforestry—A Climate-Smart Agriculture Model. In Climate Change and Agriculture Worldwide; Torquebiau, E., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2016; pp. 209–224. ISBN 978-94-017-7460-4. [Google Scholar]
- De Carvalho, A.F.; Fernandes-Filho, E.I.; Daher, M.; Gomes, L.D.C.; Cardoso, I.M.; Fernandes, R.B.A.; Schaefer, C.E.G.R. Microclimate and soil and water loss in shaded and unshaded agroforestry coffee systems. Agroforest. Syst. 2021, 95, 119–134. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, A.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Reference and Selection Guide; Centre for Agriculture and Bioscience International: Wallingford, UK, 2009. [Google Scholar]
- Meunier, Q.; Lemmens, R.; Morin, A. Alternatives to Exotic Species in Uganda: Growth and Cultivation of 85 Indigenous Trees; French Embassy in Uganda: Kampala, Uganda, 2010; ISBN 978-9970-14-402-0. [Google Scholar]
- Bitariho, R.; Byamukama, A.; Akampurira, E. Socio-Economic and Ecological Baseline Impact Study of the Reclaimed Part of Sarambwe Nature Reserve after Border Reaffirmation; GVTC: New Braunfels, TX, USA, 2019. [Google Scholar]
- Ollis, W.D.; Rhodes, C.A.; Sutherland, I.O. The extractives of Millettia dura (Dunn). Tetrahedron 1967, 23, 4741–4760. [Google Scholar] [CrossRef]
- Kakudidi, E.K. A study of plant materials used for house construction around Kibale National Park, western Uganda. Afr. J. Ecol. 2007, 45, 22–27. [Google Scholar] [CrossRef]
- Banzouzi, J.T.; Prost, A.; Rajemiarim, M.; Ongoka, P. Traditional Uses of the African Millettia species (Fabaceae). Int. J. Bot. 2008, 4, 406–420. [Google Scholar] [CrossRef] [Green Version]
- Asfaw, Z. Growt of Millettia ferruginea and its impact on soil properties of three coffee plantations of southern Ethiopia. Ethiop. J. Nat. Resour. 2008, 10, 147–160. [Google Scholar]
- Nigussie, A.; Taye, E.; Bukero, G. Survey on Potentials and Constraints of Shade Tree Species for Arabica Coffee Production in South Ethiopia. Int. J. Recent Res. Life Sci. 2014, 1, 11. [Google Scholar]
- Jena, R.; Rath, D.; Rout, S.S.; Kar, D.M. A review on genus Millettia: Traditional uses, phytochemicals and pharmacological activities. Saudi Pharm. J. 2020, 28, 1686–1703. [Google Scholar] [CrossRef]
- Fischer, G.; Nachtergaele, F.; Prieler, S.; van Velthuizen, H.T.; Verelst, L.; Wiberg, D. Global Agro-Ecological Zones Assessment for Agriculture (GAEZ 2008); IIASA: Laxenburg, Austria; FAO: Rome, Italy, 2008. [Google Scholar]
- Grieser, J.; Gommes, R.; Bernardi, M. New LocClim—The Local Climate Estimator of FAO. Geophys. Res. Abstr. 2006, 8, 2. [Google Scholar]
- Blouin, D.C.; Webster, E.P.; Bond, J.A. On the Analysis of Combined Experiments. Weed Technol. 2011, 25, 165–169. [Google Scholar] [CrossRef]
- Petersen, R.G. Agricultural Field Experiments; CRC Press: Boca Raton, FL, USA, 1994; ISBN 978-1-4822-7737-1. [Google Scholar]
- Nyombi, K.; Ssebulime, G.; Kagezi, G.; Mpiira, S.; Byabagambi, S.; Tushemereirwe, W.; Kubiriba, J.; Karamura, E.; Staver, C. Canopy management, leaf fall and litter quality of dominant tree species in the banana agroforestry system in Uganda. AJFAND 2018, 18, 13154–13170. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, G.; Shen, W.; Liu, X. Cover as a simple predictor of biomass for two shrubs in Tibet. Ecol. Indic. 2016, 64, 266–271. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of coffee growth and production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef] [Green Version]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; Sacred Africa: Nairobi, Kenya, 2002. [Google Scholar]
- Cordingley, J. Soil Fertility Survey of Rwandan Coffee Sector; Crop Nutrition Laboratory Services: Nairobi, Kenya, 2009. [Google Scholar]
- Tié, T.B.; Diby, L.N.; Seyo, E.; Assa, A. Estimating soil available nitrogen with a hot H2O2/KCl extraction. Sci. Res. Essays 2010, 5, 1455–1462. [Google Scholar]
- Kovar, J.L.; Pierzynski, G.M. Southern Cooperative Series Bulletin; North Carolina State University: Raleigh, North Carolina, USA, 2009. [Google Scholar]
- Pawar, D.R.; Randhe, D.B.; Shaikh, M.; Korde, A.K.; Shinde, K.A.; Yadav, M.P.; Chavan, A.E.; Sapkale, V.M. Laboratory Testing Procedure for Soil & Water Sample Analysis; Government of Maharashtra: Maharashtra, India, 2009. [Google Scholar]
- Langton, S. Avoiding edge effects in agroforestry experiments; the use of neighbour-balanced designs and guard areas. Agroforest. Syst. 1990, 12, 173–185. [Google Scholar] [CrossRef]
- Beuchelt, T.D.; Zeller, M. Profits and poverty: Certification’s troubled link for Nicaragua’s organic and fairtrade coffee producers. Ecol. Econ. 2011, 70, 1316–1324. [Google Scholar] [CrossRef]
- Uddin, M.T.; Dhar, A.R.; Islam, M.M. Adoption of conservation agriculture practice in Bangladesh: Impact on crop profitability and productivity. J. Bangladesh Agric. Univ. 2016, 14, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Archontoulis, S.V.; Miguez, F.E. Nonlinear Regression Models and Applications in Agricultural Research. Agron. J. 2015, 107, 786–798. [Google Scholar] [CrossRef] [Green Version]
- Omeja, P.A.; Chapman, C.A.; Obua, J.; Lwanga, J.S.; Jacob, A.L.; Wanyama, F.; Mugenyi, R. Intensive tree planting facilitates tropical forest biodiversity and biomass accumulation in Kibale National Park, Uganda. For. Ecol. Manag. 2011, 261, 703–709. [Google Scholar] [CrossRef]
- Zaim, M.; Kumar, Y.; Hallan, V.; Zaidi, A.A. Velvet bean severe mosaic virus: A distinct begomovirus species causing severe mosaic in Mucuna pruriens (L.) DC. Virus Genes 2011, 43, 138–146. [Google Scholar] [CrossRef]
- Blanchart, E.; Villenave, C.; Viallatoux, A.; Barthès, B.; Girardin, C.; Azontonde, A.; Feller, C. Long-term effect of a legume cover crop (Mucuna pruriens var. utilis) on the communities of soil macrofauna and nematofauna, under maize cultivation, in southern Benin. Eur. J. Soil Biol. 2006, 42, S136–S144. [Google Scholar] [CrossRef]
- Sims, J.T.; Simard, R.R.; Joern, B.C. Phosphorus Loss in Agricultural Drainage: Historical Perspective and Current Research. J. Environ. Qual. 1998, 27, 277–293. [Google Scholar] [CrossRef] [Green Version]
- Addiscott, T.M.; Thomas, D. Tillage, mineralization and leaching: Phosphate. Soil Tillage Res. 2000, 53, 255–273. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Mancuso, M.A.C.; Soratto, R.P.; Crusciol, C.A.C.; Castro, G.S.A. Effect of potassium sources and rates on arabica coffee yield, nutrition, and macronutrient export. Rev. Bras. Ciênc. Solo 2014, 38, 1448–1456. [Google Scholar] [CrossRef]
- Melke, A.; Ittana, F. Nutritional Requirement and Management of Arabica Coffee (Coffea arabica L.) in Ethiopia: National and Global Perspectives. AJEA 2014, 5, 400–418. [Google Scholar] [CrossRef]
- Waller, J.M.; Bigger, M.; Hillocks, R.J. Coffee Pests, Diseases and Their Management; CABI Pub: Wallingford, UK; Cambridge, MA, USA, 2007; ISBN 978-1-84593-129-2. [Google Scholar]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Wairegi, L.; van Asten, P.; Giller, K.; Fairhurst, T. Banana-Coffee System Cropping Guide; Africa Soil Health Consortium: Muscle Shoals, AL, USA, 2016; p. 104. [Google Scholar]
- Sarmiento-Soler, A.; Vaast, P.; Hoffmann, M.P.; Rötter, R.P.; Jassogne, L.; van Asten, P.J.A.; Graefe, S. Water use of Coffea arabica in open versus shaded systems under smallholder’s farm conditions in Eastern Uganda. Agric. For. Meteorol. 2019, 266–267, 231–242. [Google Scholar] [CrossRef]
- Iijima, M.; Izumi, Y.; Yuliadi, E.; Sunyoto; Afandi; Utomo, M. Erosion Control on a Steep Sloped Coffee Field in Indonesia with Alley Cropping, Intercropped Vegetables, and No-Tillage. Plant Prod. Sci. 2003, 6, 224–229. [Google Scholar] [CrossRef]


| Date. | Activity | Activity Description | Treatment |
|---|---|---|---|
| 21 January 2018 | Weed control by hoe | All weeds were cleared and turned into the soil using a hand hoe. | T1, T2, T3 and T4 |
| 12 April 2018, 16 September 2018, 12 April 2019, 16 September 2019 | T4 | ||
| 3 July 2018, 4 December 2018, 3 July 2019, and 4 December 2019 | After sampling for biomass, all weeds were cleared and turned into the soil using a hand hoe. | T4 | |
| 12 February 2018 | Planting the different treatments | Treatments were randomly allocated to the plots and planted at the spacing and plant population indicated in Figure 3. | T1, T2, T3 |
| 16 July 2018, 12 February 2019, and 16 July 2019 | Mucuna pruriens was integrated into the specific plots where it had been planted in the first growing season. The spacing and seed rate is indicated in Figure 3. | T1 and T3 | |
| 12 April 2018, 16 September 2018, 12 April 2019, and 16 September 2019 | Spot weeding | Weeds were removed by hand and left on the ground surface as mulch within the respective plots. | T1, T2 and T3 |
| 3 July 2018 and 4 December 2018 | Sampling for biomass/harvesting | Above-ground biomass cover (excluding coffee trees and M. dura trees) in each plot was cut at the root collar and was returned to the respective plot as a surface mulch. | T1, T2, T3 and T4 |
| 3 July 2019 and 4 December 2019 | Above-ground biomass cover (excluding coffee trees and M. dura trees) in each plot was cut at the root collar. Millettia dura trees were pruned according to the guidelines by Nyombi et al. [77]. All cut material was returned to the respective plot as a surface mulch. |
| Treatment | Mean Difference in Biomass | Std. Error | t Value | Pr (>|t|) |
|---|---|---|---|---|
| T2-T1 | −1.59 | 0.06 | −27.53 | 0.00 |
| T3-T1 | 0.67 | 0.06 | 11.57 | 0.00 |
| T4-T1 | −2.09 | 0.06 | −36.22 | 0.00 |
| T3-T2 | 2.26 | 0.06 | 39.10 | 0.00 |
| T4-T2 | −0.50 | 0.06 | −8.69 | 0.00 |
| T4-T3 | −2.76 | 0.06 | −47.79 | 0.00 |
| Treatment | SR1 | LR1 | SR2 | LR2 |
|---|---|---|---|---|
| T1 | 3.57 (0.05) b | 4.06 (0.05) b | 3.78 (0.05) b | 3.32 (0.10) b |
| T2 | 1.41 (0.03) c | 1.62 (0.03) c | 2.46 (0.07) c | 2.88 (0.13) c |
| T3 | 3.88 (0.05) a | 4.53 (0.04) a | 4.41 (0.06) a | 4.58 (0.05) a |
| T4 | 1.43 (0.04) c | 1.60 (0.03) c | 1.59 (0.04) d | 1.74 (0.03) d |
| Treatment | % Change in Available Soil-Plant Nutrients | ||
|---|---|---|---|
| N | P | K | |
| T1 | 82.76 | −7.41 | 26.33 |
| T2 | 58.53 | −4.57 | 16.57 |
| T3 | 69.92 | −3.05 | 30.64 |
| T4 | −0.35 | −52.87 | −2.90 |
| Treatment | SR1 | LR1 | SR2 | LR2 |
|---|---|---|---|---|
| T1 | 0.47 (0.03) a | 0.96 (0.04) a | 1.07 (0.07) a | 1.54 (0.09) b |
| T2 | 0.34 (0.02) b c | 0.53 (0.03) b | 0.67 (0.05) b | 1.22 (0.06) c |
| T3 | 0.39 (0.04) a b | 0.95 (0.04) a | 1.16 (0.07) a | 1.91 (0.08) a |
| T4 | 0.24 (0.02) c | 0.37 (0.03) c | 0.43 (0.05) c | 0.57 (0.03) d |
| Table | Mean Difference in Profit | Std. Error | t Value | Pr (>|t|) |
|---|---|---|---|---|
| T2-T1 | −421.93 | 47.56 | −8.87 | 0.00 |
| T3-T1 | −148.20 | 47.56 | −3.12 | 0.01 |
| T4-T1 | −616.16 | 47.56 | −12.96 | 0.00 |
| T3-T2 | 273.73 | 47.56 | 5.76 | 0.00 |
| T4-T2 | −194.23 | 47.56 | −4.08 | 0.00 |
| T4-T3 | −467.95 | 47.56 | −9.84 | 0.00 |
| Treatment | SR1 | LR1 | SR2 | LR2 |
|---|---|---|---|---|
| Gross Profit ($ ha−1) | ||||
| T1 | −206.7 (21.78) a | 504.9 (30.15) a | 700.6 (48.82) a | 1047.5 (63.86) b |
| T2 | −1012.9 (17.48) c | 278.2 (25.45) b | 332.8 (40.28) b | 760.5 (47.51) c |
| T3 | −1122.3 (29.71) d | 475.1 (30.99) a | 764.4 (52.04) a | 1336.3 (60.37) a |
| T4 | −331.0 (19.99) b | −188.0 (24.60) c | −3.1 (33.15) c | 103.8 (23.01) d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tibasiima, T.K.; Ekyaligonza, D.M.; Kagorora, J.P.K.; Friedel, J.K.; Melcher, A.; Bwambale, B.; Akugizibwe, E.; Freyer, B. Impact of Integrating Annual and Perennial Legumes under Coffea arabica on Sloping Land. Sustainability 2023, 15, 2453. https://doi.org/10.3390/su15032453
Tibasiima TK, Ekyaligonza DM, Kagorora JPK, Friedel JK, Melcher A, Bwambale B, Akugizibwe E, Freyer B. Impact of Integrating Annual and Perennial Legumes under Coffea arabica on Sloping Land. Sustainability. 2023; 15(3):2453. https://doi.org/10.3390/su15032453
Chicago/Turabian StyleTibasiima, Thaddeo Kahigwa, Deous Mary Ekyaligonza, John Patrick Kanahe Kagorora, Jürgen Kurt Friedel, Andreas Melcher, Bosco Bwambale, Edwin Akugizibwe, and Bernhard Freyer. 2023. "Impact of Integrating Annual and Perennial Legumes under Coffea arabica on Sloping Land" Sustainability 15, no. 3: 2453. https://doi.org/10.3390/su15032453






