Importance of Blue Carbon in Mitigating Climate Change and Plastic/Microplastic Pollution and Promoting Circular Economy
Abstract
:1. Introduction
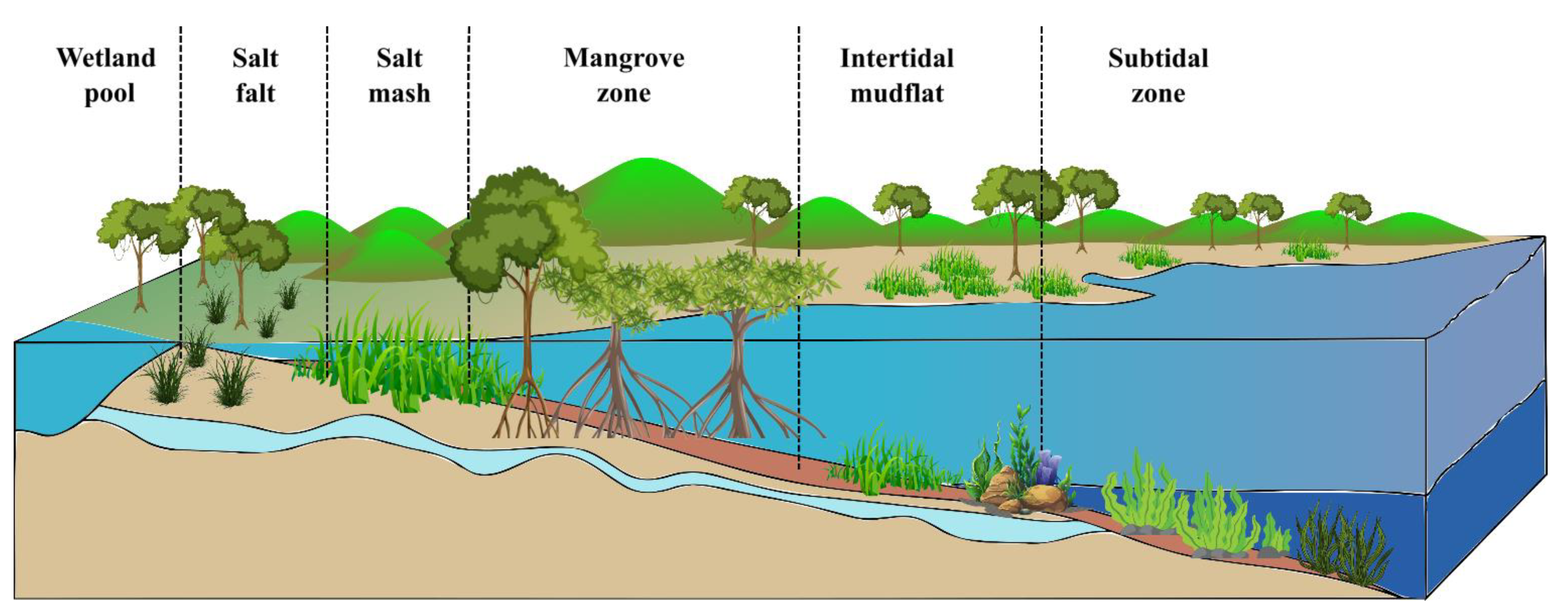
2. Spatiotemporal Distribution of Blue Carbon Ecosystems
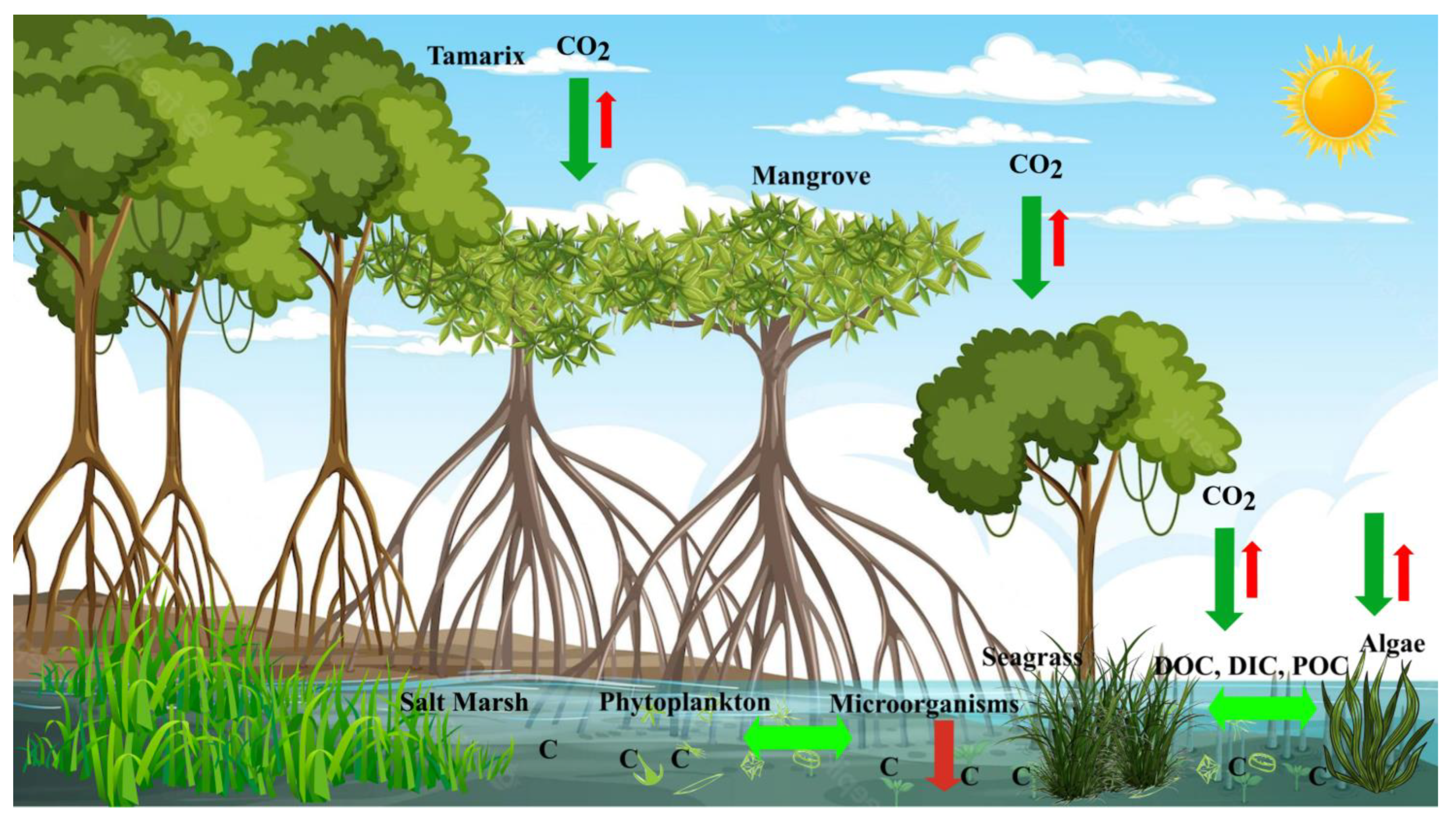
3. Role of Blue Carbon Ecosystems
3.1. Role of Blue Carbon Ecosystems in Mitigation of Climate Change
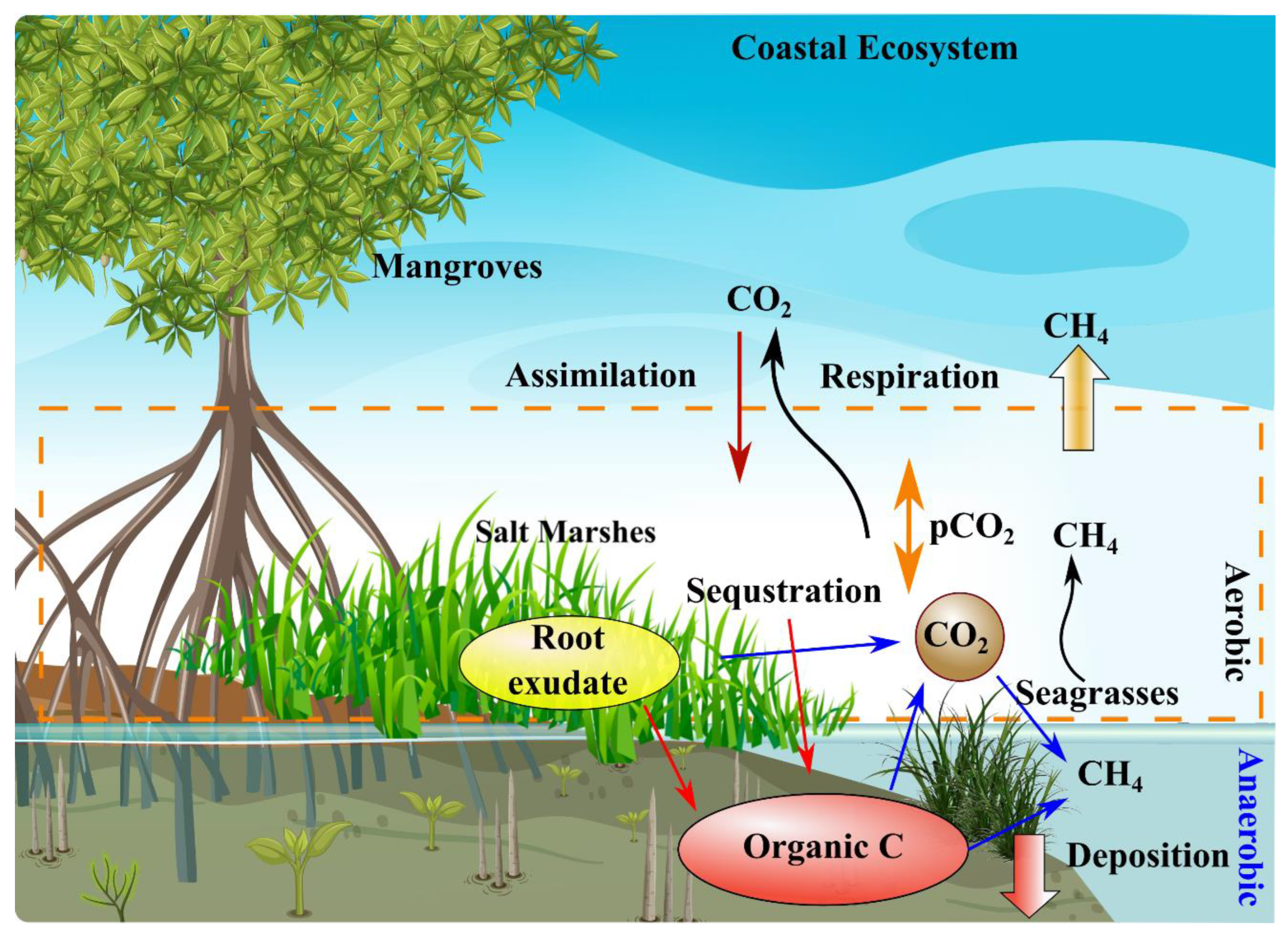
3.2. Blue Carbon Ecosystems as Carbon Sequestration and Sinks
3.3. Role of Blue Carbon Ecosystems in Mitigation of Microplastic Pollution
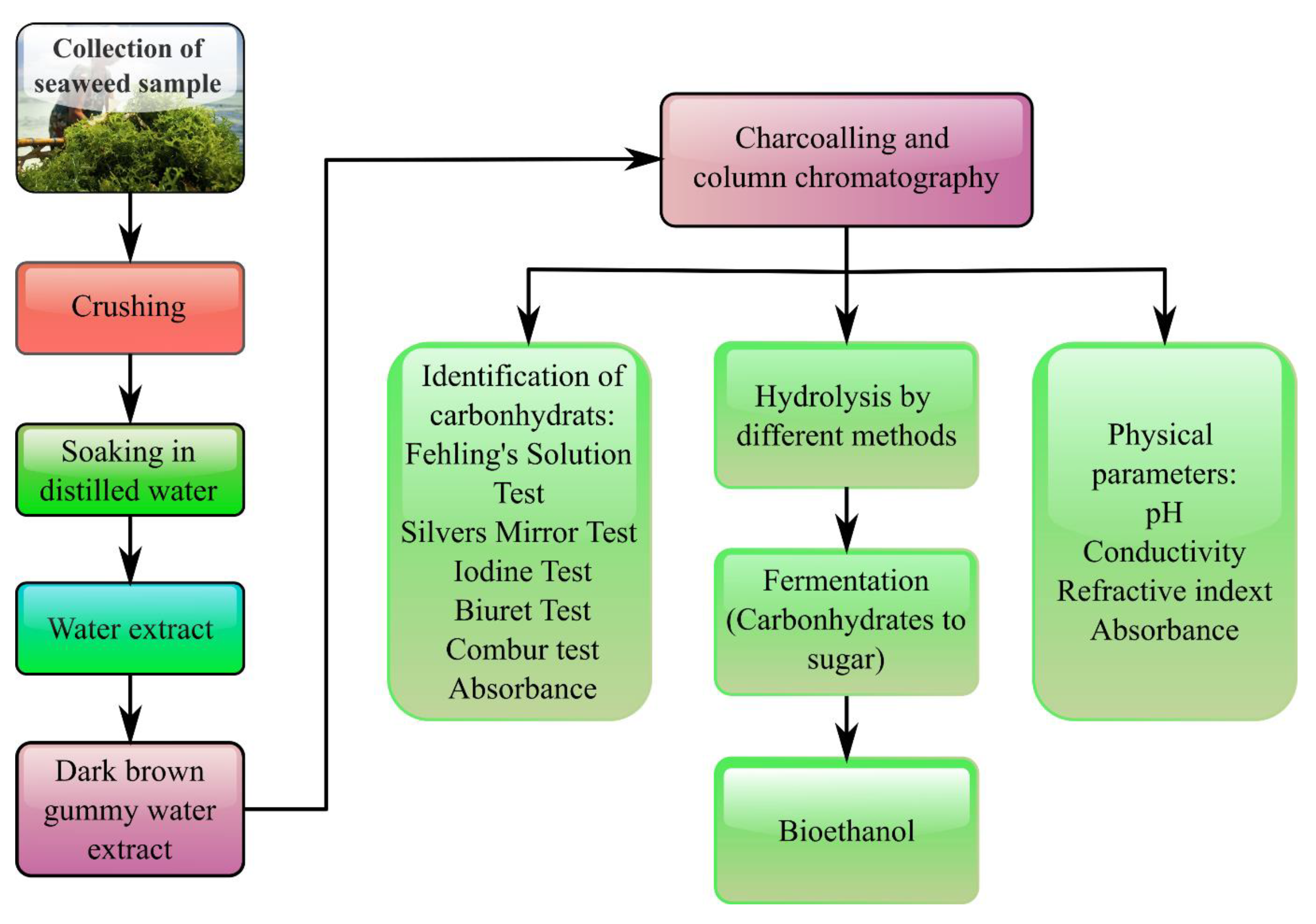
4. Blue Carbon as a Potential Source for Biofuel Production
5. Global Blue Carbon Framework for Climate Change Mitigation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdrés, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon: The Role of Healthy Oceans in Binding Carbon. A Rapid Response Assessment; United Nations Environment Programme, GRID-Arendal: Nairobi, Kenya, 2009. [Google Scholar]
- Duarte, C.M.; Kennedy, H.; Marbà, N.; Hendriks, I. Assessing the capacity of seagrass meadows for carbon burial: Current limitations and future strategies. Ocean Coast. Manag. 2013, 83, 32–38. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Nguyen, H.-H.; McAlpine, C.; Pullar, D.; Johansen, K.; Duke, N.C. The relationship of spatial–temporal changes in fringe mangrove extent and adjacent land-use: Case study of Kien Giang coast, Vietnam. Ocean Coast. Manag. 2013, 76, 12–22. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Cubit, J.D.; Keller, B.D.; Batista, V.; Burns, K.; Caffey, H.M. Ecological Effects of a Major Oil Spill on Panamanian Coastal Marine Communities. Science 1989, 243, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mimura, N. Sea-level rise caused by climate change and its implications for society. Proc. Jpn. Acad. Ser. B 2013, 89, 281–301. [Google Scholar] [CrossRef] [PubMed]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- OzCoasts. About Conceptual Diagrams 2022. Available online: https://ozcoasts.org.au/conceptual-diagrams/introduction/ (accessed on 18 March 2022).
- Ruttenberg, B.; Granek, E. Bridging the marine–terrestrial disconnect to improve marine coastal zone science and management. Mar. Ecol. Prog. Ser. 2011, 434, 203–212. [Google Scholar] [CrossRef]
- Wylie, L.; Sutton-Grier, A.E.; Moore, A. Keys to successful blue carbon projects: Lessons learned from global case studies. Mar. Policy 2016, 65, 76–84. [Google Scholar] [CrossRef]
- Phang, V.X.H.; Chou, L.M.; Friess, D.A. Ecosystem carbon stocks across a tropical intertidal habitat mosaic of mangrove forest, seagrass meadow, mudflat and sandbar. Earth Surf. Process Landf. 2015, 40, 1387–1400. [Google Scholar] [CrossRef]
- Roberts, C.M.; O’Leary, B.C.; McCauley, D.J.; Cury, P.M.; Duarte, C.M.; Lubchenco, J. Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 6167–6175. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, T.; Krug, T.; Tanabe, K.; Srivastava, N.; Baasansuren, J.; Fukuda, M. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Herr, D.; Landis, E. Coastal blue carbon ecosystems. Opportunities for Nationally Determined Contributions. Policy Brief 2016, 1–28. [Google Scholar]
- Zhou, C.; Wong, K.; Zhao, J. Coastal Wetland Vegetation in Response to Global Warming and Climate Change. In Sea Level Rise and Coastal Infrastructure; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Beck, M.W.; Lange, G.M. Guidelines for Coastal and Marine Ecosystem Accounting: Incorporating the Protective Service Values of Coral Reefs and Mangroves in National Wealth Accounts, Wealth Accounting and Valuation of Ecosystem Services; World Bank: Washington, DC, USA, 2015; pp. 1–15. [Google Scholar]
- Narayan, S.; Beck, M.W.; Reguero, B.G.; Losada, I.J.; van Wesenbeeck, B.; Pontee, N. The Effectiveness, Costs and Coastal Protection Benefits of Natural and Nature-Based Defences. PLoS ONE 2016, 11, e0154735. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Narayan, S.; Beck, M.W.; Wilson, P.; Thomas, C.; Guerrero, A.; Shepard, C. Coastal Wetlands and Flood Damage Reduction. Using Risk Industry-Based Models to Assess Natural Defenses in the Northeastern USA; Loyd’s Tercentenary Research Foundation: London, UK, 2012; pp. 1–23. [Google Scholar] [CrossRef]
- Alongi, D. The Energetics of Mangrove Forests, 1st ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Bryan, T.; Virdin, J.; Vegh, T.; Kot, C.Y.; Cleary, J.; Halpin, P.N. Blue carbon conservation in West Africa: A first assessment of feasibility. J. Coast. Conserv. 2020, 24, 8. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Alongi, D.M. The Impact of Climate Change on Mangrove Forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]
- Woodroffe, C.D.; Rogers, K.; McKee, K.L.; Lovelock, C.E.; Mendelssohn, I.A.; Saintilan, N. Mangrove Sedimentation and Response to Relative Sea-Level Rise. Ann. Rev. Mar. Sci. 2016, 8, 243–266. [Google Scholar] [CrossRef]
- Hoque, M.M.; Abu Hena, M.K.; Ahmed, O.H.; Idris, M.H.; Hoque, A.T.M.R.; Billah, M.M. Can mangroves help combat sea level rise through sediment accretion and accumulation? Malaysian J. Sci. 2015, 34, 78–86. [Google Scholar] [CrossRef]
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Chow, J. Mangrove management for climate change adaptation and sustainable development in coastal zones. J. Sustain. For. 2018, 37, 139–156. [Google Scholar] [CrossRef]
- Ramesh, R.; Banerjee, K.; Paneerselvam, A.; Raghuraman, R.; Purvaja, R.; Lakshmi, A. Importance of Seagrass Management for Effective Mitigation of Climate Change. Coast. Manag. 2019, 283–299. [Google Scholar] [CrossRef]
- Duarte, C.M. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences 2017, 14, 301–310. [Google Scholar] [CrossRef]
- Ricart, A.M.; York, P.H.; Bryant, C.V.; Rasheed, M.A.; Ierodiaconou, D.; Macreadie, P.I. High variability of Blue Carbon storage in seagrass meadows at the estuary scale. Sci. Rep. 2020, 10, 5865. [Google Scholar] [CrossRef]
- Charpy-Roubaud, C.; Sournia, A. The comparative estimation of phytoplanktonic, microphytobenthic and macrophytobenthic primary production in the oceans. Mar. Microb. Food Webs 1990, 4, 31–57. [Google Scholar]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Sundararaju, V. Why We Must Conserve the World’s Seagrasses; Wildlife Biodiversity: New Delhi, India, 2020. [Google Scholar]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S. Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef] [Green Version]
- Greiner, J.T.; McGlathery, K.J.; Gunnell, J.; McKee, B.A. Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLoS ONE 2013, 8, e72469. [Google Scholar] [CrossRef]
- Drake, K.; Halifax, H.; Adamowicz, S.C.; Craft, C. Carbon sequestration in tidal salt marshes of the Northeast United States. Environ. Manag. 2015, 56, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Poffenbarger, H.J.; Needelman, B.A.; Megonigal, J.P. Salinity influence on methane emissions from tidal marshes. Wetlands 2011, 31, 831–842. [Google Scholar] [CrossRef]
- Greenberg, R.; Maldonado, J.E.; Droege, S.A.M.; McDonald, M.V. Tidal marshes: A global perspective on the evolution and conservation of their terrestrial vertebrates. Bioscience 2006, 56, 675–685. [Google Scholar] [CrossRef]
- Wang, Z.A.; Kroeger, K.D.; Ganju, N.K.; Gonneea, M.E.; Chu, S.N. Intertidal salt marshes as an important source of inorganic carbon to the coastal ocean. Limnol. Oceanogr. 2016, 61, 1916–1931. [Google Scholar] [CrossRef]
- Mcowen, C.J.; Weatherdon, L.V.; Van Bochove, J.-W.; Sullivan, E.; Blyth, S.; Zockler, C. A global map of saltmarshes. Biodivers. Data J. 2017, 5, e11764. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Dennison, W.C.; Orth, R.J.W.; Carruthers, T.J.B. The Charisma of Coastal Ecosystems: Addressing the Imbalance. Estuaries Coasts 2008, 31, 233–238. [Google Scholar] [CrossRef]
- Doody, J.P. ‘Coastal squeeze’—An historical perspective. J. Coast. Conserv. 2004, 10, 129–138. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Huynh, T.T. Sorbent-based devices for the removal of spilled oil from water: A review. Environ. Sci. Pollut. Res. 2021, 28, 28876–28910. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, X.; Graham, T.; Wang, J. An analysis of factors affecting the severity of marine accidents. Reliab. Eng. Syst. Saf. 2021, 210, 107513. [Google Scholar] [CrossRef]
- Ghasemi, O.; Mehrdadi, N.; Baghdadi, M.; Aminzadeh, B.; Ghaseminejad, A. Spilled oil absorption from Caspian sea water by graphene/chitosan nano composite. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 2856–2872. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Spalding, M.D.; Ruffo, S.; Lacambra, C.; Meliane, I.; Hale, L.Z.; Shepard, C.C. The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean Coast. Manag. 2014, 90, 50–57. [Google Scholar] [CrossRef]
- Chisholm, J.R.M.; Barnes, D.J. Anomalies in coral reef community metabolism and their potential importance in the reef CO2 source-sink debate. Proc. Natl. Acad. Sci. USA 1998, 95, 6566–6569. [Google Scholar] [CrossRef] [PubMed]
- Kench, P.S.; Brander, R.W. Wave Processes on Coral Reef Flats: Implications for Reef Geomorphology Using Australian Case Studies. J. Coast. Res. 2006, 221, 209–223. [Google Scholar] [CrossRef]
- Monismith, S.G. Hydrodynamics of Coral Reefs. Annu. Rev. Fluid Mech. 2007, 39, 37–55. [Google Scholar] [CrossRef]
- Geraldi, N.R.; Ortega, A.; Serrano, O.; Macreadie, P.I.; Lovelock, C.E.; Krause-Jensen, D. Fingerprinting Blue Carbon: Rationale and Tools to Determine the Source of Organic Carbon in Marine Depositional Environments. Front. Mar. Sci. 2019, 6, 263. [Google Scholar] [CrossRef]
- Herr, D.; von Unger, M.; Laffoley, D.; McGivern, A. Pathways for implementation of blue carbon initiatives. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 116–129. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Reef, R. Variable Impacts of Climate Change on Blue Carbon. One Earth 2020, 3, 195–211. [Google Scholar] [CrossRef]
- García-Oliver, J.M.; Novella, R.; Micó, C.; Bin-Khalid, U. A numerical investigation of the performance of oxymethylene ethers blended with fossil diesel to reduce soot emissions in compression ignition engines. Fuel 2022, 324, 124768. [Google Scholar] [CrossRef]
- Bakır, H.; Ağbulut, Ü.; Gürel, A.E.; Yıldız, G.; Güvenç, U.; Soudagar, M.E.M. Forecasting of future greenhouse gas emissions trajectory for India using energy and economic indexes with various metaheuristic algorithms. J. Clean Prod. 2022, 360, 131946. [Google Scholar] [CrossRef]
- Wang, X.; Yan, L. Driving factors and decoupling analysis of fossil fuel related-carbon dioxide emissions in China. Fuel 2022, 314, 122869. [Google Scholar] [CrossRef]
- Chia, S.R.; Nomanbhay, S.; Ong, M.Y.; Bin, S.A.H.; Chew, K.W.; Show, P.L. Renewable diesel as fossil fuel substitution in Malaysia: A review. Fuel 2022, 314, 123137. [Google Scholar] [CrossRef]
- Szeto, W.; Leung, D.Y.C. Is hydrotreated vegetable oil a superior substitute for fossil diesel? A comprehensive review on physicochemical properties, engine performance and emissions. Fuel 2022, 327, 125065. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, M.; Anshika Gao, J.; Zhang, H.; Kota, S.H. Effect of restricted emissions during COVID-19 on air quality in India. Sci. Total Environ. 2020, 728, 138878. [Google Scholar] [CrossRef]
- Chowdhury, S.; Gaur, A.; Mohapatra, S.; Verma, S.; Mishra, S.; Dwivedi, G. Environmental pollution analysis during the lockdown imposed due to COVID-19: A case study. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 4679–4692. [Google Scholar] [CrossRef]
- Nguyen, X.P.; Hoang, A.T.; Ölçer, A.I.; Huynh, T.T. Record decline in global CO2 emissions prompted by COVID-19 pandemic and its implications on future. Energy Sources Part A Recover. Util. Environ. Eff. 2021. [CrossRef]
- Hampo, C.C.; Ojo, D.O.; Olatunde, D.E.; Isioma, O.J.; Oni, O.O.; Echendu, A.J. Life cycle assessment of renewable energy technologies in Northern Africa: A critical review. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 10248–10269. [Google Scholar] [CrossRef]
- Akarsu, B.; Serdar Genç, M. Optimization of electricity and hydrogen production with hybrid renewable energy systems. Fuel 2022, 324, 124465. [Google Scholar] [CrossRef]
- Huang, W.; Genaro Reivan Ortiz, G.; Kuo, Y.-L.; Maneengam, A.; Nassani, A.A.; Haffar, M. The Non-linear impact of renewable energy and trade on Consumption-based carbon emissions. Fuel 2022, 324, 124423. [Google Scholar] [CrossRef]
- Kuo, Y.; Maneengam, A.; Phan The, C.; Binh An, N.; Nassani, A.A.; Haffar, M. Fresh evidence on environmental quality measures using natural resources, renewable energy, non-renewable energy and economic growth for 10 Asian nations from CS-ARDL technique. Fuel 2022, 320, 123914. [Google Scholar] [CrossRef]
- Nochta, T.; Skelcher, C. Network governance in low-carbon energy transitions in European cities: A comparative analysis. Energy Policy 2020, 138, 111298. [Google Scholar] [CrossRef]
- Ramamoorthi, P.; Ramasamy, K.; Muthusamy, S. A variable wind harvesting based induction generator using variable voltage and variable frequency converter for renewable energy applications. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 8427–8444. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Esfilar, R.; Golestani, B.; Sadeghi Sadeghabad, M.; Biglaryan, M. Comparative study of seven agricultural wastes for renewable heat and power generation using integrated gasification combined cycle based on energy and exergy analyses. Fuel 2022, 317, 123430. [Google Scholar] [CrossRef]
- Hoang, A.T.; Sandro, N.i.ž.e.t.i.ć.; Olcer, A.I.; Ong, H.C.; Chen, W.-H.; Chong, C.T. Impacts of COVID-19 pandemic on the global energy system and the shift progress to renewable energy: Opportunities, challenges, and policy implications. Energy Policy 2021, 154, 112322. [Google Scholar] [CrossRef]
- Zhang, Y.; Anoopkumar, A.N.; Aneesh, E.M.; Pugazhendhi, A.; Binod, P.; Kuddus, M. Advancements in the energy-efficient brine mining technologies as a new frontier for renewable energy. Fuel 2023, 335, 127072. [Google Scholar] [CrossRef]
- Ayenew, E.; Berhanu, M. Application of artificial intelligent technique to maximize power yielding ability of wind turbine. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 2115–2132. [Google Scholar] [CrossRef]
- Mohanan, J.N.; Kumaravel, S.; Ashok, S. Diffuser augmented wind turbines—A case study for hybrid energy system applications and comparison with horizontal axis wind turbines. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 6807–6822. [Google Scholar] [CrossRef]
- Killingtveit, Å. Hydropower. In Managing Global Warming; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–315. [Google Scholar] [CrossRef]
- Kumar Singh, S.; Mittal, M.K.; Sharma, D. Thermal performance enhancement of basin-type solar still coupled with mini solar pond and shallow solar pond in closed-cycle mode. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 7237–7251. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the density and viscosity of biodiesel and the influence of biodiesel properties on a diesel engine fuel supply system. J. Mar. Eng. Technol. 2021, 20, 299–311. [Google Scholar] [CrossRef]
- Vávrová, K.; Solcova, O.; Knápek, J.; Weger, J.; Soukup, K.; Humešová, T. Economic evaluation of Hemp’s (Cannabis sativa) residual biomass for production of direct energy or biochar. Fuel 2022, 329, 125435. [Google Scholar] [CrossRef]
- Soltani, M.M.; Ahmadi, P.; Ashjaee, M. Techno-economic optimization of a biomass gasification energy system with Supercritical CO2 cycle for hydrogen fuel and electricity production. Fuel 2023, 333, 126264. [Google Scholar] [CrossRef]
- Hai, T.; Alsharif, S.; Aziz, K.H.H.; Dhahad, H.A.; Kumar Singh, P. Deep learning optimization of a biomass and biofuel-driven energy system with energy storage option for electricity, cooling, and desalinated water. Fuel 2023, 334, 126024. [Google Scholar] [CrossRef]
- Yang, C.; Kwon, H.; Bang, B.; Jeong, S.; Lee, U. Role of biomass as low-carbon energy source in the era of net zero emissions. Fuel 2022, 328, 125206. [Google Scholar] [CrossRef]
- Mridha, B.; Ramana, G.V.; Pareek, S.; Sarkar, B. An efficient sustainable smart approach to biofuel production with emphasizing the environmental and energy aspects. Fuel 2023, 336, 126896. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, G.; Yang, T.; Jia, Y.; He, N.; Zhuang, J. New insight into global blue carbon estimation under human activity in land-sea interaction area: A case study of China. Earth-Sci. Rev. 2016, 159, 36–46. [Google Scholar] [CrossRef]
- Taillardat, P.; Friess, D.A.; Lupascu, M. Mangrove blue carbon strategies for climate change mitigation are most effective at the national scale. Biol. Lett. 2018, 14, 20180251. [Google Scholar] [CrossRef]
- Gurjar, U.R.; Naidu, B.C.; Deshmukhe, G.; Xavier, K.A.M.; Jaiswar, A.K.; Nayak, B.B. Microplastics in Shrimps: A Case Study of Mumbai Waters. In Proceedings of the International Conference on Blue Economy & Aquatic Resources, Kerala, India, 28–30 November 2019. [Google Scholar]
- Sheehan, L.; Sherwood, E.T.; Moyer, R.P.; Radabaugh, K.R.; Simpson, S. Blue Carbon: An Additional Driver for Restoring and Preserving Ecological Services of Coastal Wetlands in Tampa Bay (Florida, USA). Wetlands 2019, 39, 1317–1328. [Google Scholar] [CrossRef]
- Connor, R. The United Nations World Water Development Report 2015: Water for a Sustainable World; UNESCO publishing: Paris, France, 2015; Volume 1. [Google Scholar]
- Zommers, Z.; van der Geest, K.; De Sherbinin, A.; Kienberger, S.; Roberts, E.; Harootunian, G. Loss and Damage: The Role of Ecosystem Services; United Nations Environment Programme: Nairobi, Kenya, 2016. [Google Scholar]
- Siikamäki, J.; Sanchirico, J.N.; Jardine, S.; McLaughlin, D.; Morris, D.F. Blue Carbon: Global Options for Reducing Emissions from the Degradation and Development of Coastal Ecosystems; Resoucres for the Future: Washington, DC, USA, 2012; pp. 1–74. [Google Scholar]
- Smoak, J.M.; Breithaupt, J.L.; Smith, T.J.; Sanders, C.J. Sediment accretion and organic carbon burial relative to sea-level rise and storm events in two mangrove forests in Everglades National Park. CATENA 2013, 104, 58–66. [Google Scholar] [CrossRef]
- Langley, J.A.; McKee, K.L.; Cahoon, D.R.; Cherry, J.A.; Megonigal, J.P. Elevated CO2 stimulates marsh elevation gain, counterbalancing sea-level rise. Proc. Natl. Acad. Sci. USA 2009, 106, 6182–6186. [Google Scholar] [CrossRef]
- López-Medellín, X.; Ezcurra, E.; González-Abraham, C.; Hak, J.; Santiago, L.S.; Sickman, J.O. Oceanographic anomalies and sea-level rise drive mangroves inland in the Pacific coast of Mexico. J. Veg. Sci. 2011, 22, 143–151. [Google Scholar] [CrossRef]
- Sanders, C.J.; Smoak, J.M.; Waters, M.N.; Sanders, L.M.; Brandini, N.; Patchineelam, S.R. Organic matter content and particle size modifications in mangrove sediments as responses to sea level rise. Mar. Environ. Res. 2012, 77, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Gonneea, M.E.; Maio, C.V.; Kroeger, K.D.; Hawkes, A.D.; Mora, J.; Sullivan, R. Salt marsh ecosystem restructuring enhances elevation resilience and carbon storage during accelerating relative sea-level rise. Estuar. Coast. Shelf Sci. 2019, 217, 56–68. [Google Scholar] [CrossRef]
- Mudd, S.M.; Howell, S.M.; Morris, J.T. Impact of dynamic feedbacks between sedimentation, sea-level rise, and biomass production on near-surface marsh stratigraphy and carbon accumulation. Estuar. Coast. Shelf Sci. 2009, 82, 377–389. [Google Scholar] [CrossRef]
- Traill, L.W.; Perhans, K.; Lovelock, C.E.; Prohaska, A.; McFallan, S.; Rhodes, J.R. Managing for change: Wetland transitions under sea-level rise and outcomes for threatened species. Divers. Distrib. 2011, 17, 1225–1233. [Google Scholar] [CrossRef]
- Oliver, T.S.N.; Rogers, K.; Chafer, C.J.; Woodroffe, C.D. Measuring, mapping and modelling: An integrated approach to the management of mangrove and saltmarsh in the Minnamurra River estuary, southeast Australia. Wetl. Ecol. Manag. 2012, 20, 353–371. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; Mackenzie, R.A. Impacts of climate change on mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Hickey, S.; Ball, M.C. Mangrove dieback during fluctuating sea levels. Sci. Rep. 2017, 7, 1680. [Google Scholar] [CrossRef]
- Howard, J.; Sutton-Grier, A.; Herr, D.; Kleypas, J.; Landis, E.; Mcleod, E. Clarifying the role of coastal and marine systems in climate mitigation. Front. Ecol. Environ. 2017, 15, 42–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cui, Q.; Fan, W.; Qi, J.; Chen, Y. Processes of coastal ecosystem carbon sequestration and approaches for increasing carbon sink. Sci. China Earth Sci. 2017, 60, 809–820. [Google Scholar] [CrossRef]
- Tang, J.; Ye, S.; Chen, X.; Yang, H.; Sun, X.; Wang, F. Coastal blue carbon: Concept, study method, and the application to ecological restoration. Sci. China Earth Sci. 2018, 61, 637–646. [Google Scholar] [CrossRef]
- Duarte, C.M.; Cebrián, J. The fate of marine autotrophic production. Limnol. Oceanogr. 1996, 41, 1758–1766. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Quintana-Alcantara, C.E. Carbon Sequestration in Tidal Salt Marshes and Mangrove Ecosystems. Master’s thesis, University of Sac Francisco, San Francisco, CA, USA, 2014. [Google Scholar]
- Abril, G.; Borges, A.V. Carbon Dioxide and Methane Emissions from Estuaries. In Greenhouse Gas Emissions—Fluxes and Processes—Hydroelectric Reservoirs and Natural Environments; Tremblay, A., Varfalvy, L., Roehm, C., Garneau, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 187–207. [Google Scholar] [CrossRef]
- Livesley, S.J.; Andrusiak, S.M. Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuar. Coast. Shelf Sci. 2012, 97, 19–27. [Google Scholar] [CrossRef]
- Thompson, K.; Miller, K.; Johnston, P.; Santillo, D. Storage of carbon by marine ecosystems and their contribution to climate change mitigation. Greenpeace Res. Lab. Tech. Rep. 2017, 3–2017. [Google Scholar]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Ann. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Ezcurra, P.; Ezcurra, E.; Garcillán, P.P.; Costa, M.T.; Aburto-Oropeza, O. Coastal landforms and accumulation of mangrove peat increase carbon sequestration and storage. Proc. Natl. Acad. Sci. USA 2016, 113, 4404–4409. [Google Scholar] [CrossRef]
- Fennessy, S.M.; Lei, G. Wetland restoration for climate change resilience. Ramsar Brief. Note 2018, 10, 1–11. [Google Scholar]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17, 1–12. [Google Scholar] [CrossRef]
- Heckbert, S.; Costanza, R.; Poloczanska, E.S.; Richardson, A.J. Climate Regulation as a Service from Estuarine and Coastal Ecosystems. In Treatise on Estuarine and Coastal Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 199–216. [Google Scholar] [CrossRef]
- Qiu, G.; Lin, H.-J.; Li, Z.-S.; Fan, H.-Q.; Zhou, H.-L.; Liu, G.-H. Seagrass ecosystems: Contributions to and mechanisms of carbon sequestration. Yingyong Shengtai Xuebao 2014, 25, 1825–1832. [Google Scholar] [PubMed]
- Kennedy, H.; Björk, M. Seagrass meadows. In The Management of Natural Coastal Carbon Sinks; Laffoley, D., Grimsditch, G., Eds.; IUCN: Gland, Switzerland, 2009; pp. 23–29. [Google Scholar]
- Hejnowicz, A.P.; Kennedy, H.; Rudd, M.A.; Huxham, M.R. Harnessing the climate mitigation, conservation and poverty alleviation potential of seagrasses: Prospects for developing blue carbon initiatives and payment for ecosystem service programmes. Front. Mar. Sci. 2015, 2, 32. [Google Scholar] [CrossRef]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 465–570. [Google Scholar]
- Epple, C.; García Rangel, S.; Jenkins, M.; Guth, M. Managing ecosystems in the context of climate change mitigation: A review of current knowledge and recommendations to support ecosystem-based mitigation actions that look beyond terrestrial forests. Tech. Ser. 2016, 55. [Google Scholar]
- Hill, R.; Bellgrove, A.; Macreadie, P.I.; Petrou, K.; Beardall, J.; Steven, A. Can macroalgae contribute to blue carbon? An Australian perspective. Limnol. Oceanogr. 2015, 60, 1689–1706. [Google Scholar] [CrossRef]
- Queirós, A.M.; Stephens, N.; Widdicombe, S.; Tait, K.; McCoy, S.J.; Ingels, J. Connected macroalgal-sediment systems: Blue carbon and food webs in the deep coastal ocean. Ecol. Monogr. 2019, 89, e01366. [Google Scholar] [CrossRef]
- Smith, S.V. Marine Macrophytes as a Global Carbon Sink. Science 1981, 211, 838–840. [Google Scholar] [CrossRef]
- Ortega, A.; Geraldi, N.R.; Alam, I.; Kamau, A.A.; Acinas, S.G.; Logares, R. Important contribution of macroalgae to oceanic carbon sequestration. Nat. Geosci. 2019, 12, 748–754. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Substantial blue carbon in overlooked Australian kelp forests. Sci. Rep. 2020, 10, 12341. [Google Scholar] [CrossRef]
- Trevathan-Tackett, S.M.; Kelleway, J.; Macreadie, P.I.; Beardall, J.; Ralph, P.; Bellgrove, A. Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology 2015, 96, 3043–3057. [Google Scholar] [CrossRef] [PubMed]
- Pessarrodona, A.; Moore, P.J.; Sayer, M.D.J.; Smale, D.A. Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Glob. Chang. Biol. 2018, 24, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Nur Hidayah, N.H.; Mohammad Rozaimi, M.R.; Mohd Nadzir, M.S. Sumber Penyumbang Karbon ke dalam Sedimen Dataran Rumput Laut di Muara Sungai Pulai, Johor, Malaysia. Sains Malays. 2019, 48, 2405–2413. [Google Scholar] [CrossRef]
- Rozaimi, M.; Lavery, P.S.; Serrano, O.; Kyrwood, D. Long-term carbon storage and its recent loss in an estuarine Posidonia australis meadow (Albany, Western Australia). Estuar. Coast. Shelf Sci. 2016, 171, 58–65. [Google Scholar] [CrossRef]
- Arina, N.; Raynusha, C.; Hidayah, N.; Zainee, N.F.A.; Prathep, A.; Rozaimi, M. Coralline macroalgae contribution to ecological services of carbon storage in a disturbed seagrass meadow. Mar. Environ. Res. 2020, 162, 105156. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.C.; Brzezinski, M.A. Kelp forests. In The Management of Natural Coastal Carbon Sinks; IUCN: Gland, Switzerland, 2009; p. 31. [Google Scholar]
- King, N.G.; Moore, P.J.; Pessarrodona, A.; Burrows, M.T.; Porter, J.; Bue, M. Ecological performance differs between range centre and trailing edge populations of a cold-water kelp: Implications for estimating net primary productivity. Mar. Biol. 2020, 167, 137. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A. The future of Blue Carbon science. Nat. Commun. 2019, 10, 3998. [Google Scholar] [CrossRef]
- Ellison, J.C. How South Pacific Mangroves May Respond to Predicted Climate Change and Sea-level Rise. In Climate Change in the South Pacific: Impacts and Responses in Australia, New Zealand, and Small Island States, 1st ed.; Gillespie, A., Burns, W.C.G., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 289–300. [Google Scholar] [CrossRef]
- Arias-Ortiz, A.; Serrano, O.; Masqué, P.; Lavery, P.S.; Mueller, U.; Kendrick, G.A. A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat. Clim. Chang. 2018, 8, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Xie, S.-P.; Hu, K.; Huang, G.; Huang, R. Patterns of the seasonal response of tropical rainfall to global warming. Nat. Geosci. 2013, 6, 357–361. [Google Scholar] [CrossRef]
- Sanders, C.J.; Maher, D.T.; Tait, D.R.; Williams, D.; Holloway, C.; Sippo, J.Z. Are global mangrove carbon stocks driven by rainfall? J. Geophys. Res. Biogeosciences 2016, 121, 2600–2609. [Google Scholar] [CrossRef]
- Lee, R.Y.; Porubsky, W.P.; Feller, I.C.; McKee, K.L.; Joye, S.B. Porewater biogeochemistry and soil metabolism in dwarf red mangrove habitats (Twin Cays, Belize). Biogeochemistry 2008, 87, 181–198. [Google Scholar] [CrossRef]
- Lewis, D.B.; Brown, J.A.; Jimenez, K.L. Effects of flooding and warming on soil organic matter mineralization in Avicennia germinans mangrove forests and Juncus roemerianus salt marshes. Estuar. Coast. Shelf Sci. 2014, 139, 11–19. [Google Scholar] [CrossRef]
- Schuerch, M.; Spencer, T.; Temmerman, S.; Kirwan, M.L.; Wolff, C.; Lincke, D. Future response of global coastal wetlands to sea-level rise. Nature 2018, 561, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, N.; Hamidian, A.H.; Wu, C.; Zhang, Y.; Yang, M. Scientific studies on microplastics pollution in Iran: An in-depth review of the published articles. Mar. Pollut. Bull. 2021, 162, 111901. [Google Scholar] [CrossRef]
- Chau, M.Q.; Hoang, A.T.; Truong, T.T.; Nguyen, X.P. Endless story about the alarming reality of plastic waste in Vietnam. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–9. [Google Scholar] [CrossRef]
- Ya, H.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.; Wang, X. Recent advances on ecological effects of microplastics on soil environment. Sci. Total Environ. 2021, 798, 149338. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, C.; Xu, X.; Jin, R.; Li, D.; Christakos, G. Underestimated PAH accumulation potential of blue carbon vegetation: Evidence from sedimentary records of saltmarsh and mangrove in Yueqing Bay, China. Sci. Total Environ. 2022, 817, 152887. [Google Scholar] [CrossRef]
- Li, Q.; Su, L.; Ma, C.; Feng, Z.; Shi, H. Plastic debris in coastal macroalgae. Environ. Res. 2022, 205, 112464. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, M.; Chen, X.; Hu, L.; Xu, Y.; Fu, W. A comparative review of microplastics in lake systems from different countries and regions. Chemosphere 2022, 286, 131806. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.; Fauziah, S. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Almahasheer, H.; Duarte, C.M. Mangrove forests as traps for marine litter. Environ. Pollut. 2019, 247, 499–508. [Google Scholar] [CrossRef]
- de Smit, J.C.; Anton, A.; Martin, C.; Rossbach, S.; Bouma, T.J.; Duarte, C.M. Habitat-forming species trap microplastics into coastal sediment sinks. Sci. Total Environ. 2021, 772, 145520. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Al-Ansari, T. Investigation of co-pyrolysis blends of camel manure, date pits and plastic waste into value added products using Aspen Plus. Fuel 2023, 340, 127474. [Google Scholar] [CrossRef]
- Veerasingam, S.; Saha, M.; Suneel, V.; Vethamony, P.; Rodrigues, A.C.; Bhattacharyya, S. Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India. Chemosphere 2016, 159, 496–505. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Chen, L.; Wang, X. Ecological interception effect of mangroves on microplastics. J. Hazard Mater. 2022, 423, 127231. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Vuik, V.; Visser, P.J.; Soens, T.; van Wesenbeeck, B.; van de Koppel, J. Historic storms and the hidden value of coastal wetlands for nature-based flood defence. Nat. Sustain. 2020, 3, 853–862. [Google Scholar] [CrossRef]
- Temmerman, S.; Meire, P.; Bouma, T.J.; Herman, P.M.J.; Ysebaert, T.; De Vriend, H.J. Ecosystem-based coastal defence in the face of global change. Nature 2013, 504, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sanders, C.J.; Santos, I.R.; Tang, J.; Schuerch, M.; Kirwan, M.L. Global blue carbon accumulation in tidal wetlands increases with climate change. Natl. Sci. Rev. 2021, 8, nwaa296. [Google Scholar] [CrossRef]
- Temmink, R.J.M.; Lamers, L.P.M.; Angelini, C.; Bouma, T.J.; Fritz, C.; van de Koppel, J. Recovering wetland biogeomorphic feedbacks to restore the world’s biotic carbon hotspots. Science 2022, 376, eabn1479. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Cao, W.; Jiang, F.; Zhao, C.; Ding, H. Vertical distribution of microplastics in bay sediment reflecting effects of sedimentation dynamics and anthropogenic activities. Mar. Pollut. Bull. 2020, 152, 110885. [Google Scholar] [CrossRef]
- Mao, R.; Song, J.; Yan, P.; Ouyang, Z.; Wu, R.; Liu, S. Horizontal and vertical distribution of microplastics in the Wuliangsuhai Lake sediment, northern China. Sci. Total Environ. 2021, 754, 142426. [Google Scholar] [CrossRef]
- Lin, L.; Pan, X.; Zhang, S.; Li, D.; Zhai, W.; Wang, Z. Distribution and source of microplastics in China’s second largest reservoir—Danjiangkou Reservoir. J. Environ. Sci. 2021, 102, 74–84. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Hassan Kazmi, S.S.U.; Zhao, Y.; Chen, M.; Wang, J. A review of microplastic pollution in seawater, sediments and organisms of the Chinese coastal and marginal seas. Chemosphere 2022, 286, 131677. [Google Scholar] [CrossRef]
- Dubaish, F.; Liebezeit, G. Suspended Microplastics and Black Carbon Particles in the Jade System, Southern North Sea. Water Air Soil Pollut. 2013, 224, 1352. [Google Scholar] [CrossRef]
- Ouyang, X.; Duarte, C.M.; Cheung, S.-G.; Tam, N.F.-Y.; Cannicci, S.; Martin, C. Fate and Effects of Macro- and Microplastics in Coastal Wetlands. Environ. Sci. Technol. 2022, 56, 2386–2397. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Baalkhuyur, F.; Valluzzi, L.; Saderne, V.; Cusack, M.; Almahasheer, H. Exponential increase of plastic burial in mangrove sediments as a major plastic sink. Sci. Adv. 2020, 6, eaaz5593. [Google Scholar] [CrossRef] [PubMed]
- de los Santos, C.B.; Krång, A.-S.; Infantes, E. Microplastic retention by marine vegetated canopies: Simulations with seagrass meadows in a hydraulic flume. Environ. Pollut. 2021, 269, 116050. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 2012, 48, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Nor, N.H.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, J.; Feng, D.; Zhao, Y.; Sun, W.; Yu, H. Microplastics pollution in mangrove ecosystems: A critical review of current knowledge and future directions. Sci. Total Environ. 2021, 753, 142041. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, X.; Effiong, K.; Xu, C.; Su, Z.; Hu, J. New Insights into the Microplastic Enrichment in the Blue Carbon Ecosystem: Evidence from Seagrass Meadows and Mangrove Forests in Coastal South China Sea. Environ. Sci. Technol. 2021, 55, 4804–4812. [Google Scholar] [CrossRef]
- Furukawa, K.; Wolanski, E.; Mueller, H. Currents and Sediment Transport in Mangrove Forests. Estuar. Coast. Shelf Sci. 1997, 44, 301–310. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat Bot 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Van Melkebeke, M.; Janssen, C.; De Meester, S. Characteristics and Sinking Behavior of Typical Microplastics Including the Potential Effect of Biofouling: Implications for Remediation. Environ. Sci. Technol. 2020, 54, 8668–8680. [Google Scholar] [CrossRef] [PubMed]
- Waldschläger, K.; Schüttrumpf, H. Effects of Particle Properties on the Settling and Rise Velocities of Microplastics in Freshwater under Laboratory Conditions. Environ. Sci. Technol. 2019, 53, 1958–1966. [Google Scholar] [CrossRef]
- Duan, J.; Han, J.; Cheung, S.G.; Chong, R.K.Y.; Lo, C.-M.; Lee, F.W.-F. How mangrove plants affect microplastic distribution in sediments of coastal wetlands: Case study in Shenzhen Bay, South China. Sci. Total Environ. 2021, 767, 144695. [Google Scholar] [CrossRef] [PubMed]
- Butman, C.A.; Grassle, J.P.; Webb, C.M. Substrate choices made by marine larvae settling in still water and in a flume flow. Nature 1988, 333, 771–773. [Google Scholar] [CrossRef]
- Boström, C.; Törnroos, A.; Bonsdorff, E. Invertebrate dispersal and habitat heterogeneity: Expression of biological traits in a seagrass landscape. J. Exp. Mar. Bio Ecol. 2010, 390, 106–117. [Google Scholar] [CrossRef]
- Gacia, E.; Granata, T.; Duarte, C. An approach to measurement of particle flux and sediment retention within seagrass (Posidonia oceanica) meadows. Aquat. Bot. 1999, 65, 255–268. [Google Scholar] [CrossRef]
- Datu, S.S.; Supriadi, S.; Tahir, A. Microplastic in Cymodocea rotundata Seagrass Blades. Int. J. Environ. Agric. Biotechnol. 2019, 4, 1758–1761. [Google Scholar] [CrossRef] [Green Version]
- Goss, H.; Jaskiel, J.; Rotjan, R. Thalassia testudinum as a potential vector for incorporating microplastics into benthic marine food webs. Mar. Pollut. Bull. 2018, 135, 1085–1089. [Google Scholar] [CrossRef]
- Miyajima, T.; Hori, M.; Hamaguchi, M.; Shimabukuro, H.; Adachi, H.; Yamano, H. Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows. Glob. Biogeochem. Cycles 2015, 29, 397–415. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Short, F.T.; Polidoro, B.; Livingstone, S.R.; Carpenter, K.E.; Bandeira, S.; Bujang, J.S. Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 2011, 144, 1961–1971. [Google Scholar] [CrossRef]
- Dahlgren, C.; Kellison, G.; Adams, A.; Gillanders, B.; Kendall, M.; Layman, C. Marine nurseries and effective juvenile habitats: Concepts and applications. Mar. Ecol. Prog. Ser. 2006, 312, 291–295. [Google Scholar] [CrossRef]
- Sanchez-Vidal, A.; Canals, M.; de Haan, W.P.; Romero, J.; Veny, M. Seagrasses provide a novel ecosystem service by trapping marine plastics. Sci. Rep. 2021, 11, 254. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, X.; Xu, C.; Perianen, Y.D.; Hu, J.; Holmer, M. Seagrass beds acting as a trap of microplastics—Emerging hotspot in the coastal region? Environ. Pollut. 2020, 257, 113450. [Google Scholar] [CrossRef]
- Gullström, M.; Lyimo, L.D.; Dahl, M.; Samuelsson, G.S.; Eggertsen, M.; Anderberg, E. Blue Carbon Storage in Tropical Seagrass Meadows Relates to Carbonate Stock Dynamics, Plant–Sediment Processes, and Landscape Context: Insights from the Western Indian Ocean. Ecosystems 2018, 21, 551–566. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Al-Muhtaseb, A.H. Challenges and perspectives on innovative technologies for biofuel production and sustainable environmental management. Fuel 2022, 325, 124845. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Biruntha, M.; Krishnan, R.Y.; Muthusamy, G.; Karmegam, N. Recent development patterns, utilization and prospective of biofuel production: Emerging nanotechnological intervention for environmental sustainability—A review. Fuel 2022, 314, 122757. [Google Scholar] [CrossRef]
- Son Le, H.; Said, Z.; Tuan Pham, M.; Hieu Le, T.; Veza, I.; Nhanh Nguyen, V. Production of HMF and DMF biofuel from carbohydrates through catalytic pathways as a sustainable strategy for the future energy sector. Fuel 2022, 324, 124474. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Rajendran, S.; Vasseghian, Y.; Dragoi, E.-N. Advanced integrated nanocatalytic routes for converting biomass to biofuels: A comprehensive review. Fuel 2022, 314, 122762. [Google Scholar] [CrossRef]
- Bamisaye, A.; Ige, A.R.; Adegoke, I.A.; Ogunbiyi, E.O.; Bamidele, M.O.; Adeleke, O. Eco-friendly de-lignified and raw Celosia argentea waste solid biofuel: Comparative studies and machine learning modelling. Fuel 2023, 340, 127412. [Google Scholar] [CrossRef]
- Maaoui, A.; Ben Hassen Trabelsi, A.; Hamdi, M.; Chagtmi, R.; Jamaaoui, F.; Lopez, G. Towards local circular economy through Opuntia Ficus Indica cladodes conversion into renewable biofuels and biochars: Product distribution and kinetic modelling. Fuel 2023, 332, 126056. [Google Scholar] [CrossRef]
- Tuan Hoang, A.; Nižetić, S.; Ölçer, A.I.; Chyuan Ong, H. Synthesis pathway and combustion mechanism of a sustainable biofuel 2,5-Dimethylfuran: Progress and prospective. Fuel 2021, 286, 119337. [Google Scholar] [CrossRef]
- Bin, Y.; Yu, Z.; Huang, Z.; Li, M.; Zhang, Y.; Ma, X. Investigation on the co-pyrolysis of municipal solid waste and sawdust: Pyrolysis behaviors, kinetics, and thermodynamic analysis. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 8001–8011. [Google Scholar] [CrossRef]
- Kowthaman, C.N.; Senthil Kumar, P.; Arul Mozhi Selvan, V.; Ganesh, D. A comprehensive insight from microalgae production process to characterization of biofuel for the sustainable energy. Fuel 2022, 310, 122320. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Jiang, X.; Lin, J.; Lin, X.; Zhao, S. Valorisation of harmful algae bloom (Enteromorpha prolifera) for polysaccharide and crude bio-oil production. Fuel 2022, 324, 124482. [Google Scholar] [CrossRef]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Sekar, S.; Kandasamy, S.; Ramasamy Subramanian, K.P. A review on hydrothermal liquefaction of algal biomass on process parameters, purification and applications. Fuel 2022, 313, 122679. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, F.; Abed, A.M.; Le, B.N.; Dai, L.; Elhosiny Ali, H. Macroalgae as a potential source of biomass for generation of biofuel: Artificial intelligence, challenges, and future insights towards a sustainable environment. Fuel 2023, 336, 126826. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Mumtaz, M.; Baqar, Z.; Hussain, N.; Afifa Bilal, M.; Azam, H.M.H. Application of nanomaterials for enhanced production of biodiesel, biooil, biogas, bioethanol, and biohydrogen via lignocellulosic biomass transformation. Fuel 2022, 315, 122840. [Google Scholar] [CrossRef]
- Kota, K.B.; Shenbagaraj, S.; Sharma, P.K.; Sharma, A.K.; Ghodke, P.K.; Chen, W.-H. Biomass torrefaction: An overview of process and technology assessment based on global readiness level. Fuel 2022, 324, 124663. [Google Scholar] [CrossRef]
- Wang, P.; Xu, P.; Wang, B.; Shen, C.; Shen, L. Green ammonia production via microalgae steam catalytic gasification process over LaFeO3 perovskite. Fuel 2022, 318, 123322. [Google Scholar] [CrossRef]
- Xu, D.; Lin, J.; Ma, R.; Hou, J.; Sun, S.; Ma, N. Fast pyrolysis of algae model compounds for bio-oil: In-depth insights into the volatile interaction mechanisms based on DFT calculations. Fuel 2023, 333, 126449. [Google Scholar] [CrossRef]
- Agrawal, R.; Bhadana, B.; Singh Chauhan, P.; Adsul, M.; Kumar, R.; Gupta, R.P. Understanding the effects of low enzyme dosage and high solid loading on the enzyme inhibition and strategies to improve hydrolysis yields of pilot scale pretreated rice straw. Fuel 2022, 327, 125114. [Google Scholar] [CrossRef]
- Yuan, C.; Zhao, S.; Ni, J.; He, Y.; Cao, B.; Hu, Y. Integrated route of fast hydrothermal liquefaction of microalgae and sludge by recycling the waste aqueous phase for microalgal growth. Fuel 2023, 334, 126488. [Google Scholar] [CrossRef]
- Marín, D.; Méndez, L.; Suero, I.; Díaz, I.; Blanco, S.; Fdz-Polanco, M. Anaerobic digestion of food waste coupled with biogas upgrading in an outdoors algal-bacterial photobioreactor at pilot scale. Fuel 2022, 324, 124554. [Google Scholar] [CrossRef]
- Germec, M.; Karhan, M.; Demirci, A.; Turhan, I. Kinetic modeling, sensitivity analysis, and techno-economic feasibility of ethanol fermentation from non-sterile carob extract-based media in Saccharomyces cerevisiae biofilm reactor under a repeated-batch fermentation process. Fuel 2022, 324, 124729. [Google Scholar] [CrossRef]
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel production in Ireland—An approach to 2020 targets with a focus on algal biomass. Energies 2013, 6, 6391–6412. [Google Scholar] [CrossRef]
- Mushlihah, S.; Langford, A.; Tassakka, A.C.M.A.R. Ozonolysis as an effective pretreatment strategy for bioethanol production from marine algae. BioEnergy Res. 2020, 13, 1269–1279. [Google Scholar]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Ibrahim, H.A.H.; Barakat, K.M.; Shaltout, N.A.; Sayed, W.M.M.E.L.; Abou-Shanab, R.A.I. Potential of Marine Biota and Bio-Waste Materials as Feedstock for Biofuel Production; Waste Management; CRC Press: Boca Raton, FL, USA, 2022; pp. 123–139. [Google Scholar]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Arita, C.; Shinde, S.; Kim, S.; Monroe, E.; George, A.; Quinn, J.C. Bioproducts from high-protein algal biomass: An economic and environmental sustainability review and risk analysis. Sustain. Energy Fuels 2022, 6, 2398–2422. [Google Scholar] [CrossRef]
- Özçimen, D.; Inan, B. An Overview of Bioethanol Production From Algae. In Biofuels—Status and Perspective; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Chelf, P.; Brown, L.M.; Wyman, C.E. Aquatic biomass resources and carbon dioxide trapping. Biomass Bioenergy 1993, 4, 175–183. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Gunasekaran, M.; Kumar, G.; Banu, J.R.; Zhen, G. Biohydrogen production from seagrass via novel energetically efficient ozone coupled rotor stator homogenization. Int. J. Hydrogen Energy 2020, 45, 5881–5889. [Google Scholar] [CrossRef]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Alnaqi, A.A.; Alsarraf, J.; Al-Rashed, A.A.A.A. The waste heat of a biofuel-powered SOFC for green hydrogen production using thermochemical cycle; Economic, environmental analysis, and tri-criteria optimization. Fuel 2023, 335, 126599. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Wahia, H.; Zhang, L.; Zhou, C.; Ma, H. State-of-the-art co-pyrolysis of lignocellulosic and macroalgae biomass feedstocks for improved bio-oil production—A review. Fuel 2023, 332, 126071. [Google Scholar] [CrossRef]
- Yameen, M.Z.; AlMohamadi, H.; Naqvi, S.R.; Noor, T.; Chen, W.-H.; Amin, N.A.S. Advances in production & activation of marine macroalgae-derived biochar catalyst for sustainable biodiesel production. Fuel 2023, 337, 127215. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Keerthana Devi, M.; Senthil Kumar, P. Algal biofuels: Technological perspective on cultivation, fuel extraction and engineering genetic pathway for enhancing productivity. Fuel 2022, 320, 123814. [Google Scholar] [CrossRef]
- Nanda, M.; Jaiswal, K.K.; Negi, J.; de Farias Neves, F.; Ranjitha, J.; Vlaskin, M.S. A sustainable approach to produce yeast lipid by utilizing marine macroalgae biomass. Fuel 2023, 338, 127214. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Andrade, C.; Martins, P.L.; Duarte, L.C.; Oliveira, A.C.; Carvalheiro, F. Development of an innovative macroalgae biorefinery: Oligosaccharides as pivotal compounds. Fuel 2022, 320, 123780. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Chang, Y.K.; Kim, D. Lipid production under a nutrient-sufficient condition outperforms starvation conditions due to a natural polarization of lipid content in algal biofilm. Fuel 2022, 126902. [Google Scholar] [CrossRef]
- Peter, A.P.; Chew, K.W.; Pandey, A.; Lau, S.Y.; Rajendran, S.; Ting, H.Y. Artificial intelligence model for monitoring biomass growth in semi-batch Chlorella vulgaris cultivation. Fuel 2023, 333, 126438. [Google Scholar] [CrossRef]
- Sathya, A.B.; Thirunavukkarasu, A.; Nithya, R.; Nandan, A.; Sakthishobana, K.; Kola, A.K. Microalgal biofuel production: Potential challenges and prospective research. Fuel 2023, 332, 126199. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Priya, A.K.; Dutta, K.; Rajendran, S.; Vasseghian, Y. Engineering strategies and opportunities of next generation biofuel from microalgae: A perspective review on the potential bioenergy feedstock. Fuel 2022, 312, 122827. [Google Scholar] [CrossRef]
- Marquez, G.P.B.; Reichardt, W.T.; Azanza, R.V.; Klocke, M.; Montaño, M.N.E. Thalassic biogas production from sea wrack biomass using different microbial seeds: Cow manure, marine sediment and sea wrack-associated microflora. Bioresour Technol. 2013, 133, 612–617. [Google Scholar] [CrossRef]
- Pechsiri, J.S.; Thomas, J.-B.E.; Risén, E.; Ribeiro, M.S.; Malmström, M.E.; Nylund, G.M. Energy performance and greenhouse gas emissions of kelp cultivation for biogas and fertilizer recovery in Sweden. Sci. Total Environ. 2016, 573, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.; Gonçalves, P.R.; Nobre, A.; Alves, M.M. Biomethanation potential of macroalgae Ulva spp. and Gracilaria spp. and in co-digestion with waste activated sludge. Bioresour Technol. 2012, 114, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Peu, P.; Sassi, J.-F.; Girault, R.; Picard, S.; Saint-Cast, P.; Béline, F. Sulphur fate and anaerobic biodegradation potential during co-digestion of seaweed biomass (Ulva sp.) with pig slurry. Bioresour Technol. 2011, 102, 10794–10802. [Google Scholar] [CrossRef]
- Thakur, N.; Salama, E.-S.; Sharma, M.; Sharma, P.; Sharma, D.; Li, X. Efficient utilization and management of seaweed biomass for biogas production. Mater Today Sustain. 2022, 18, 100120. [Google Scholar] [CrossRef]
- Banu, J.R.; Tamilarasan, K.; Rani, R.U.; Gunasekaran, M.; Cho, S.-K.; Ala’a, H. Dispersion aided tenside disintegration of seagrass Syringodium isoetifolium: Towards biomethanation, kinetics, energy exploration and evaluation. Bioresour Technol. 2019, 277, 62–67. [Google Scholar] [CrossRef]
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.-J.; Bae, H.-J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol. 2009, 100, 6658–6660. [Google Scholar] [CrossRef]
- Alam, S.N.; Khalid, Z.; Guldhe, A.; Singh, B.; Korstad, J. Harvesting and pretreatment techniques of aquatic macrophytes and macroalgae for production of biofuels. Environ. Sustain. 2021, 4, 299–316. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; El-Naggar, A.H.; Baeshen, A.A. Potential of macroalgae for biodiesel production: Screening and evaluation studies. J. Biosci Bioeng 2018, 125, 231–237. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Azhar, S.H.M.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Bibi, R.; Ahmad, Z.; Imran, M.; Hussain, S.; Ditta, A.; Mahmood, S. Algal bioethanol production technology: A trend towards sustainable development. Renew Sustain. Energy Rev. 2017, 71, 976–985. [Google Scholar] [CrossRef]
- Maceiras, R.; Rodrı, M.; Cancela, A.; Urréjola, S.; Sánchez, A. Macroalgae: Raw material for biodiesel production. Appl. Energy 2011, 88, 3318–3323. [Google Scholar] [CrossRef]
- Li, R.; Zhong, Z.; Jin, B.; Zheng, A. Selection of temperature for bio-oil production from pyrolysis of algae from lake blooms. Energy Fuels 2012, 26, 2996–3002. [Google Scholar] [CrossRef]
- Ye Lee, J.; Li, P.; Lee, J.; Ryu, H.J.; Oh, K.K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, N.; Hoang, A.T.; Nižetić, S.; Balasubramanian, D.; Kamaraj, S.; Pandian, P.L. Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel 2022, 329, 125362. [Google Scholar] [CrossRef]
- Ravikumar, S.; Gokulakrishnan, R.; Kanagavel, M.; Thajuddin, N. Production of biofuel ethanol from pretreated seagrass by using Saccharomyces cerevisiae. Indian J. Sci. Technol. 2011, 4, 1087–1089. [Google Scholar] [CrossRef]
- Viola, E.; Cardinale, M.; Santarcangelo, R.; Villone, A.; Zimbardi, F. Ethanol from eel grass via steam explosion and enzymatic hydrolysis. Biomass Bioenergy 2008, 32, 613–618. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A. Effects of climate change on global seaweed communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef]
- Masri, M.A.; Younes, S.; Haack, M.; Qoura, F.; Mehlmer, N.; Brück, T. A Seagrass-Based Biorefinery for Generation of Single-Cell Oils for Biofuel and Oleochemical Production. Energy Technol. 2018, 6, 1026–1038. [Google Scholar] [CrossRef] [Green Version]
- Uchida, M.; Miyoshi, T.; Kaneniwa, M.; Ishihara, K.; Nakashimada, Y.; Urano, N. Production of 16.5% v/v ethanol from seagrass seeds. J. Biosci Bioeng 2014, 118, 646–650. [Google Scholar] [CrossRef]
- Ghazal, M.A.; Ibrahim, H.A.H.; Shaltout, N.A.; Ali, A.E. Biodiesel and bioethanol production from Ulva fasciata Delie biomass via enzymatic pretreatment using marine-derived Aspergillus niger. Int. J. Pure App. Biosci. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Gallagher, J.A.; Donnison, I.S. Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J. Appl. Phycol. 2009, 21, 569–574. [Google Scholar] [CrossRef]
- Ge, L.; Wang, P.; Mou, H. Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew. Energy 2011, 36, 84–89. [Google Scholar] [CrossRef]
- Horn, S.J.; Aasen, I.M.; Østgaard, K. Production of ethanol from mannitol by Zymobacter palmae. J. Ind. Microbiol. Biotechnol. 2000, 24, 51–57. [Google Scholar] [CrossRef]
- El-Sayed, W.M.M.; Ibrahim, H.A.H.; Abdul-Raouf, U.M.; El-Nagar, M.M. Evaluation of bioethanol production from Ulva lactuca by Saccharomyces cerevisiae. J. Biotechnol. Biomater. 2016, 6, 2. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Kuo, L.-J.; Urquhart, L.; Gill, G.A.; Roesijadi, G. Acetone-butanol fermentation of marine macroalgae. Bioresour. Technol. 2012, 108, 305–309. [Google Scholar] [CrossRef]
- Potts, T.; Du, J.; Paul, M.; May, P.; Beitle, R.; Hestekin, J. The production of butanol from Jamaica bay macro algae. Environ. Prog. Sustain. Energy 2012, 31, 29–36. [Google Scholar] [CrossRef]
- De Rosa, S.; Zavodnik, N.; De Stefano, S.; Fiaccavento, R.; Travizi, A. Seasonal Changes of Biomass and Soluble Carbohydrates in the Seagrass Zostera noltii Hornem; Walter de Gruyter: Berlin, Germany, 1990. [Google Scholar]
- Syed, F.; Zakaria, M.H.; Bujang, J.S.; Ramaiya, S.D.; Hayashizaki, K. Physicochemical properties of starches from seed and rhizome of Enhalus acoroides. Phil J. Nat. Sci. 2019, 24, 27–33. [Google Scholar]
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Raw plant-based biorefinery: A new paradigm shift towards biotechnological approach to sustainable manufacturing of HMF. Biotechnol. Adv. 2019, 37, 107422. [Google Scholar] [CrossRef]
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Toward sustainable hydroxymethylfurfural production using seaweeds. Trends Biotechnol. 2020, 38, 487–496. [Google Scholar] [CrossRef]
- Tamilarasan, S.; Sahadevan, R. Ultrasonic assisted acid base transesterification of algal oil from marine macroalgae Caulerpa peltata: Optimization and characterization studies. Fuel 2014, 128, 347–355. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.Y.; Oh, Y.R.; Park, J.M. Production of biodiesel from carbon sources of macroalgae, Laminaria japonica. Bioresour. Technol. 2014, 169, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Daroch, M.; Geng, S.; Wang, G. Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar] [CrossRef]
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 2020, 274, 117841. [Google Scholar] [CrossRef]
- Khan, M.; Raza Naqvi, S.; Ullah, Z.; Ali Ammar Taqvi, S.; Nouman Aslam Khan, M.; Farooq, W. Applications of machine learning in thermochemical conversion of biomass-A review. Fuel 2023, 332, 126055. [Google Scholar] [CrossRef]
- Siwal, S.S.; Sheoran, K.; Saini, A.K.; Vo, D.-V.N.; Wang, Q.; Thakur, V.K. Advanced thermochemical conversion technologies used for energy generation: Advancement and prospects. Fuel 2022, 321, 124107. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Jaiswal, K.K.; Kumar, V.; Kumar, S. Recent advances and viability in sustainable thermochemical conversion of sludge to bio-fuel production. Fuel 2022, 316, 123351. [Google Scholar] [CrossRef]
- Xu, K.; Li, J.; Zeng, K.; Zhong, D.; Peng, J.; Qiu, Y. The characteristics and evolution of nitrogen in bio-oil from microalgae pyrolysis in molten salt. Fuel 2023, 331, 125903. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Chen, S.; Zhang, X.; Chen, F.; Ye, N. Comparative evaluation of the pyrolytic and kinetic characteristics of a macroalga (Sargassum thunbergii) and a freshwater plant (Potamogeton crispus). Fuel 2012, 96, 185–191. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Jiang, X.; Han, X.; Ji, H. Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Convers. Manag. 2013, 68, 273–280. [Google Scholar] [CrossRef]
- Kan, T.; Grierson, S.; De Nys, R.; Strezov, V. Comparative assessment of the thermochemical conversion of freshwater and marine micro-and macroalgae. Energy Fuels 2014, 28, 104–114. [Google Scholar] [CrossRef]
- Ngoc Bao Dung, T.; Lay, C.-H.; Nguyen, D.D.; Chang, S.W.; Rajesh Banu, J.; Hong, Y. Improving the biohydrogen production potential of macroalgal biomass through mild acid dispersion pretreatment. Fuel 2023, 332, 125895. [Google Scholar] [CrossRef]
- Bae, Y.J.; Ryu, C.; Jeon, J.-K.; Park, J.; Suh, D.J.; Suh, Y.-W. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for bio-crude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377. [Google Scholar] [CrossRef]
- Moazezi, M.R.; Bayat, H.; Tavakoli, O.; Hallajisani, A. Hydrothermal liquefaction of Chlorella vulgaris and catalytic upgrading of product: Effect of process parameter on bio-oil yield and thermodynamics modeling. Fuel 2022, 318, 123595. [Google Scholar] [CrossRef]
- Elliott, D.C.; Sealock, L.J., Jr.; Butner, R.S. Product analysis from direct liquefaction of several high-moisture biomass feedstocks. In Pyrolysis Oils from Biomass: Producing, Analyzing, and Upgrading; ACS Publications: Washington, DC, USA, 1988; pp. 179–188. [Google Scholar]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Neveux, N.; Yuen, A.K.L.; Jazrawi, C.; Magnusson, M.; Haynes, B.S.; Masters, A.F. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresour. Technol. 2014, 155, 334–341. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Li, B.; Magoua Mbeugang, C.F.; Xie, X.; Wei, J.; Zhang, S.; Zhang, L. Catalysis/CO2 sorption enhanced pyrolysis-gasification of biomass for H2-rich gas production: Effects of activated carbon, NiO active component and calcined dolomite. Fuel 2023, 334, 126842. [Google Scholar] [CrossRef]
- Singh, M.; Salaudeen, S.A.; Gilroyed, B.H.; Dutta, A. Simulation of biomass-plastic co-gasification in a fluidized bed reactor using Aspen plus. Fuel 2022, 319, 123708. [Google Scholar] [CrossRef]
- Shahbaz, M.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Sudhakar, K.; Rajesh, M.; Premalatha, M. A mathematical model to assess the potential of algal bio-fuels in India. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 1114–1120. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Murto, M. Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J. Environ. Manag. 2010, 91, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A. Biofuel production through micro- and macroalgae pyrolysis—A review of pyrolysis methods and process parameters. J. Anal. Appl. Pyrolysis 2019, 142, 104599. [Google Scholar] [CrossRef]
- Bax, N.; Sands, C.J.; Gogarty, B.; Downey, R.V.; Moreau, C.V.E.; Moreno, B. Perspective: Increasing blue carbon around Antarctica is an ecosystem service of considerable societal and economic value worth protecting. Glob. Chang. Biol. 2021, 27, 5–12. [Google Scholar] [CrossRef]
- Buditama, A. Blue carbon for reducing the impacts of climate change: An Indonesian case study. Glob. Ecol. Biogeogr. 2016, 68–88. [Google Scholar]
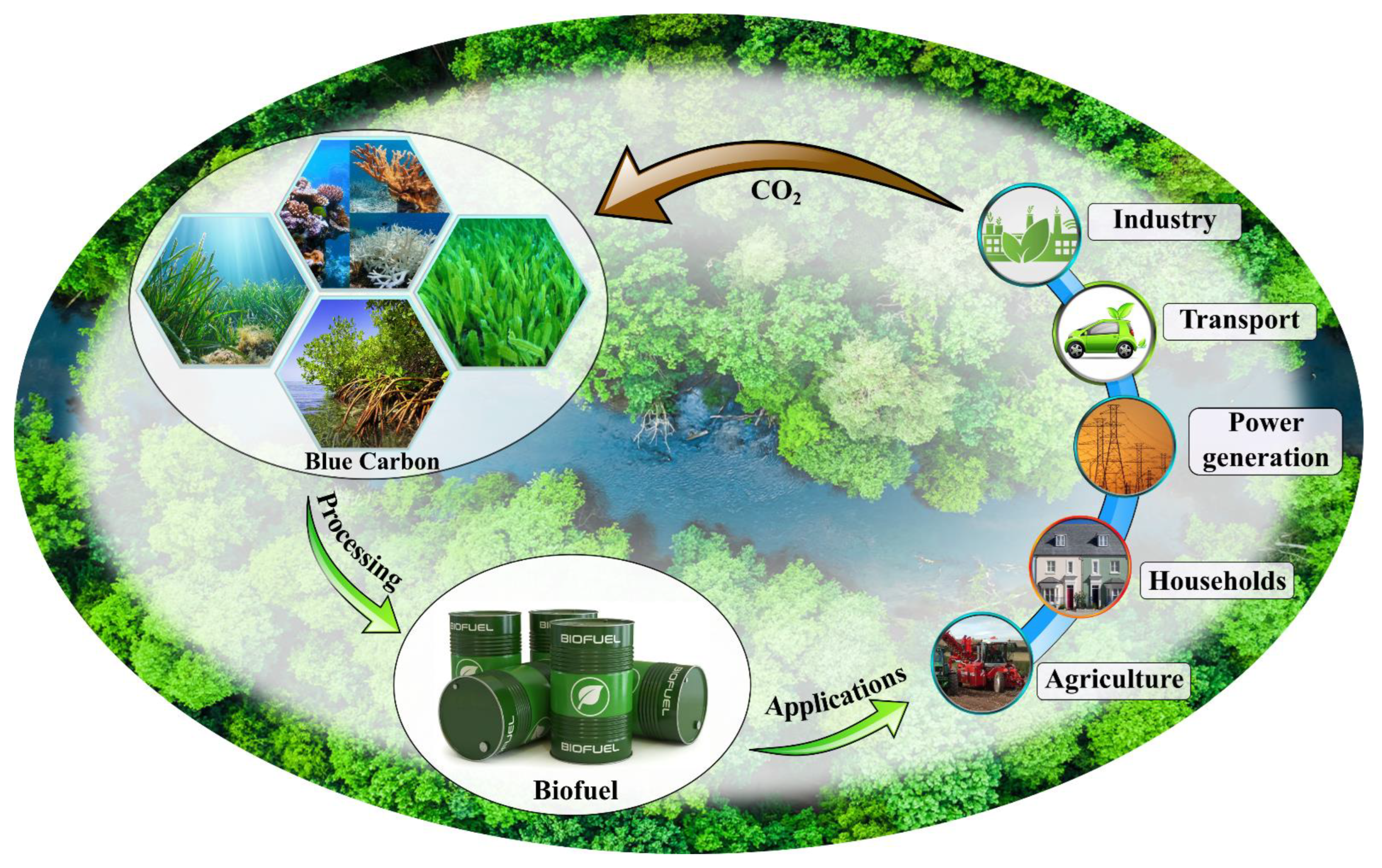
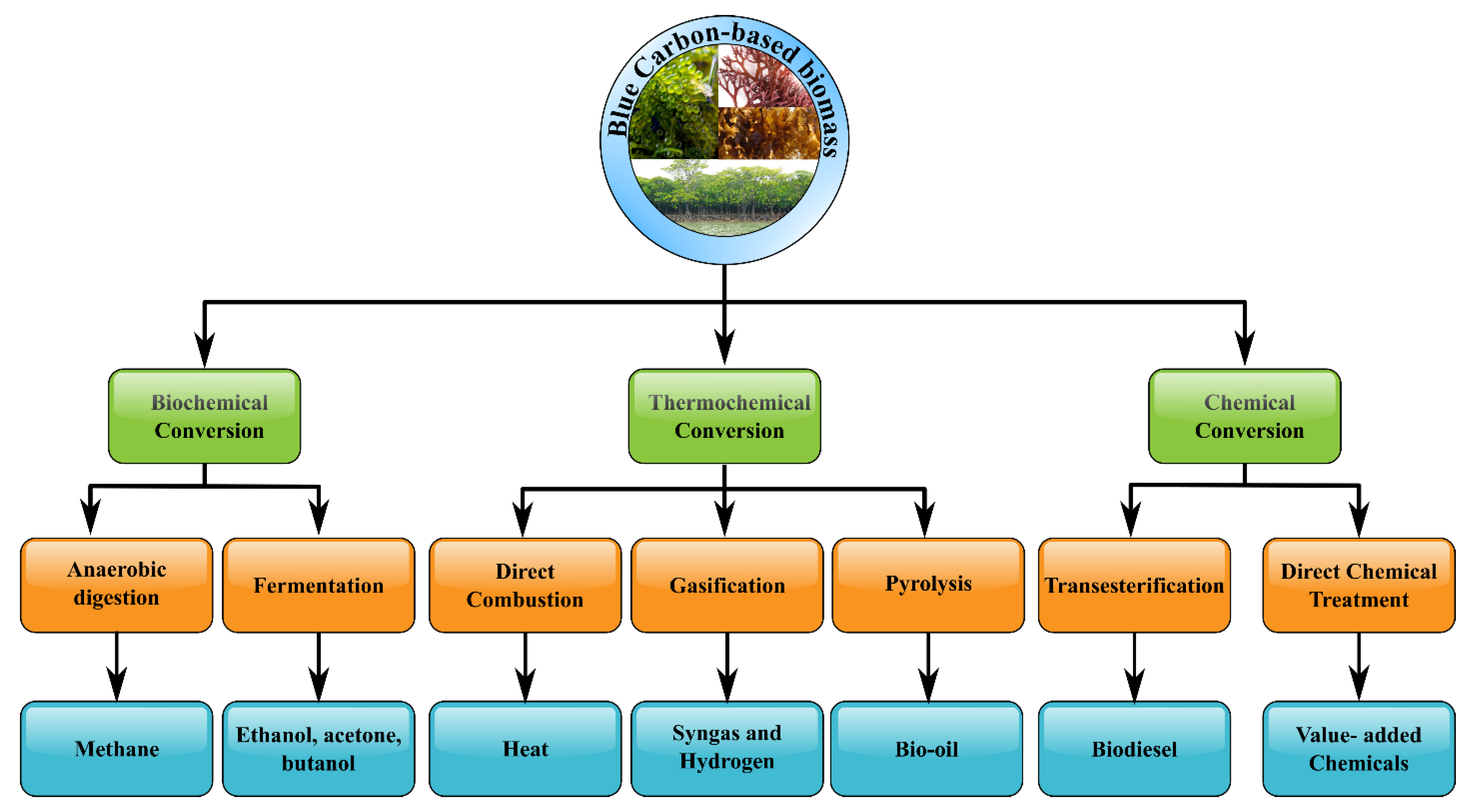
| Processing Techniques | Target Products | Benefits | Drawbacks |
|---|---|---|---|
| Anaerobic digestion | Biogas | Finishing technology without drying process | High inhibition and salt |
| Fermentation | Bioethanol/biobutanol | High content of carbohydrate | Low efficiency in forming various mixed sugars |
| Transesterification | Biodiesel | No required the dewatering process | Low yield |
| Pyrolysis/Gasification/Liquefaction | Bio-oil, syngas, hydrogen, bio-char | Fast rate without required chemicals | High energy consumption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandh, S.A.; Malla, F.A.; Qayoom, I.; Mohi-Ud-Din, H.; Butt, A.K.; Altaf, A.; Wani, S.A.; Betts, R.; Truong, T.H.; Pham, N.D.K.; et al. Importance of Blue Carbon in Mitigating Climate Change and Plastic/Microplastic Pollution and Promoting Circular Economy. Sustainability 2023, 15, 2682. https://doi.org/10.3390/su15032682
Bandh SA, Malla FA, Qayoom I, Mohi-Ud-Din H, Butt AK, Altaf A, Wani SA, Betts R, Truong TH, Pham NDK, et al. Importance of Blue Carbon in Mitigating Climate Change and Plastic/Microplastic Pollution and Promoting Circular Economy. Sustainability. 2023; 15(3):2682. https://doi.org/10.3390/su15032682
Chicago/Turabian StyleBandh, Suhaib A., Fayaz A. Malla, Irteza Qayoom, Haika Mohi-Ud-Din, Aqsa Khursheed Butt, Aashia Altaf, Shahid A. Wani, Richard Betts, Thanh Hai Truong, Nguyen Dang Khoa Pham, and et al. 2023. "Importance of Blue Carbon in Mitigating Climate Change and Plastic/Microplastic Pollution and Promoting Circular Economy" Sustainability 15, no. 3: 2682. https://doi.org/10.3390/su15032682







