Potential Use of Bio-Oleogel as Phase Change Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Bio-Oleogel
2.2. Methods
2.2.1. Attenuated Total Reflectance Infrared Spectroscopy (ATR–IR)
2.2.2. Differential Scanning Calorimetry (DSC)
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Viscosity
3. Results and Discussion
3.1. Structural Characterization of Oleogel by ATR-IR
3.2. Analysis of Thermal Properties
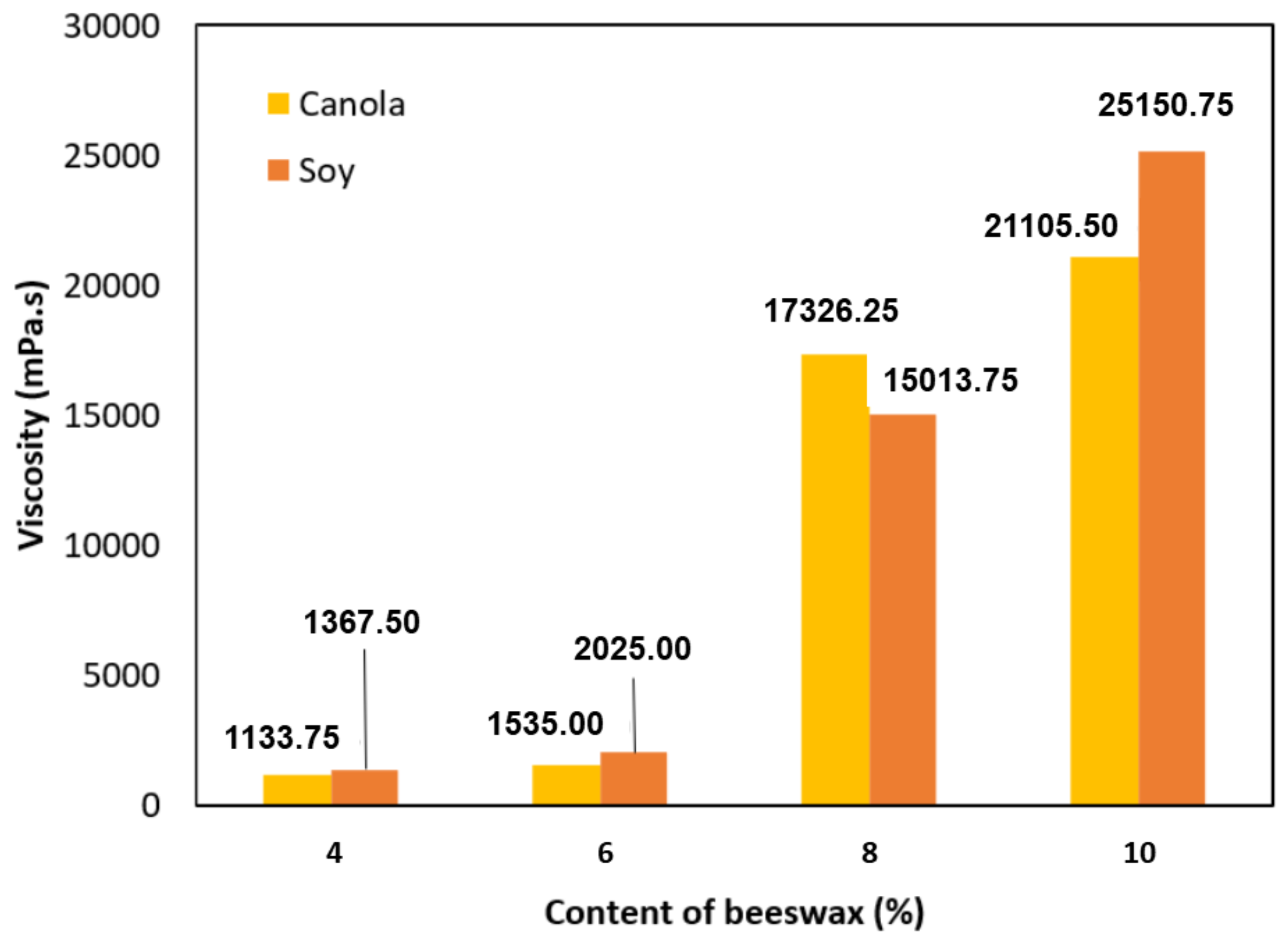
3.3. Viscosity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.; Rismanchi, B.; Ng, H.M.; Hasan, M.H.; Metselaar, H.S.; Muraza, O.; Aditiya, H.B. A review on insulation materials for energy conservation in buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Boer, D.; Segarra, M.; Fernández, A.I.; Vallès, M.; Mateu, C.; Cabeza, L.F. Approach for the analysis of TES technologies aiming towards a circular economy: Case study of building-like cubicles. Renew. Energy 2020, 150, 589–597. [Google Scholar] [CrossRef]
- Saxena, R.; Rakshit, D.; Kaushik, S.C. Phase change material (PCM) incorporated bricks for energy conservation in composite climate: A sustainable building solution. Sol. Energy 2019, 183, 276–284. [Google Scholar] [CrossRef]
- Shi, X.; Memon, S.A.; Tang, W.; Cui, H.; Xing, F. Experimental Assessment of Position of Macro Encapsulated Phase Change Material in Concrete Walls on Indoor Temperatures and Humidity Levels; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 71. [Google Scholar] [CrossRef]
- Stritih, U.; Butala, V. Experimental investigation of energy saving in buildings with PCM cold storage. Int. J. Refrig. 2010, 33, 1676–1683. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Li, L.; Zhao, M. Experimental assessment on the use of phase change materials (PCMs)-bricks in the exterior wall of a full-scale room. Energy Convers. Manag. 2016, 120, 81–89. [Google Scholar] [CrossRef]
- Da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Alam, T.E.; Dhau, J.S.; Goswami, D.Y.; Stefanakos, E. Macroencapsulation and characterization of phase change materials for latent heat thermal energy storage systems. Appl. Energy 2015, 154, 92–101. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Sivasamy, P.; Devaraju, A.; Harikrishnan, S. Review on Heat Transfer Enhancement of Phase Change Materials (PCMs). Mater. Today Proc. 2018, 5, 14423–14431. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.; Shang, M.; Zhong, Y. Research progress of packaging indicating materials for real-time monitoring of food quality. Mater. Express 2019, 9, 377–396. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Phase change materials for advanced cooling packaging. Environ. Chem. Lett. 2018, 16, 845–859. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, M.; Lee, Y.S. Temperature-regulating materials for advanced food packaging applications: A review. J. Food Meas. Charact. 2018, 12, 588–601. [Google Scholar] [CrossRef]
- Arjenaki, N.O.; Soltanizadeh, N.; Hamdami, N. Designing an active phase change material package for thermal and qualitative protection of meat. Food Packag. Shelf Life 2019, 21, 100362. [Google Scholar] [CrossRef]
- Hoang, H.M.; Leducq, D.; Pérez Masia, R.; Lagaron, J.; Alvarez, G. Heat transfer modelling of encapsulated phase change materials for food packaging. In Proceedings of the 3rd IIR International Conference on Sustainability and the Cold Chain, ICCC 2014, London, UK, 23–25 June 2014; International Institute of Refrigeration: London, UK, 2014; pp. 405–411. [Google Scholar]
- Hoang, H.; Leducq, D.; Pérez-Masia, R.; Lagaron, J.; Gogou, E.; Taoukis, P.; Alvarez, G. Heat transfer study of submicro-encapsulated PCM plate for food packaging application. Int. J. Refrig. 2015, 52, 151–160. [Google Scholar] [CrossRef]
- Leducq, D.; Ndoye, F.T.; Alvarez, G. ScienceDirect Phase change material for the thermal protection of ice cream during storage and transportation riau a changement de phase pour la protection Mat e me glac e e pendant l’entreposage et le thermique de cr e transport. Int. J. Refrig. 2014, 52, 133–139. [Google Scholar] [CrossRef]
- Melone, L.; Altomare, L.; Cigada, A.; De Nardo, L. Phase change material cellulosic composites for the cold storage of perishable products: From material preparation to computational evaluation. Appl. Energy 2012, 89, 339–346. [Google Scholar] [CrossRef]
- Ünal, M.; Konuklu, Y.; Paksoy, H. Thermal buffering effect of a packaging design with microencapsulated phase change material. Int. J. Energy Res. 2019, 43, 4495–4505. [Google Scholar] [CrossRef]
- Jeong, S.G.; Jeon, J.; Cha, J.; Kim, J.; Kim, S. Preparation and evaluation of thermal enhanced silica fume by incorporating organic PCM, for application to concrete. Energy Build. 2013, 62, 190–195. [Google Scholar] [CrossRef]
- Kabeel, A.E.; El-Samadony, Y.A.; El-Maghlany, W.M. Comparative study on the solar still performance utilizing different PCM. Desalination 2018, 432, 89–96. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A. Development and thermal performance of pumice/organic PCM/gypsum composite plasters for thermal energy storage in buildings. Sol. Energy Mater. Sol. Cells 2016, 149, 19–28. [Google Scholar] [CrossRef]
- Rasta, I.M.; Suamir, I.N. Study on Thermal Properties of Bio-PCM Candidates in Comparison with Propylene Glycol and Salt Based PCM for sub-Zero Energy Storage Applications. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012024. [Google Scholar] [CrossRef]
- Bot, A.; Veldhuizen, Y.S.; den Adel, R.; Roijers, E.C. Non-TAG structuring of edible oils and emulsions. Food Hydrocoll. 2009, 23, 1184–1189. [Google Scholar] [CrossRef]
- O’Sullivan, C.M.; Barbut, S.; Marangoni, A.G. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef]
- Pernetti, M.; van Malssen, K.; Kalnin, D.; Flöter, E. Structuring edible oil with lecithin and sorbitan tri-stearate. Food Hydrocoll. 2007, 21, 855–861. [Google Scholar] [CrossRef]
- Anonim. DIN 51005:2022-03. Thermal Analysis (TA)—Vocabulary; Text in German and English; Technical report; Anonim: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Anonim. DIN 51007:2019-04. Thermal Analysis—Differential Thermal Analysis (DTA) and Differential Scanning Calorimetry (DSC)—General Principles; Technical report; Anonim: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Gschwander, S.; Haussmann, T.; Hagelstein, G.; Sole, A.; Diarce, G.; Hohenauer, W.; Lager, D.; Rathgeber, C.; Hennemann, P.; Lazaro, A.; et al. IEA SHC Task 42/ECES Annex 29, DSC 4229 PCM Standard. Standard to Determine the Heat Storage Capacity of PCM Using hf-DSC with Constant Heating/Cooling Rate (Dynamic Mode))—A Technical Report of Subtask A2.1 of IEA-SHC 42/ECES Annex 29. Technical report, 2015. Available online: https://task42.iea-shc.org/Data/Sites/1/publications/Task4224-Standard-to-determine-the-heat-storage-capacity-of-PCM-vers150326.pdf (accessed on 15 December 2022).
- Rommel, M.; Hauer, A.; Helden, W. IEA SHC Task 42 / ECES Annex 29 Compact Thermal Energy Storage. Energy Procedia 2016, 91, 226–230. [Google Scholar] [CrossRef]
- Sam, M.N.; Caggiano, A.; Mankel, C.; Koenders, E. A comparative study on the thermal energy storage performance of bio-based and paraffin-based PCMs using DSC procedures. Materials 2020, 13, 1705. [Google Scholar] [CrossRef]
- Gendy, A.M.; Jun, H.W.; Kassem, A.A. In vitro release studies of flurbiprofen from different topical formulations. Drug Dev. Ind. Pharm. 2002, 28, 823–831. [Google Scholar] [CrossRef]
- Mukherjee, S.; Majee, S.B.; Biswas, G.R. Formulation and in vitro characterisation of soybean oil-hpmck4m based bigel matrix for topical drug delivery. Int. J. Appl. Pharm. 2019, 11, 33–38. [Google Scholar] [CrossRef]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; Prđun, S.; Bubalo, D.; Gajger, I.T. N approach for routine analytical detection of beeswax adulteration using ftir-atr spectroscopy. J. Apic. Sci. 2015, 59, 37–49. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, H.; Ma, J.; Tuchiya, T.; Miao, Y. Determination of the degree of degradation of frying rapeseed oil using fourier-transform infrared spectroscopy combined with partial least-squares regression. Int. J. Anal. Chem. 2015, 2015, 185367. [Google Scholar] [CrossRef] [PubMed]
- Lumakso, F.A.; Rohman, A.; Handoy, M.; Riyanto, S.; Yusuf, F.M. Detection and quantification of soybean and corn oils as adulterants in avocado oil using fourier transform mid infrared (FT-MIR) spectroscopy aided with multivariate calibration. J. Teknol. 2015, 77, 251–255. [Google Scholar] [CrossRef]
- Li, J.; Guo, R.; Wang, M.; Bi, Y.; Zhang, H.; Xu, X. Development and Characterization of Compound Oleogels Based on Monoglycerides and Edible Waxes. ACS Food Sci. Technol. 2022, 2, 302–314. [Google Scholar] [CrossRef]
- Gin, B.; Farid, M.M. The use of PCM panels to improve storage condition of frozen food. J. Food Eng. 2010, 100, 372–376. [Google Scholar] [CrossRef]
- Kozak, Y.; Farid, M.; Ziskind, G. Experimental and comprehensive theoretical study of cold storage packages containing PCM. Appl. Therm. Eng. 2017, 115, 899–912. [Google Scholar] [CrossRef]
- Schalbart, P.; Leducq, D.; Alvarez, G. Ice-cream storage energy efficiency with model predictive control of a refrigeration system coupled to a PCM tank. Int. J. Refrig. 2015, 52, 140–150. [Google Scholar] [CrossRef]
- Rojas, E.E.G.; Coimbra, J.S.; Telis-Romero, J. Thermophysical Properties of Cotton, Canola, Sunflower and Soybean Oils as a Function of Temperature. Int. J. Food Prop. 2013, 16, 1620–1629. [Google Scholar] [CrossRef]
- Holey, S.A.; Sekhar, K.P.C.; Mishra, S.S.; Kanjilal, S.; Nayak, R.R. Effect of oil unsaturation and wax composition on stability, properties and food applicability of oleogels. J. Am. Oil Chem. Soc. 2021, 98, 1189–1203. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Schaake, R.; van Miltenburg, J.; de Kruif, C. Thermodynamic properties of the normal alkanoic acids I. Molar heat capacities of seven odd-numbered normal alkanoic acids. J. Chem. Thermodyn. 1982, 14, 763–769. [Google Scholar] [CrossRef]
- Feldman, D.; Shapiro, M.M.; Banu, D.; Fuks, C.J. Fatty acids and their mixtures as phase-change materials for thermal energy storage. Sol. Energy Mater. 1989, 18, 201–216. [Google Scholar] [CrossRef]
- Hawes, D.W.; Feldman, D.; Banu, D. Latent heat storage in building materials. Energy Build. 1993, 20, 77–86. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Himran, S.; Suwono, A.; Mansoori, G.A. Characterization of alkanes and paraffin waxes for application as phase change energy storage medium. Energy Sources 1994, 16, 117–128. [Google Scholar] [CrossRef]
- Paris, J.; Falardeau, M.; Villeneuve, C. Thermal storage by latent heat: A viable option for energy conservation in buildings. Energy Sources 1993, 15, 85–93. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Chen, C.R.; Dwivedi, S. Development of phase change materials for building applications. Energy Build. 2013, 64, 403–407. [Google Scholar] [CrossRef]
- Sari, A.; Sari, H.; Önal, A. Thermal properties and thermal reliability of eutectic mixtures of some fatty acids as latent heat storage materials. Energy Convers. Manag. 2004, 45, 365–376. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Eroǧlu, R.; Biçer, A. Erythritol tetra myristate and erythritol tetra laurate as novel phase change materials for low temperature thermal energy storage. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 1285–1295. [Google Scholar] [CrossRef]
- Barone, G.; Della Gatta, G.; Ferro, D.; Piacente, V. Enthalpies and entropies of sublimation, vaporization and fusion of nine polyhydric alcohols. J. Chem. Soc. Faraday Trans. 1990, 86, 75–79. [Google Scholar] [CrossRef]
- Aydin, A.A.; Aydin, A. High-chain fatty acid esters of 1-hexadecanol for low temperature thermal energy storage with phase change materials. Sol. Energy Mater. Sol. Cells 2012, 96, 93–100. [Google Scholar] [CrossRef]
- Haillot, D.; Bauer, T.; Kröner, U.; Tamme, R. Thermal analysis of phase change materials in the temperature range 120–150 °C. Thermochim. Acta 2011, 513, 49–59. [Google Scholar] [CrossRef]
- Da Silva, T.L.; Arellano, D.B.; Martini, S. Physical Properties of Candelilla Wax, Monoacylglycerols, and Fully Hydrogenated Oil Oleogels. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 797–811. [Google Scholar] [CrossRef]
- Da Silva, T.L.T.; Arellano, D.B.; Martini, S. Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Res. Int. 2019, 121, 900–909. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef]
- Yi, B.R.; Kim, M.J.; Lee, S.Y.; Lee, J.H. Physicochemical properties and oxidative stability of oleogels made of carnauba wax with canola oil or beeswax with grapeseed oil. Food Sci. Biotechnol. 2017, 26, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Öğütcü, M.; Arifoğlu, N.; Yılmaz, E. Preparation and characterization of virgin olive oil-beeswax oleogel emulsion products. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Putra, N.; Prawiro, E.; Amin, M. Thermal properties of beeswax/CuO nano phase-change material used for thermal energy storage. Int. J. Technol. 2016, 7, 244–253. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative Study on the Ability of Different Oleogelators to Structure Sunflower Oil. Food Biophys. 2020, 15, 42–49. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Castellón, C.; Günther, E.; Mehling, H.; Hiebler, S.; Cabeza, L.F. Determination of the enthalpy of PCM as a function of temperature using a heat-flux DSC—A study of different measurement procedures and their accuracy. Int. J. Energy Res. 2008, 32, 1258–1265. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F. Palm oil-based bio-PCM for energy efficient building applications: Multipurpose thermal investigation and life cycle assessment. J. Energy Storage 2020, 28, 101129. [Google Scholar] [CrossRef]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of the PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17–21. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B. Differential scanning calorimetric analysis of edible oils: Comparison of thermal properties and chemical composition. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 143–155. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.J.; Stamatiou, A.; Worlitschek, J. Investigation of lactones as innovative bio-sourced phase change materials for latent heat storage. Molecules 2019, 24, 1300. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. International Journal of Heat and Mass Transfer Experimental investigation of the effects of mass fraction and temperature on the viscosity of microencapsulated PCM slurry. Int. J. Heat Mass Transf. 2018, 126, 390–399. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental research of viscosity of microencapsulated PCM slurry at the phase change temperature. Int. J. Heat Mass Transf. 2019, 134, 1209–1217. [Google Scholar] [CrossRef]
- Ferrer, G.; Barreneche, C.; Palacios, A.; Solé, A.; Fernández, A.I.; Cabeza, L.F. Empirical equations for viscosity and specific heat capacity determination of fatty acids. J. Energy Storage 2017, 10, 20–27. [Google Scholar] [CrossRef]
- Ferrer, G.; Gschwander, S.; Solé, A.; Barreneche, C.; Fernández, A.I.; Schossig, P.; Cabeza, L.F. Empirical equation to estimate viscosity of paraffin. J. Energy Storage 2017, 11, 154–161. [Google Scholar] [CrossRef]
- Huang, L.; Doetsch, C.; Pollerberg, C. Low temperature paraffin phase change emulsions. Int. J. Refrig. 2010, 33, 1583–1589. [Google Scholar] [CrossRef]
- Mu, M.; Basheer, P.A.M.; Sha, W.; Bai, Y.; Mcnally, T. Shape stabilised phase change materials based on a high melt viscosity HDPE and paraffin waxes. Appl. Energy 2016, 162, 68–82. [Google Scholar] [CrossRef]
- Wang, F.; Fang, X.; Zhang, Z. Preparation of phase change material emulsions with good stability and little supercooling by using a mixed polymeric emulsifier for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 381–390. [Google Scholar] [CrossRef]
- Rudra Murthy, V.B.; Gumtapure, V. Thermo-physical analysis of natural shellac wax as novel bio-phase change material for thermal energy storage applications. J. Energy Storage 2020, 29, 101390. [Google Scholar] [CrossRef]
- Calligaris, S.; Mirolo, G.; Da Pieve, S.; Arrighetti, G.; Nicoli, M.C. Effect of Oil Type on Formation, Structure and Thermal Properties of γ-oryzanol and β-sitosterol-Based Organogels. Food Biophys. 2014, 9, 69–75. [Google Scholar] [CrossRef]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M. A Novel Propolis Wax-Based Organogel: Effect of Oil Type on Its Formation, Crystal Structure and Thermal Properties. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 47–55. [Google Scholar] [CrossRef]
- Laredo, T.; Barbut, S.; Marangoni, A.G. Molecular interactions of polymer oleogelation. Soft Matter 2011, 7, 2734–2743. [Google Scholar] [CrossRef]

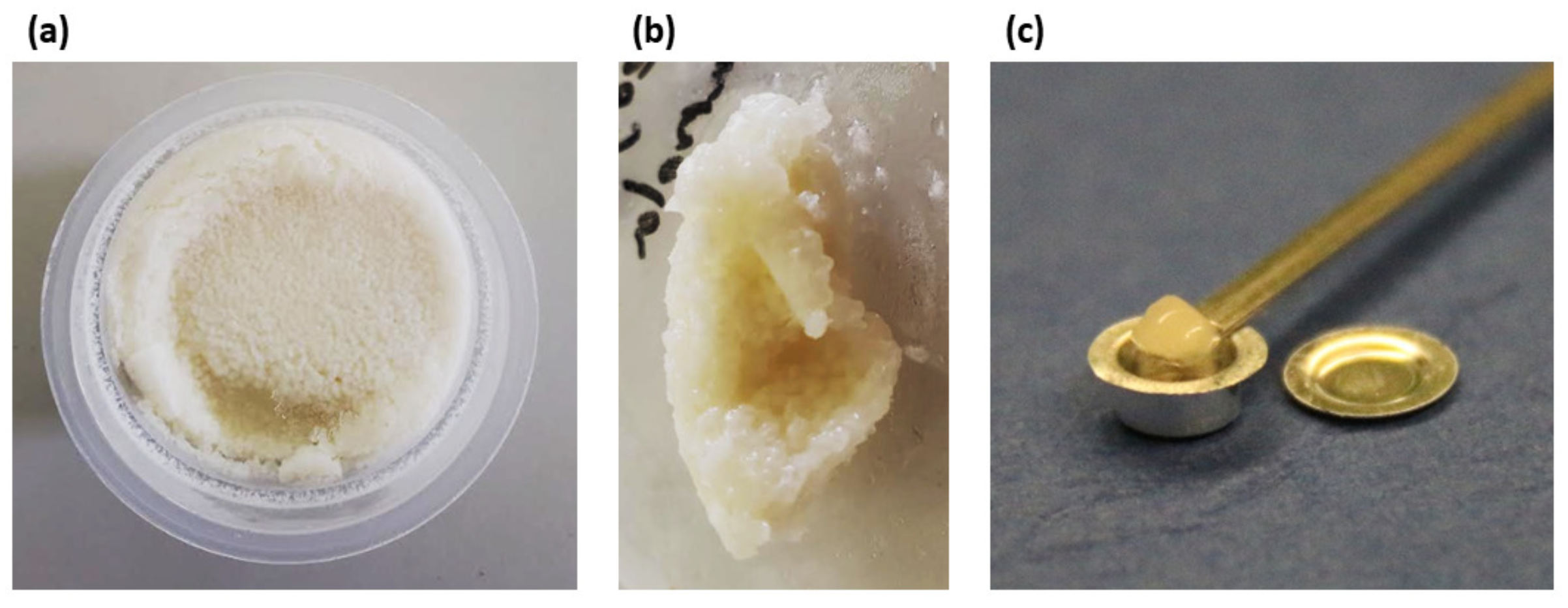
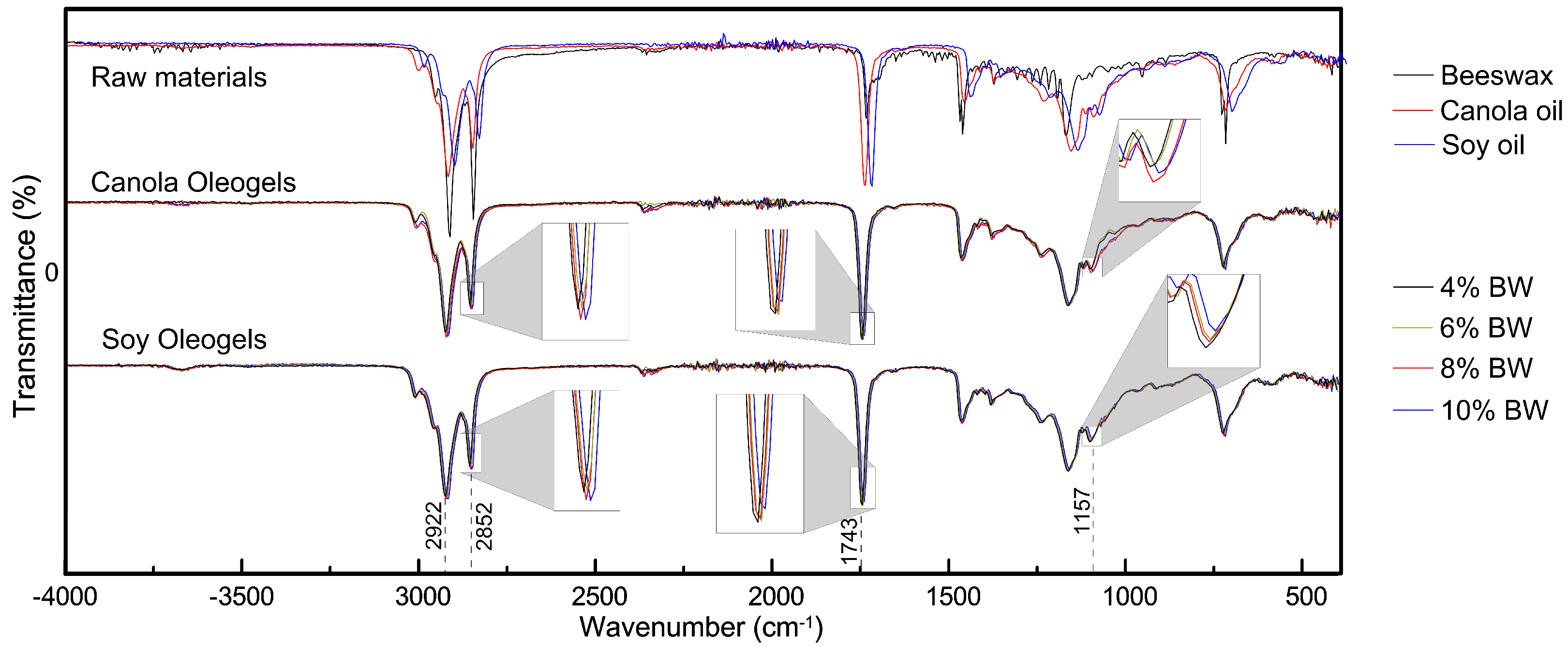



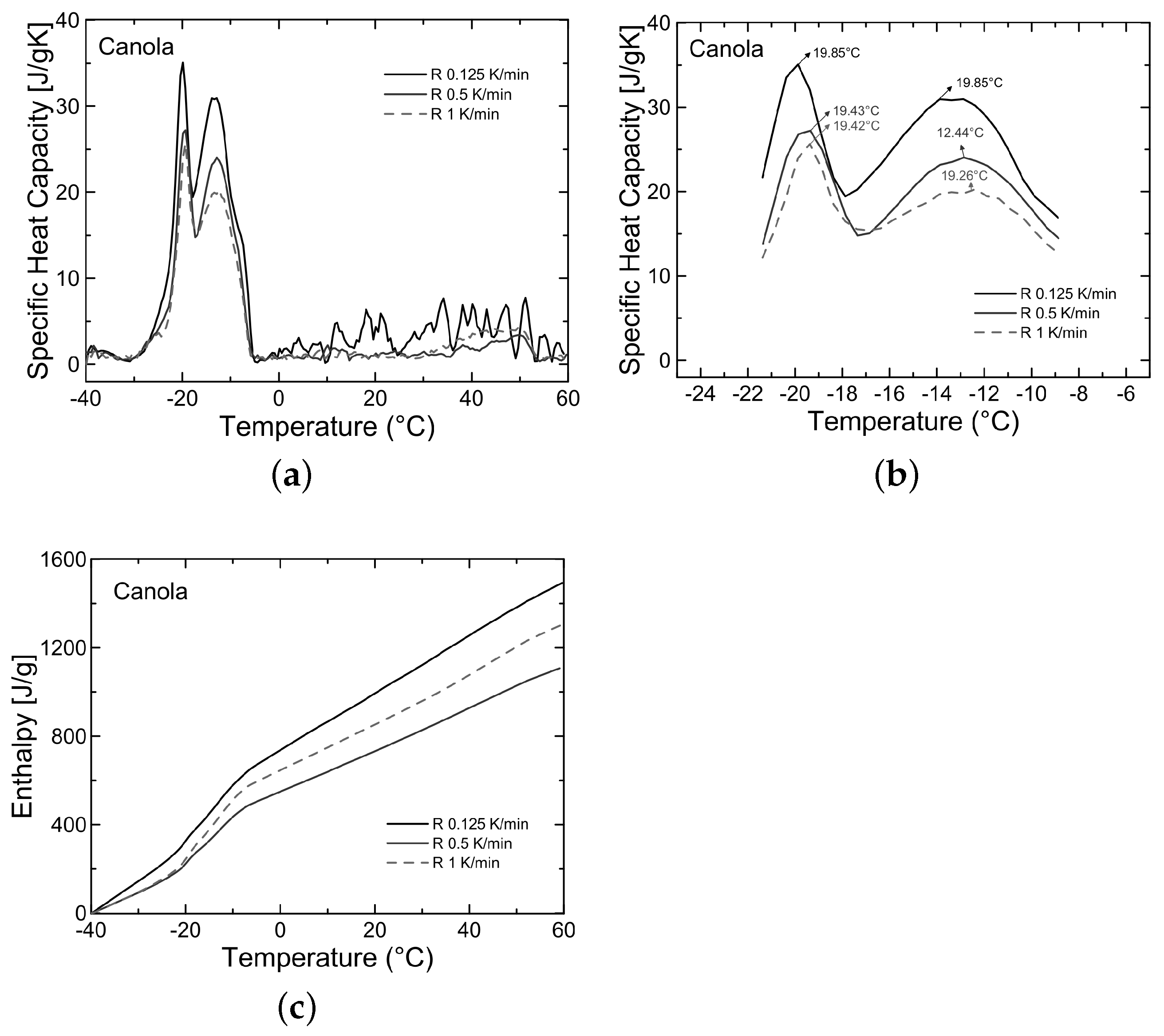
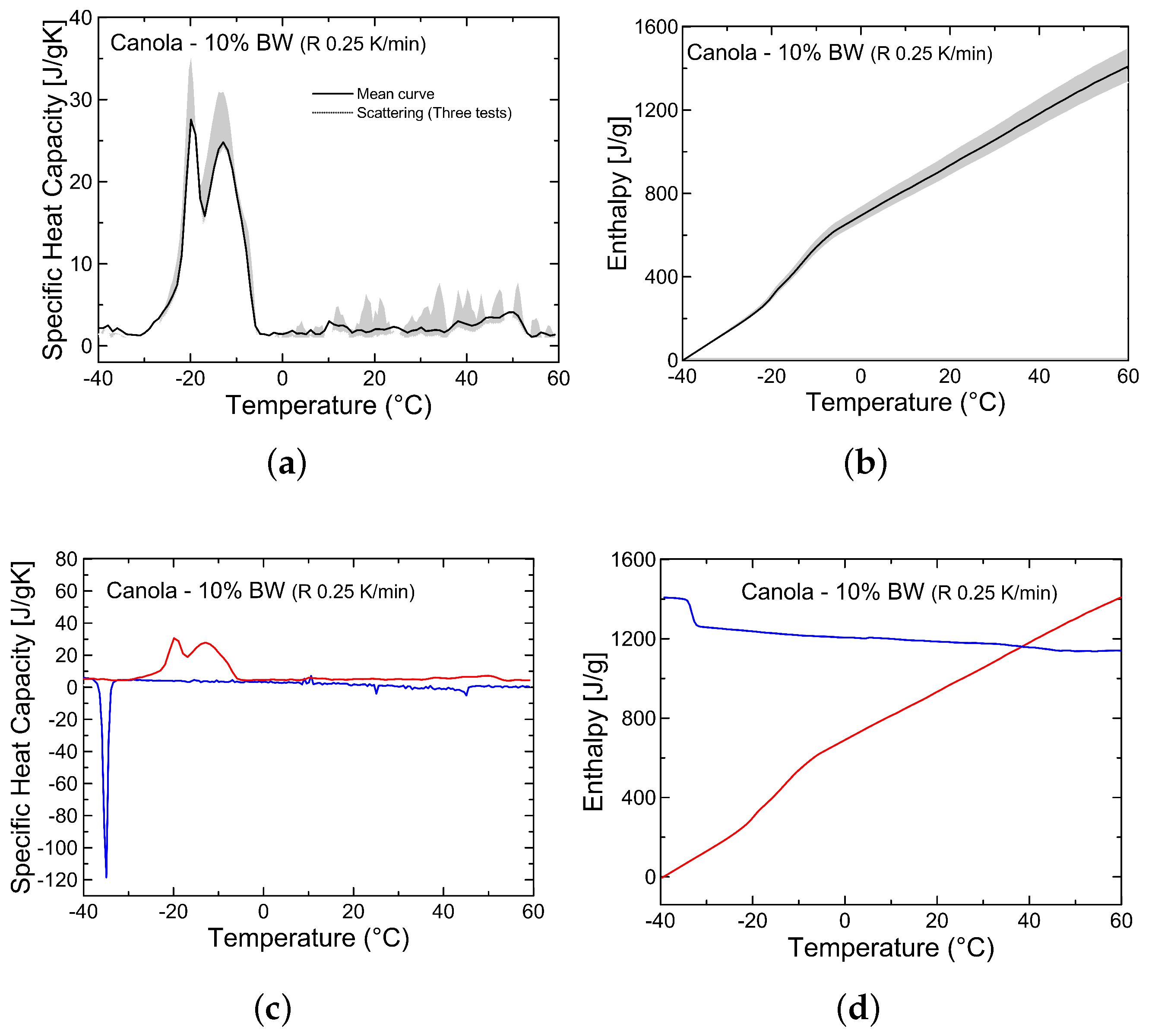
| Oleogels | Beeswax (%) | Soybean (%) | Canola (%) |
|---|---|---|---|
| BS4 | 4 | 96 | - |
| BS6 | 6 | 94 | - |
| BS8 | 8 | 92 | - |
| BS10 | 10 | 90 | - |
| BC4 | 4 | - | 96 |
| BC6 | 6 | - | 94 |
| BC8 | 8 | - | 92 |
| BC10 | 10 | - | 90 |
| PCM | Sample Mass (mg) | Total Latent Heat (J/g) | Melting Temperature (Tm) |
|---|---|---|---|
| Canola 10% BW | 14 | 367.6 | −13.6 |
| Soybean 10% BW | 10 | 22.9 | −16.7 |
| Beeswax (%) | Sample Mass (mg) | Latent Heat (J/g) |
|---|---|---|
| 4 | 23.2 | 112.5 |
| 6 | 23.3 | 251.8 |
| 8 | 17.4 | 334.1 |
| Heating Rate (K/min) | Sample Mass (mg) | Latent HEAT (J/g) |
|---|---|---|
| 0.25 | 19.2 | 128.7 |
| 0.5 | 19.1 | 298.2 |
| 1 | 19.2 | 359.4 |
| PCM | h (J/g) | Tm Peak 1/ Peak 2 (°C) | Tm Onset (°C) | Tm Offset (°C) | Tc (°C) | Tc Onset (°C) | Tc Offset (°C) |
|---|---|---|---|---|---|---|---|
| BC10 (Canola 10% BW) | 367.6 | −19.9/−13.6 | −24.6 | −4.1 | 168.1 | −36.8 | −34.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, N.D.; Kuhn, L.S.; Guimarães, T.C.; Sam, M.N.; Mankel, C.; Caggiano, A.; Koenders, E.; Nunes, C.A.; Ferreira, S.R. Potential Use of Bio-Oleogel as Phase Change Material. Sustainability 2023, 15, 2534. https://doi.org/10.3390/su15032534
Lorenzo ND, Kuhn LS, Guimarães TC, Sam MN, Mankel C, Caggiano A, Koenders E, Nunes CA, Ferreira SR. Potential Use of Bio-Oleogel as Phase Change Material. Sustainability. 2023; 15(3):2534. https://doi.org/10.3390/su15032534
Chicago/Turabian StyleLorenzo, Natasha Dantas, Leonardo Seibert Kuhn, Túlio Caetano Guimarães, Mona Nazari Sam, Christoph Mankel, Antonio Caggiano, Eduardus Koenders, Cleiton Antonio Nunes, and Saulo Rocha Ferreira. 2023. "Potential Use of Bio-Oleogel as Phase Change Material" Sustainability 15, no. 3: 2534. https://doi.org/10.3390/su15032534
APA StyleLorenzo, N. D., Kuhn, L. S., Guimarães, T. C., Sam, M. N., Mankel, C., Caggiano, A., Koenders, E., Nunes, C. A., & Ferreira, S. R. (2023). Potential Use of Bio-Oleogel as Phase Change Material. Sustainability, 15(3), 2534. https://doi.org/10.3390/su15032534








