Genotoxicity of Synthetic Food Colors on Nitrogen-Fixing Bacteria in Agricultural Lands Irrigated with Wastewater of Corresponding Industries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Morphological Identification of Bacterial Isolates
2.3. Application of Dilution of Synthetic Food Colors
2.4. Genomic DNA Extraction
2.5. Quantification of Genomic DNA
2.6. Comet Assay
Quantification and Visualization of DNA Damage
2.7. Statistical Analyses
3. Results
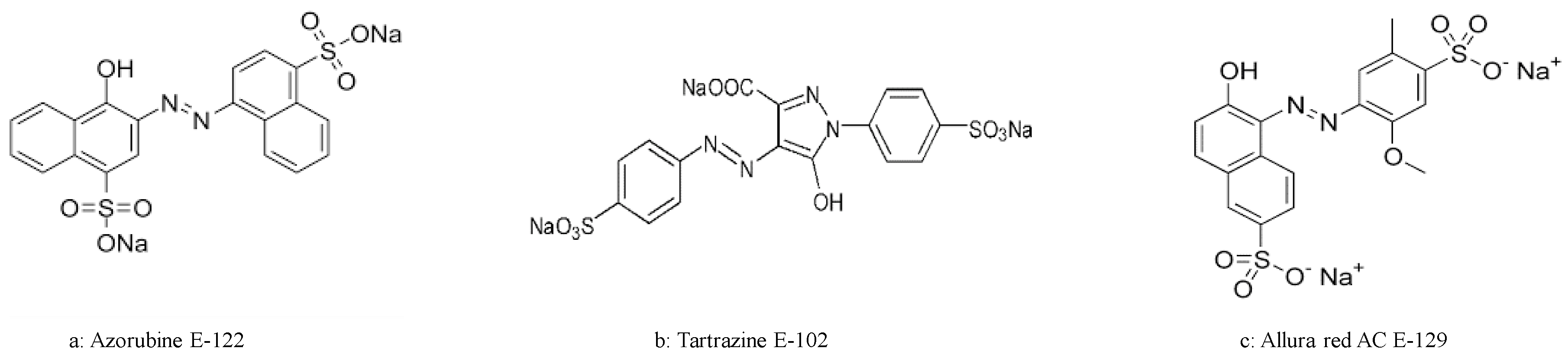
3.1. Quantification of DNA after Treatment with Azorubine E-122, Tartrazine E-102 and Allura Red AC E-129
3.2. Quantification of DNA through Agarose Gel Electrophoresis
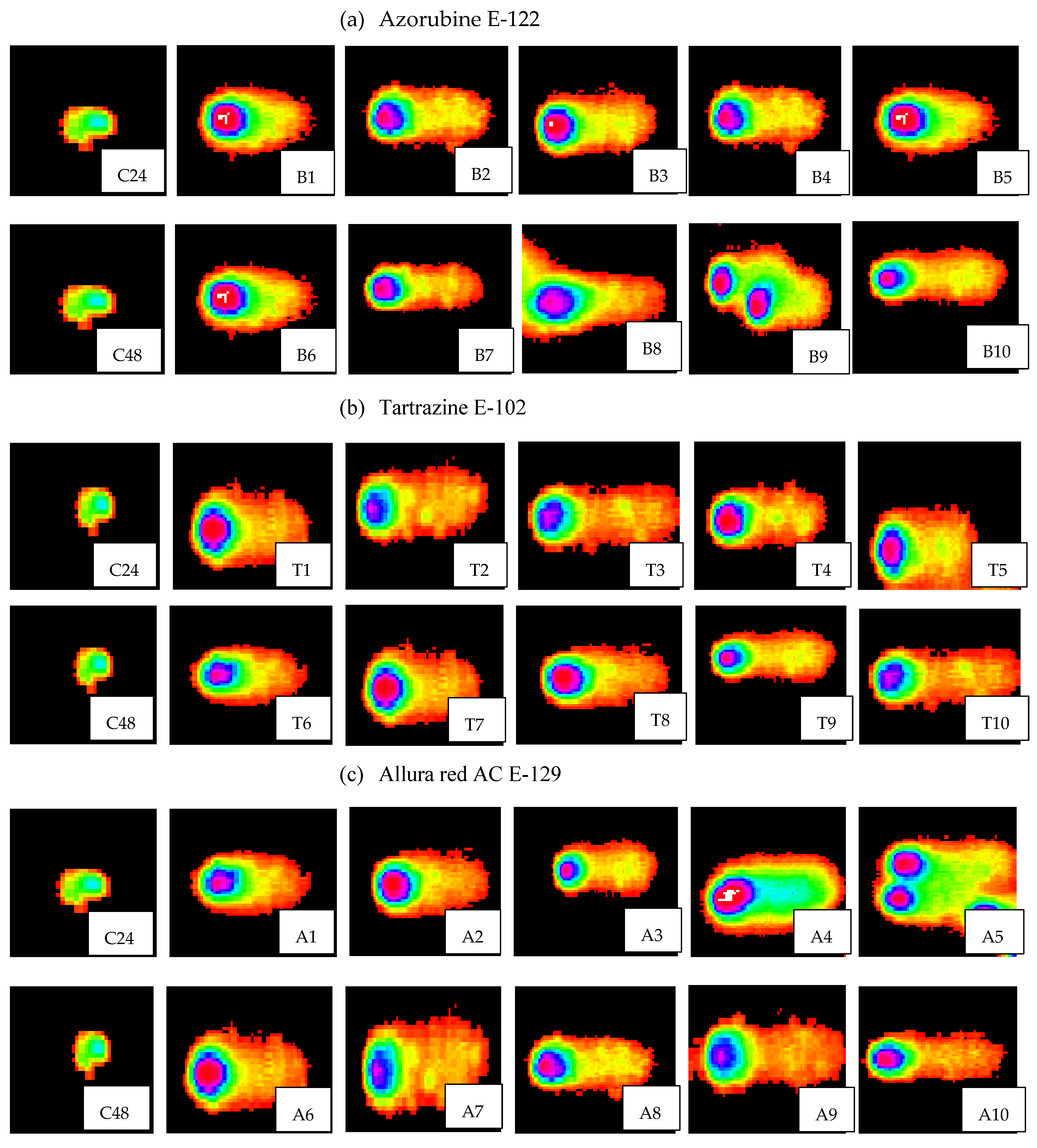
3.3. Image Analysis by Using Comet Scoring Software
3.4. Implementation of Comet Measurement to Evaluate the Rate of Genotoxicity on DNA Caused by Synthetic Food Colors
3.5. Evaluation of DNA Damage through Statistical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.S.; Ali, M.N.; Hamid, R.; Ganie, S.A. Genotoxic effect of two commonly used food dyes metanil yellow and carmoisine using Allium cepa L. as indicator. Toxicology 2020, 7, 370–375. [Google Scholar] [CrossRef]
- Subhashish, D.; Nagababu, B.H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. 2022, 1, 100019. [Google Scholar]
- Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Natural blue food colorants: Consumer acceptance, current alternatives, trends, challenges, and future strategies. Food Sci. Technol. 2021, 112, 163173. [Google Scholar]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed]
- AbuKhader, M.; Dhanalekshmi, U.M.; Nazmi, A. Identification and Prevalence of Food Colors in Candies Commonly Consumed by Children in Muscat, Oman. IJNPND 2021, 11, 128. [Google Scholar] [CrossRef]
- Hallagan, J.B.; Allen, D.C.; Borzelleca, J.F. The safety and regulatory status of food, drug and cosmetics colour additives exempt from certification. FCT 1995, 33, 515–528. [Google Scholar] [CrossRef]
- Punjab Pure Food Regulation. Available online: https://foodscienceuniverse.com/wp-content/uploads/2020/10/Punjab-Pure-Food-Regulations-2018.pdf (accessed on 20 November 2022).
- European Parliament and Council Directive 94/36/EC. Off. J. Eur. Communities 1994, 10, 13–29.
- Kroger, M.; Meister, K.; Kava, R. Low-calorie Sweeteners and Other Sugar Substitutes: A Review of the Safety Issues. IFT 2006, 5, 27–47. [Google Scholar] [CrossRef]
- Lhotta, K.; Höfle, G.; Gasser, R.; Finkenstedt, G. Hypokalemia, hyperreninemia and osteoporosis in a patient ingesting large amounts of cider vinegar. Nephron 1998, 80, 242–243. [Google Scholar] [CrossRef]
- El-Borm, H.; Badawy, G.; Hassab El-Nabi, S.; El-Sherif, W.; Atallah, M. Toxicity of sunset yellow fcf and tartrazine dyes on dna and cell cycle of liver and kidneys of the chick embryo: The alleviative effects of curcumin. Egypt. J. Zool. 2020, 74, 43–55. [Google Scholar] [CrossRef]
- John, A.; Yang, H.-H.; Muhammad, S.; Khan, Z.I.; Yu, H.; Luqman, M.; Tofail, M.; Hussain, M.I.; Awan, M.U.F. Cross Talk between Synthetic Food Colors (Azo Dyes), Oral Flora, and Cardiovascular Disorders. Appl. Sci. 2022, 12, 7084. [Google Scholar] [CrossRef]
- Reza, M.S.A.; Hasan, M.M.; Kamruzzaman, M.; Hossain, M.I.; Zubair, M.A.; Bari, L.; Abedin, M.Z.; Reza, M.A.; Khalid-Bin-Ferdaus, K.M.; Haque, K.M.F.; et al. Study of a common azo food dye in mice model: Toxicity reports and its relation to carcinogenicity. Food Sci. Nutr. 2019, 7, 667–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef] [PubMed]
- Oyeniran, D.O.; Sogbanmu, T.O.; Adesalu, T.A. Antibiotics, algal evaluations and subacute effects of abattoir wastewater on liver function enzymes, genetic and haematologic biomarkers in the freshwater fish, Clarias gariepinus. Ecotoxicol. Environ. Saf. 2021, 212, 111982. [Google Scholar] [CrossRef]
- Jiku, M.A.S.; Singha, A.; Faruquee, M.; Rahaman, M.A.; Alam, M.A.; Ehsanullah, M. Toxic wastewater status for irrigation usage at Gazipur and Savar industrial vicinity of Bangladesh. Acta Ecol. Sin. 2021, 41, 358–364. [Google Scholar] [CrossRef]
- Ao, S.; Zayed, T. Impact of sewer overflow on public health: A comprehensive scientometric analysis and systematic review. Environ. Res. 2022, 203, 111609. [Google Scholar]
- Padervand, M.; Mazloum, M.; Bargahi, A.; Arsalani, N. CQDs/BiOCl Photocatalysts for the Efficient Treatment of Congo Red Aqueous Solution under Visible Light. J. Nanostruct. 2021, 11, 790–801. [Google Scholar] [CrossRef]
- Padervand, M.; Rhimi, B.; Wang, C. One-pot synthesis of novel ternary Fe3N/Fe2O3/C3N4 photocatalyst for efficient removal of rhodamine B and CO2 reduction. J. Alloys Compd. 2021, 852, 156955. [Google Scholar] [CrossRef]
- Padervand, M.; Nasiri, F.; Hajiahmadi, S.; Bargahi, A.; Esmaeili, S.; Amini, M.; Nami, R.K.; Shahsavari, Z.; Karima, S. Ag@Ag2MoO4 decorated polyoxomolybdate/C3N4 nanostructures as highly efficient photocatalysts for the wastewater treatment and cancer cells killing under visible light. Inorg. Chem. Commun. 2022, 141, 109500. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of Synthetic Dyes of Textile Effluent by Microorganisms: An Environmentally and Economically Sustainable Approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Raffi, M.M.; Charyulu, P.B.B.N. Azospirillum-biofertilizer for sustainable cereal crop production: Current status. In Recent Developments in Applied Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 193–209. [Google Scholar]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- El-Mageed, A.; Taia, A.; El-Mageed, A.; Shimaa, A.; El-Saadony, M.T.; Abdelaziz, S.; Abdou, N.M. Plant growth-promoting rhizobacteria improve growth, morph-physiological responses, water productivity, and yield of rice plants under full and deficit drip irrigation. Rice 2022, 15, 1–15. [Google Scholar] [CrossRef]
- Kaloterakis, N.; Van Delden, S.H.; Hartley, S.; De Deyn, G.B. Silicon application and plant growth promoting rhizobacteria consisting of six pure Bacillus species alleviate salinity stress in cucumber (Cucumis sativus L). Sci. Hortic. 2021, 288, 110383. [Google Scholar] [CrossRef]
- He, A.; Niu, S.; Yang, D.; Ren, W.; Zhao, L.; Sun, Y.; Meng, L.; Zhao, Q.; Paré, P.W.; Zhang, J. Two PGPR strains from the rhizosphere of Haloxylon ammodendron promoted growth and enhanced drought tolerance of ryegrass. Plant Physiol. Biochem. 2021, 161, 74–85. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 096–102. [Google Scholar]
- Yadav, A.N. Biodiversity and bioprospecting of extremophilic microbiomes for agro-environmental sustainability. J. Appl. Biol. 2021, 9, 1–6. [Google Scholar]
- Gupta, V.K.; Mittal, A.; Malviya, A.; Mittal, J. Adsorption of carmoisine A from wastewater using waste materials-bottom ash and deoiled soya. J. Colloid Interface Sci. 2009, 335, 24–33. [Google Scholar] [CrossRef]
- FAO. Tartrazine. Allura Red AC. Compendium of Food Additive Specifications. Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82nd Meeting 2016. FAO JECFA Monographs 19. FAO/WHO 2016. Available online: http://www.fao.org (accessed on 12 September 2021).
- Barragán, B.E.; Costa, C.; Marquez, M.C. Biodegradation of azo dyes by bacteria inoculated on solid media. Dyes Pigm. 2007, 75, 73–81. [Google Scholar] [CrossRef]
- Maza, F.; Maldonado, J.; Vásquez-Dean, J.; Mandakovic, D.; Gaete, A.; Cambiazo, V.; González, M. Soil Bacterial Communities from the Chilean Andean Highlands: Taxonomic Composition and Culturability. Front. Bioeng. Biotechnol. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in Legume-Rhizobium Technology; Springer: New York, NY, USA, 1994. [Google Scholar]
- Vincent, J.M.; Humphrey, B. Taxonomically significant group antigens in Rhizobium. J. Gen. Microbiol. 1970, 63, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Bergey, D.H.; Holt, J.G.; Noel, R.K. Bergey’s Manual of Systematic Bacteriology, 9th ed.; Williams & Wilkins: Baltimore, MD, USA, 1994; Volume 1, pp. 1935–2045. [Google Scholar]
- Kirsop, B.E.; Doyle, A. Maintenance of Microorganisms and Cultured Cells: A Manual of Laboratory Methods, 2nd ed.; Academic Press: London, UK, 1991. [Google Scholar]
- Shu, Y.; Wan-Ting, J.; Ya-Ning, Y.; Yu-Han, F. An Optimized CTAB Method for Genomic DNA Extraction from Freshly-picked Pinnae of Fern, Adiantum capillus-veneris L. Bio-Protocol 2018, 8, e2906. [Google Scholar] [CrossRef] [Green Version]
- Nzilibili, S.M.M.; Ekodiyanto, M.K.H.; Hardjanto, P.; Yudianto, A. Concentration and Purity DNA Spectrophotometer: Sodium Monofluorophosphate forensic impended effect. Egypt J. Forensic Sci. 2018, 8, 34. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 2012, 62, 3923. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Iyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Lovell, D.P. Statistical analysis of comet assay data. In The Comet Assay in Toxicology; Dhawan, A., Anderson, D., Eds.; RSC: Cambridge, UK, 2009; pp. 424–450. [Google Scholar]
- Raza, A.; Ejaz, S.; Saleem, M.S.; Hejnak, V.; Ahmad, F.; Ahmed, M.A.A.; Alotaibi, S.S.; El-Shehawi, M.A.; Alsubeie, M.S.; Zuan, A.T.K. Plant growth promoting rhizobacteria improve growth and yield related attributes of chili under low nitrogen availability. PLoS ONE 2021, 16, e0261468. [Google Scholar] [CrossRef]
- Sheetal, K.; Halady, P.; Swapna, B. Genotoxicity Induced by Food Coloring Dyes on Meristematic Cells Root Tips of Allium Cepa. Int. J. Trend Sci. Res. Dev. 2019, 3, 116–118. [Google Scholar] [CrossRef]
- Sadar, P.; Dande, P.; Kulkarni, N.; Pachori, R. Evaluation of toxicity of synthetic food colors on human normal flora and yeast. Int. J. Health Sci. Res. 2017, 7, 110–114. [Google Scholar]
- Gallart, M.; Paungfoo-Lonhienne, C.; Gonzalez, A.; Trueman, S.J. Nitrogen source influences the effect of plant growth-promoting rhizobacteria (Pgpr) on macadamia integrifolia. J. Agron. 2021, 11, 1064. [Google Scholar] [CrossRef]
- Fernandes, F.H.; de Aragão Umbuzeiro, G.; Fávero Salvadori, D.M. Genotoxicity of textile dye C.I. Disperse Blue 291 in mouse bone marrow. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 837, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Pan, J.; Xue, X.; Zhang, J.; Guo, Q. A Bibliometric Review of Plant Growth-Promoting Rhizobacteria in Salt-Affected Soils. Agronomy 2022, 12, 2304. [Google Scholar] [CrossRef]
- Chequer, F.M.D.; de Paula Venâncio, V.; Bianchi, M.D.L.P.; Antunes, L.M.G. Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells. Food Chem. Toxicol. 2012, 50, 3447–3451. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Ogimoto, M.; Uematsu, Y.; Suzuki, K.; Kabashima, J.; Nakazato, M. Shokuhin eiseigaku zasshi. J. Food Hyg. Soc. Jpn. 2009, 50, 256–260. [Google Scholar] [CrossRef]
- Kiziltan, T.; Baran, A.; Kankaynar, M.; Şenol, O.; Sulukan, E.; Yildirim, S.; Ceyhun, S.B. Effects of the food colorant carmoisine on zebrafish embryos at a wide range of concentrations. Arch. Toxicol. 2022, 96, 1089–1099. [Google Scholar] [CrossRef]
- Zeid, H.A.A.; El-Zayat, M.M.; Abdrabouh, A.E.S. Ecotoxicological impacts of industrial effluents on irrigation water quality, animal health and the role of calcium alginate in effluents treatment. Environ Monit Assess. 2022, 194, 1–25. [Google Scholar] [CrossRef]
- Othman, Y.A.; Al-Assaf, A.; Tadros, M.J.; Albalawneh, A. Heavy Metals and Microbes Accumulation in Soil and Food Crops Irrigated with Wastewater and the Potential Human Health Risk: A Metadata Analysis. Water 2021, 13, 3405. [Google Scholar] [CrossRef]
- Suryavathi, V.; Sharma, S.; Sharma, S.; Saxena, P.; Pandey, S.; Grover, R.; Kumar, S.; Sharma, K.P. Acute toxicity of textile dye wastewaters (untreated and treated) of Sanganer on male reproductive systems of albino rats and mice. Reprod. Toxicol. 2005, 19, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.A.; Saigo, K.; Koyanagi, M.; Hayashi, S.M. Magnesium stearate, a widely-used food additive, exhibits a lack of in vitro and in vivo genotoxic potential. Toxicology 2017, 4, 554–559. [Google Scholar] [CrossRef]
- Fernandes, A.; Pinto, B.; Bonardo, L.; Royo, B.; Robalo, M.P.; Martins, L.O. Wasteful Azo Dyes as a Source of Biologically Active Building Blocks. Front. Bioeng. Biotechnol. 2021, 9, 672436. [Google Scholar] [CrossRef]
- Biswas, M.M.; Taylor, K.E.; Bewtra, J.K.; Biswas, N. Enzymatic treatment of sulfonated aromatic amines generated from reductive degradation of reactive azo dyes. Water Environ. Res. 2007, 79, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tuormaa, T.E. The adverse effects of food additive on health. J. Orthomol. Med. 1994, 9, 225–243. [Google Scholar]
- New Jersey Department of Health and Senior Services. Hazardous Substances Fact Sheet [CAS Number: 91-59-8]. Revised 2004. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1324.pdf (accessed on 12 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7057, 2-Naphthylamine. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Naphthylamine (accessed on 31 December 2022).

| Time | Dilutions | Azorubine E-122 | Tartrazine E-102 | Allura Red AC E-129 | |||
|---|---|---|---|---|---|---|---|
| Concentration (μg/mL) | Ratio 260/280 | Concentration (μg/mL) | Ratio 260/280 | Concentration (μg/mL) | Ratio 260/280 | ||
| 24 h | Control | 48 | 1.83 | 52 | 1.89 | 39 | 1.82 |
| 0.25% | 40 | 1.75 | 36 | 1.4 | 53 | 1.6 | |
| 0.50% | 29 | 1.59 | 40 | 1.83 | 24 | 2.2 | |
| 0.75% | 42 | 1.66 | 52 | 1.75 | 28 | 1.4 | |
| 1.00% | 37 | 1.5 | 48 | 1.63 | 38 | 1.93 | |
| 1.25% | 36 | 2.2 | 33 | 1.66 | 42 | 1.6 | |
| 48 h | Control | 34 | 1.81 | 37 | 1.79 | 60 | 1.8 |
| 0.25% | 40 | 1.6 | 42 | 1.5 | 25 | 1.5 | |
| 0.50% | 30 | 1.5 | 27 | 1.75 | 53 | 1.63 | |
| 0.75% | 41 | 1.22 | 50 | 1.73 | 44 | 1.57 | |
| 1.00% | 50 | 1.96 | 34 | 2.2 | 34 | 2.2 | |
| 1.25% | 44 | 1.99 | 29 | 1.8 | 49 | 1.75 | |
| Time | Concentration of Dilution | Azorubine E-122 | Tartrazine E-102 | Allura Red AC E-129 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head Length | Tail Length | Comet Length | DNA Head | DNA Tail | Head Length | Tail Length | Comet Length | DNA Head | DNA Tail | Head Length | Tail Length | Comet Length | DNA Head | DNA Tail | ||
| 24 h | Control | 15 | 3 | 18 | 100 | −0.1 | 8.0 | 1.0 | 9.0 | 100 | −0.1 | 5 | 3 | 6.0 | 100 | −0.1 |
| 0.25% | 10 | 4 | 12 | 100 | 3 | 7.0 | 2.0 | 9.0 | 83 | 18 | 10 | 3 | 12 | 99 | 1 | |
| 0.50% | 8 | 39 | 46 | 18 | 83 | 14 | 14 | 28 | 61 | 40 | 9 | 7 | 14 | 69 | 32 | |
| 0.75% | 19 | 35 | 54 | 30 | 71 | 31 | 35 | 66 | 50 | 52 | 20 | 24 | 44 | 61 | 41 | |
| 1.00% | 10 | 25 | 35 | 31 | 70 | 10 | 25 | 35 | 31 | 70 | 23 | 55 | 78 | 73 | 28 | |
| 1.25% | 4 | 15 | 18 | 15 | 86 | 5.0 | 11 | 16 | 36 | 65 | 11 | 33 | 44 | 28 | 73 | |
| 48 h | Control | 33 | 3.0 | 36 | 100 | −0.1 | 20 | 1.0 | 21 | 100 | −0.1 | 14 | 2 | 15 | 100 | −0.1 |
| 0.25% | 5 | 15 | 19 | 88 | 13 | 15 | 4.0 | 19 | 88 | 13 | 13 | 33 | 46 | 81 | 20 | |
| 0.50% | 16 | 17 | 33 | 36 | 65 | 14 | 15 | 29 | 54 | 47 | 7 | 15 | 21 | 25 | 76 | |
| 0.75% | 20 | 23 | 43 | 31 | 70 | 19 | 19 | 38 | 21 | 80 | 10 | 19 | 29 | 45 | 56 | |
| 1.00% | 3 | 19 | 21 | 11 | 90 | 10 | 12 | 22 | 24 | 77 | 10 | 34 | 43 | 22 | 79 | |
| 1.25% | 6 | 52 | 57 | 8 | 93 | 4.0 | 9.0 | 13 | 22 | 79 | 8 | 45 | 52 | 9 | 91 | |
| Groups of Treatment | Source of Variation | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|---|
| 24 h | Between groups | 103 | 2 | 778 | 0.753 | 0.488 |
| Within groups | 20,446 | 15 | 1034 | |||
| Total | 20,550 | 17 | ||||
| 48 h | Between groups | 103 | 2 | 51.7 | 0.0379 | 0.963 |
| Within groups | 20,446 | 15 | 1363 | |||
| Total | 20,550 | 17 |
| Groups | Count | Sum | Average | Variance | |
|---|---|---|---|---|---|
| E-122 | 6 | 308 | 51.4 | 1617 | |
| 24 h | E-102 | 6 | 242 | 40.4 | 738.4 |
| E-129 | 6 | 172 | 28.6 | 747.5 | |
| E-122 | 6 | 327 | 54.6 | 1525 | |
| 48 h | E-102 | 6 | 294 | 48.9 | 1252 |
| E-129 | 6 | 319 | 53.2 | 1311 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, A.; Luqman, M.; Muhammad, S.; Hanif, U.; Sardar, A.A.; Ali, S.; Hasnain, A.; Tufail, M.; Khan, Z.I.; Hussain, M.I.; et al. Genotoxicity of Synthetic Food Colors on Nitrogen-Fixing Bacteria in Agricultural Lands Irrigated with Wastewater of Corresponding Industries. Sustainability 2023, 15, 2897. https://doi.org/10.3390/su15042897
John A, Luqman M, Muhammad S, Hanif U, Sardar AA, Ali S, Hasnain A, Tufail M, Khan ZI, Hussain MI, et al. Genotoxicity of Synthetic Food Colors on Nitrogen-Fixing Bacteria in Agricultural Lands Irrigated with Wastewater of Corresponding Industries. Sustainability. 2023; 15(4):2897. https://doi.org/10.3390/su15042897
Chicago/Turabian StyleJohn, Arooba, Muhammad Luqman, Sohaib Muhammad, Uzma Hanif, Andleeb Anwar Sardar, Shaukat Ali, Ali Hasnain, Matiba Tufail, Zafar Iqbal Khan, Muhammad Iftikhar Hussain, and et al. 2023. "Genotoxicity of Synthetic Food Colors on Nitrogen-Fixing Bacteria in Agricultural Lands Irrigated with Wastewater of Corresponding Industries" Sustainability 15, no. 4: 2897. https://doi.org/10.3390/su15042897








