Current and Future Trends for Crude Glycerol Upgrading to High Value-Added Products
Abstract
1. Introduction
2. Glycerol and Its Properties
3. Various Glycerol Resources
3.1. Saponification of Soap Manufacturing
3.2. Crude Glycerol from Transesterification of Biodiesel Production
3.3. Hydrolysis Reactions in Oleochemical Plants
3.4. Biodiesel Technology Wastewater
3.5. Synthetic Glycerol
4. Glycerol Upgrading
4.1. Biochemical Approaches
4.1.1. Microbial Fermentation—Anaerobic and Aerobic Digestion
- 1.
- Bacteria
| Microbes | Strain | Outcomes | Ref. | |
|---|---|---|---|---|
| End-Products | Yield (mol/mol Glycerol) | |||
| Bacteria | ||||
| Clostridium butyricum VPI 1718 | 1,3-PDO | 0.55 | [87] | |
| C. butyricum VPI 3266 | 1,3-PDO | 0.65 | [88] | |
| C. butyricum DSM 10702 | 1,3-PDO | 0.51 | [86] | |
| C. butyricum AKR102a | 1,3-PDO | 0.52 | [89] | |
| C. pasteurianum | 1,3-PDO | 0.17 | [91] | |
| C. pasteurianum | Butanol | 0.65 | [92] | |
| C. pasteurianum | n-butanol | 0.28 | [90] | |
| C. pasteurianum | n-butanol | 0.43 | [106] | |
| Clostridium sp. Strain CT7 | Butanol | 0.40 | [107] | |
| Klebsiella oxytoca | 1,3-PDO | 0.47 | [93] | |
| K. oxytoca | 1,3-PDO | 0.53 | [94] | |
| K. pneumonia DSMZ 2026 | 1,3-PDO | 0.42 | [38] | |
| K. pneumonia mutant | 1,3-PDO | 0.53 | [96] | |
| K.pneumonia ATCC 8724 | 1,3-PDO | 0.73 | [98] | |
| K.pneumonia M5al | 1,3-PDO | 0.53 | [95] | |
| K. pneumonia | 2-butanol | 0.01 | [108] | |
| Citrobacter freundii AD970 | 1,3-PDO | 0.49 | [100] | |
| C. freundii FMCC-B294 | 1,3-PDO | 0.48 | [99] | |
| C. werkmanii DSM17579 | 1,3-PDO | 0.62 | [101] | |
| Lactobacillus brevis N1E9.3.3 | 1,3-PDO | 0.89 | [102] | |
| L. reuteri CH53 | 1,3-PDO | 0.83 | [109] | |
| L. casei NCIM 2125 | Lactic acid | 0.16 | [110] | |
| Enterobacter sp. Strain MU-01 | 1,3-PDO | 0.24 | [39] | |
| E. aerogenes TISTR 1468 | Ethanol | 0.59 | [5] | |
| Escherichia coli | 1,2-PDO | 0.21 | [103] | |
| E. coli K-12 ER2925 | 1,3-PDO | 0.90 | [105] | |
| E. coli | n-butanol | 0.35 | [42] | |
| E. coli | D-lactic acid | 0.85 | [111] | |
| E. coli | L-lactic acid | 0.93 | [112] | |
| E. coli AC-521 | Lactic acid | 0.88 | [113] | |
| E. coli SS1 | Bioethanol | 0.88 | [37] | |
| E. coli SS1 | Ethanol | 1.0 | [114] | |
| Other bacteria and mixed culture | ||||
| Komagataella phafii Glpard | Lactic acid | 0.67 | [115] | |
| Pachysolen tannophilus | Ethanol | 0.56 | [36] | |
| Rhodopseudomonas palustris CGA009 | H2 | 0.60 | [116] | |
| Paenibacillus macerans | H2 | 0.81 | [117] | |
| Thermoanaerobacterium sp. | H2 | 0.30 | [8] | |
| Mixed culture | H2 | 0.52 | [118] | |
| Mixed culture | H2 | 0.96 | [41] | |
| Fungi | ||||
| Lentinula edodes | SCO | 0.10 | [84] | |
| Aspergillus niger | Oxalic acid | 0.62 | [84] | |
| Galactomyces geotrichum | SCO | 0.44 | [119] | |
| Thamnidium elegans | SCO | 0.48 | [87] | |
| Yeast | ||||
| Saccharomyces cerevisiae | D-lactic acid | 0.80 | [34] | |
| S. cerevisiae | Ethanol | 0.12 | [120] | |
| Yarrowia lipolytica NG40/UV7 | Citric acid | 0.90 | [121] | |
| Y. lipolytica NG40/UV5 | Citric acid | 0.90 | [122] | |
| Y. lipolytica A-101–1.22 | Citric acid | 0.64 | [123] | |
| Y. lipolytica | Succinic acid | 0.45 | [124] | |
| Microalgae | Schizochytrium limacium SR21 | Docosahexanoic acid | 0.23 | [125] |
- 2.
- Microbial mixed cultures and other bacteria
- 3.
- Fungi
- 4.
- Yeast
4.1.2. Bio-Electrochemical Fermentation
4.2. Thermochemical Approaches
4.2.1. Gasification Pyrolysis
4.2.2. Fast Pyrolysis
4.2.3. Supercritical Fluids
4.2.4. Steam Reforming
4.2.5. Aqueous Phase Reforming
4.2.6. Microwave-Assisted Pyrolysis
4.3. Physicochemical Techniques
4.3.1. Esterification
4.3.2. Transesterification
4.3.3. Catalytic Etherification
4.4. Electrochemical Approaches—Electrolysis
5. Future Outlooks for Crude Glycerol Upgrading
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA Renewable Electricity Growth Is Accelerating Faster than Ever Worldwide, Supporting the Emergence of the New Global Energy Economy. Available online: https://www.iea.org/news/renewable-electricity-growth-is-accelerating-faster-than-ever-worldwide-supporting-the-emergence-of-the-new-global-energy-economy (accessed on 25 July 2022).
- International Energy Agency. World Energy Outlook; OECD/IEA: Paris, France, 2009; ISBN 926428205X. [Google Scholar]
- Gielen, D.; Gorini, R.; Wagner, N.; Leme, R.; Gutierrez, L.; Prakash, G.; Asmelash, E.; Janeiro, L.; Gallina, G.; Vale, G.; et al. Global Energy Transformation: A Roadmap to 2050; IRENA: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- Loaces, I.; Rodríguez, C.; Amarelle, V.; Fabiano, E.; Noya, F. Improved Glycerol to Ethanol Conversion by E. Coli Using a Metagenomic Fragment Isolated from an Anaerobic Reactor. J. Ind. Microbiol. Biotechnol. 2016, 43, 1405–1416. [Google Scholar] [CrossRef]
- Boonyawanich, S.; Haosagul, S.; Pisutpaisal, N. Ethanol Production from Crude glycerol Using Glucose as Co-Carbon Source. Biomass Convers. Biorefinery 2021, 2021, 1–10. [Google Scholar]
- Haider, M.H.; Dummer, N.F.; Knight, D.W.; Jenkins, R.L.; Howard, M.; Moulijn, J.; Taylor, S.H.; Hutchings, G.J. Efficient Green Methanol Synthesis from Glycerol. Nat. Chem. 2015, 7, 1028–1032. [Google Scholar] [CrossRef]
- Hulteberg, C.; Nörregård, Ö.; Brandin, J.; Leveau, A. Bio Propane: Tailoring WO3/ZrO2 Catalyst for the Dehydration of Glycerol to Acrolein. In Proceedings of the 17th Nordic Symposium on Catalysis, Lund, Sweden, 14–16 June 2016; Lund University Publications: Lund, Sweden, 2016. [Google Scholar]
- Sittijunda, S.; Reungsang, A. Media Optimization for Biohydrogen Production from Crude glycerol by Anaerobic Thermophilic Mixed Cultures. Int. J. Hydrogen Energy 2012, 37, 15473–15482. [Google Scholar] [CrossRef]
- Yu-Wu, Q.M.; Weiss-Hortala, E.; Barna, R.; Boucard, H.; Bulza, S. Glycerol and Bioglycerol Conversion in Supercritical Water for Hydrogen Production. Environ. Technol. 2012, 33, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, Consumption, Prices, Characterization and New Trends in Combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Ennetta, R.; Soyhan, H.S.; Koyunoğlu, C.; Demir, V.G. Current Technologies and Future Trends for Biodiesel Production: A Review. Arab. J. Sci. Eng. 2022, 47, 15133–15151. [Google Scholar] [CrossRef]
- Varanda, M.G.; Pinto, G.; Martins, F. Life Cycle Analysis of Biodiesel Production. Fuel Process. Technol. 2011, 92, 1087–1094. [Google Scholar] [CrossRef]
- Nitayavardhana, S.; Khanal, S.K. Biodiesel-Derived Crude Glycerol Bioconversion to Animal Feed: A Sustainable Option for a Biodiesel Refinery. Bioresour. Technol. 2011, 102, 5808–5814. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, S.; Hou, Z.; Zhang, M.; Hou, Z. Transesterification of Glycerol with Dimethyl Carbonate over Mg-Al Hydrotalcites. Chin. J. Catal. 2014, 35, 310–318. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.; Aziz, N.; Kim, J.; Fernando, W. Solid Heterogeneous Catalysts for Transesterification of Triglycerides with Methanol: A Review. Appl. Catal. Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical Review on the Current Scenario and Significance of Crude Glycerol Resulting from Biodiesel Industry towards More Sustainable Renewable Energy Industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Christoph, R.; Schmidt, B.; Steinberner, U.; Dilla, W.; Karinen, R. Glycerol. In Ullmann’s Encyclopedia of Industrial Chemistry; Verlag Chemie: Hoboken, NJ, USA, 2000. [Google Scholar]
- Gupta, M.; Kumar, N. Scope and Opportunities of Using Glycerol as an Energy Source. Renew. Sustain. Energy Rev. 2012, 16, 4551–4556. [Google Scholar] [CrossRef]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.-T. Dealing with the Surplus of Glycerol Production from Biodiesel Industry through Catalytic Upgrading to Polyglycerols and Other Value-Added Products. Renew. Sustain. Energy Rev. 2014, 39, 327–341. [Google Scholar] [CrossRef]
- Samudrala, S.P. Glycerol Transformation to Value-Added 1,3-Propanediol Production: A Paradigm for a Sustainable Biorefinery Process. In Glycerine Production and Transformation—An Innovative Platform for Sustainable Biorefinery and Energy; BoD—Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Kenar, J.A. Glycerol as a Platform Chemical: Sweet Opportunities on the Horizon? Lipid Technol. 2007, 19, 249–253. [Google Scholar] [CrossRef]
- Tan, H.; Aziz, A.A.; Aroua, M. Glycerol Production and Its Applications as a Raw Material: A Review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Israel, A.; Obot, I.; Asuquo, J. Recovery of Glycerol from Spent Soap LyeBy-Product of Soap Manufacture. E—J. Chem. 2008, 5, 940–945. [Google Scholar] [CrossRef]
- Hasheminejad, M.; Tabatabaei, M.; Mansourpanah, Y.; Javani, A. Upstream and Downstream Strategies to Economize Biodiesel Production. Bioresour. Technol. 2011, 102, 461–468. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the Glycerol Market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Gunstone, F. The Chemistry of Oils and Fats: Sources, Composition, Properties and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 1-4051-5002-5. [Google Scholar]
- Guo, X.Y.; Ci, B.B.; Li, S.G.; Hu, W.; Yu, J.L.; Tang, X.H. Study on Upgrading and Further Usage of Glycerol from Waste Water. Adv. Mater. Res. 2012, 383–390, 4511–4515. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Echeta, K.C. Current Developments in Sugar Alcohols: Chemistry, Nutrition, and Health Concerns of Sorbitol, Xylitol, Glycerol, Arabitol, Inositol, Maltitol, and Lactitol. Int. J. Adv. Acad. Res. 2019, 5, 1–33. [Google Scholar]
- Eierdanz, H. Oleochemistry: Processes and Products; The American Oil Chemists Society: Urbana, IL, USA, 1993; p. 217. [Google Scholar]
- Yeong, S.K.; Idris, Z.; Hassan, H.A. Palm Oleochemicals in Non-Food Applications. In Palm Oil; Elsevier: Amsterdam, The Netherlands, 2012; pp. 587–624. [Google Scholar]
- Hejna, A.; Kosmela, P.; Formela, K.; Piszczyk, Ł.; Haponiuk, J.T. Potential Applications of Crude Glycerol in Polymer Technology–Current State and Perspectives. Renew. Sustain. Energy Rev. 2016, 66, 449–475. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, X.; Shen, Y. Commodity Chemicals Derived from Glycerol, an Important Biorefinery Feedstock. Chem. Rev. 2008, 110, 1807. [Google Scholar] [CrossRef]
- Sharninghausen, L.S.; Campos, J.; Manas, M.G.; Crabtree, R.H. Efficient Selective and Atom Economic Catalytic Conversion of Glycerol to Lactic Acid. Nat. Commun. 2014, 5, 5084. [Google Scholar] [CrossRef]
- Baek, S.-H.; Kwon, E.Y.; Kim, Y.H.; Hahn, J.-S. Metabolic Engineering and Adaptive Evolution for Efficient Production of D-Lactic Acid in Saccharomyces Cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 2737–2748. [Google Scholar] [CrossRef]
- Rangel, A.E.T.; Reyes, L.H.; Ramírez, J.M.G.; Barrios, A.F.G. Optimization of Glycerol Consumption in Wild-type Escherichia coli Using Central Carbon Modeling as an Alternative Approach. Biofuels Bioprod. Biorefin. 2021, 15, 825–839. [Google Scholar] [CrossRef]
- Liu, X.; Jensen, P.R.; Workman, M. Bioconversion of Crude Glycerol Feedstocks into Ethanol by Pachysolen Tannophilus. Bioresour. Technol. 2012, 104, 579–586. [Google Scholar] [CrossRef]
- Adnan, N.A.A.; Suhaimi, S.N.; Abd-Aziz, S.; Hassan, M.A.; Phang, L.-Y. Optimization of Bioethanol Production from Glycerol by Escherichia coli SS1. Renew. Energy 2014, 66, 625–633. [Google Scholar] [CrossRef]
- Laura, M.; Monica, T.; Dan-Cristian, V. The Effect of Crude Glycerol Impurities on 1,3-Propanediol Biosynthesis by Klebsiella Pneumoniae DSMZ 2026. Renew. Energy 2020, 153, 1418–1427. [Google Scholar] [CrossRef]
- Kongjan, P.; Jariyaboon, R.; Reungsang, A.; Sittijunda, S. Co-Fermentation of 1,3-Propanediol and 2,3-Butanediol from Crude Glycerol Derived from the Biodiesel Production Process by Newly Isolated Enterobacter sp.: Optimization Factors Affecting. Bioresour. Technol. Rep. 2021, 13, 100616. [Google Scholar] [CrossRef]
- Rodrigues, C.V.; Santana, K.O.; Nespeca, M.G.; Rodrigues, A.V.; Pires, L.O.; Maintinguer, S.I. Energy Valorization of Crude Glycerol and Sanitary Sewage in Hydrogen Generation by Biological Processes. Int. J. Hydrogen Energy 2020, 45, 11943–11953. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Y.; Wang, J. Comparison of Fermentative Hydrogen Production from Glycerol Using Immobilized and Suspended Mixed Cultures. Int. J. Hydrogen Energy 2021, 46, 8986–8994. [Google Scholar] [CrossRef]
- Saini, M.; Wang, Z.W.; Chiang, C.-J.; Chao, Y.-P. Metabolic Engineering of Escherichia coli for Production of N-Butanol from Crude Glycerol. Biotechnol. Biofuels 2017, 10, 173. [Google Scholar] [CrossRef]
- Xafenias, N.; Anunobi, M.O.; Mapelli, V. Electrochemical Startup Increases 1,3-Propanediol Titers in Mixed-Culture Glycerol Fermentations. Process Biochem. 2015, 50, 1499–1508. [Google Scholar] [CrossRef]
- Dennis, P.G.; Harnisch, F.; Yeoh, Y.K.; Tyson, G.W.; Rabaey, K. Dynamics of Cathode-Associated Microbial Communities and Metabolite Profiles in a Glycerol-Fed Bioelectrochemical System. Appl. Environ. Microbiol. 2013, 79, 4008–4014. [Google Scholar] [CrossRef]
- Roume, H.; Arends, J.B.; Ameril, C.P.; Patil, S.A.; Rabaey, K. Enhanced Product Recovery from Glycerol Fermentation into 3-Carbon Compounds in a Bioelectrochemical System Combined with in Situ Extraction. Front. Bioeng. Biotechnol. 2016, 4, 73. [Google Scholar] [CrossRef]
- Blass, S.D.; Hermann, R.J.; Persson, N.E.; Bhan, A.; Schmidt, L.D. Conversion of Glycerol to Light Olefins and Gasoline Precursors. Appl. Catal. Gen. 2014, 475, 10–15. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C.C. Catalytic Conversion of Glycerol for Sustainable Production of Solketal as a Fuel Additive: A Review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Skoulou, V.K.; Zabaniotou, A.A. Co-Gasification of Crude Glycerol with Lignocellulosic Biomass for Enhanced Syngas Production. J. Anal. Appl. Pyrolysis 2013, 99, 110–116. [Google Scholar] [CrossRef]
- He, S.; Muizebelt, I.; Heeres, A.; Schenk, N.; Blees, R.; Heeres, H. Catalytic Pyrolysis of Crude Glycerol over Shaped ZSM-5/Bentonite Catalysts for Bio-BTX Synthesis. Appl. Catal. B Environ. 2018, 235, 45–55. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of Bio-Oil from Biomass Fast Pyrolysis in China: A Review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Cui, M.; Mi, M.; Zhang, Y.; Xu, W.; Wang, M.; Shao, R.; Ding, J. Dehydration of Glycerol over H6P2W18O62/γ-Al2O3 Prepared by Supercritical Method. New J. Chem. 2022, 47, 1342–1348. [Google Scholar] [CrossRef]
- Ott, L.; Bicker, M.; Vogel, H. Catalytic Dehydration of Glycerol in Sub-and Supercritical Water: A New Chemical Process for Acrolein Production. Green Chem. 2006, 8, 214–220. [Google Scholar] [CrossRef]
- Ezhova, N.; Korosteleva, I.; Kolesnichenko, N.; Kuz’min, A.; Khadzhiev, S.; Vasil’eva, M.; Voronina, Z. Glycerol Carboxylation to Glycerol Carbonate in the Presence of Rhodium Complexes with Phosphine Ligands. Pet. Chem. 2012, 52, 91–96. [Google Scholar] [CrossRef]
- Okoye, P.; Hameed, B. Review on Recent Progress in Catalytic Carboxylation and Acetylation of Glycerol as a Byproduct of Biodiesel Production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Ye, X.P. Lactic Acid Production from Glycerol Using CaO as Solid Base Catalyst. Fuel Process. Technol. 2014, 120, 40–47. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P. Simultaneous Production of Lactic Acid and Propylene Glycol from Glycerol Using Solid Catalysts without External Hydrogen. Fuel Process. Technol. 2015, 137, 55–65. [Google Scholar] [CrossRef]
- Charisiou, N.; Papageridis, K.; Siakavelas, G.; Tzounis, L.; Kousi, K.; Baker, M.; Hinder, S.; Sebastian, V.; Polychronopoulou, K.; Goula, M. Glycerol Steam Reforming for Hydrogen Production over Nickel Supported on Alumina, Zirconia and Silica Catalysts. Top. Catal. 2017, 60, 1226–1250. [Google Scholar] [CrossRef]
- Charisiou, N.; Papageridis, K.; Tzounis, L.; Sebastian, V.; Hinder, S.; Baker, M.; AlKetbi, M.; Polychronopoulou, K.; Goula, M. Ni Supported on CaO-MgO-Al2O3 as a Highly Selective and Stable Catalyst for H2 Production via the Glycerol Steam Reforming Reaction. Int. J. Hydrogen Energy 2019, 44, 256–273. [Google Scholar] [CrossRef]
- Chen, D.; Wang, W.; Liu, C. Hydrogen Production through Glycerol Steam Reforming over Beehive-Biomimetic Graphene-Encapsulated Nickel Catalysts. Renew. Energy 2020, 145, 2647–2657. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, B.; Yan, J.; Hong, J.; Wang, L.; Zhang, Y.; Li, J.; Jing, F.; Chu, W. Plasma Assisted Preparation of Nickel-Based Catalysts Supported on CeO2 with Different Morphologies for Hydrogen Production by Glycerol Steam Reforming. Powder Technol. 2019, 354, 324–332. [Google Scholar] [CrossRef]
- Callison, J.; Subramanian, N.; Rogers, S.; Chutia, A.; Gianolio, D.; Catlow, C.R.A.; Wells, P.P.; Dimitratos, N. Directed Aqueous-Phase Reforming of Glycerol through Tailored Platinum Nanoparticles. Appl. Catal. B Environ. 2018, 238, 618–628. [Google Scholar] [CrossRef]
- Ciftci, A.; Ligthart, D.M.; Hensen, E.J. Influence of Pt Particle Size and Re Addition by Catalytic Reduction on Aqueous Phase Reforming of Glycerol for Carbon-Supported Pt (Re) Catalysts. Appl. Catal. B Environ. 2015, 174, 126–135. [Google Scholar] [CrossRef]
- Liu, F.; Okolie, C.; Ravenelle, R.M.; Crittenden, J.C.; Sievers, C.; Bruijnincx, P.C.; Weckhuysen, B.M. Silica Deposition as an Approach for Improving the Hydrothermal Stability of an Alumina Support during Glycerol Aqueous Phase Reforming. Appl. Catal. Gen. 2018, 551, 13–22. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. A Thermodynamic Analysis of Hydrogen Production via Aqueous Phase Reforming of Glycerol. Fuel Process. Technol. 2015, 134, 107–115. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Manara, P.; Zabaniotou, A. Effect of Microwave Pretreatment on Pyrolysis of Crude Glycerol–Olive Kernel Alternative Fuels. Energy Convers. Manag. 2016, 110, 287–295. [Google Scholar] [CrossRef]
- Leong, S.K.; Lam, S.S.; Ani, F.N.; Ng, J.-H.; Chong, C.T. Production of Pyrolyzed Oil from Crude Glycerol Using a Microwave Heating Technique. Int. J. Technol. 2016, 7, 323–331. [Google Scholar] [CrossRef]
- Ng, J.-H.; Leong, S.K.; Lam, S.S.; Ani, F.N.; Chong, C.T. Microwave-Assisted and Carbonaceous Catalytic Pyrolysis of Crude Glycerol from Biodiesel Waste for Energy Production. Energy Convers. Manag. 2017, 143, 399–409. [Google Scholar] [CrossRef]
- Leng, L.; Yuan, X.; Zeng, G.; Chen, X.; Wang, H.; Li, H.; Fu, L.; Xiao, Z.; Jiang, L.; Lai, C. Rhamnolipid Based Glycerol-in-Diesel Microemulsion Fuel: Formation and Characterization. Fuel 2015, 147, 76–81. [Google Scholar] [CrossRef]
- Sedghi, R.; Shahbeik, H.; Rastegari, H.; Rafiee, S.; Peng, W.; Nizami, A.-S.; Gupta, V.K.; Chen, W.-H.; Lam, S.S.; Pan, J. Turning Biodiesel Glycerol into Oxygenated Fuel Additives and Their Effects on the Behavior of Internal Combustion Engines: A Comprehensive Systematic Review. Renew. Sustain. Energy Rev. 2022, 167, 112805. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, H. Stability of Emulsion Fuels Prepared from Fast Pyrolysis Bio-Oil and Glycerol. Fuel 2017, 206, 230–238. [Google Scholar] [CrossRef]
- Álvarez, M.; Frey, A.; Bitter, J.; Segarra, A.; De Jong, K.; Medina, F. On the Role of the Activation Procedure of Supported Hydrotalcites for Base Catalyzed Reactions: Glycerol to Glycerol Carbonate and Self-Condensation of Acetone. Appl. Catal. B Environ. 2013, 134, 231–237. [Google Scholar] [CrossRef]
- Hu, K.; Wang, H.; Liu, Y.; Yang, C. KNO3/CaO as Cost-Effective Heterogeneous Catalyst for the Synthesis of Glycerol Carbonate from Glycerol and Dimethyl Carbonate. J. Ind. Eng. Chem. 2015, 28, 334–343. [Google Scholar] [CrossRef]
- Khayoon, M.; Hameed, B.H. Mg1+xCa1−xO2 as Reusable and Efficient Heterogeneous Catalyst for the Synthesis of Glycerol Carbonate via the Transesterification of Glycerol with Dimethyl Carbonate. Appl. Catal. Gen. 2013, 466, 272–281. [Google Scholar] [CrossRef]
- Chang, J.-S.; Chen, D.-H. Optimization on the Etherification of Glycerol with Tert-Butyl Alcohol. J. Taiwan Inst. Chem. Eng. 2011, 42, 760–767. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; Di Blasi, O. Catalytic Etherification of Glycerol by Tert-Butyl Alcohol to Produce Oxygenated Additives for Diesel Fuel. Appl. Catal. Gen. 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Frusteri, F.; Frusteri, L.; Cannilla, C.; Bonura, G. Catalytic Etherification of Glycerol to Produce Biofuels over Novel Spherical Silica Supported Hyflon® Catalysts. Bioresour. Technol. 2012, 118, 350–358. [Google Scholar] [CrossRef]
- Bott-Neto, J.L.; Garcia, A.C.; Oliveira, V.L.; de Souza, N.E.; Tremiliosi-Filho, G. Au/C Catalysts Prepared by a Green Method towards C3 Alcohol Electrooxidation: A Cyclic Voltammetry and in Situ FTIR Spectroscopy Study. J. Electroanal. Chem. 2014, 735, 57–62. [Google Scholar] [CrossRef]
- Dai, C.; Sun, L.; Liao, H.; Khezri, B.; Webster, R.D.; Fisher, A.C.; Xu, Z.J. Electrochemical Production of Lactic Acid from Glycerol Oxidation Catalyzed by AuPt Nanoparticles. J. Catal. 2017, 356, 14–21. [Google Scholar] [CrossRef]
- Garcia, A.C.; Kolb, M.J.; van Nierop y Sanchez, C.; Vos, J.; Birdja, Y.Y.; Kwon, Y.; Tremiliosi-Filho, G.; Koper, M.T. Strong Impact of Platinum Surface Structure on Primary and Secondary Alcohol Oxidation during Electro-Oxidation of Glycerol. ACS Catal. 2016, 6, 4491–4500. [Google Scholar] [CrossRef]
- Gomes, J.F.; Tremiliosi-Filho, G. Spectroscopic Studies of the Glycerol Electro-Oxidation on Polycrystalline Au and Pt Surfaces in Acidic and Alkaline Media. Electrocatalysis 2011, 2, 96–105. [Google Scholar] [CrossRef]
- Kwon, Y.; Hersbach, T.J.; Koper, M. Electro-Oxidation of Glycerol on Platinum Modified by Adatoms: Activity and Selectivity Effects. Top. Catal. 2014, 57, 1272–1276. [Google Scholar] [CrossRef]
- Sauter, W.; Bergmann, O.L.; Schröder, U. Hydroxyacetone: A Glycerol-Based Platform for Electrocatalytic Hydrogenation and Hydrodeoxygenation Processes. ChemSusChem 2017, 10, 3105–3110. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-G.; Ho, P.-Y.; Chiang, C.-Y. Operando Mechanistic Studies of Selective Oxidation of Glycerol to Dihydroxyacetone over Amorphous Cobalt Oxide. Appl. Catal. B Environ. 2022, 300, 120723. [Google Scholar] [CrossRef]
- André, A.; Diamantopoulou, P.; Philippoussis, A.; Sarris, D.; Komaitis, M.; Papanikolaou, S. Biotechnological Conversions of Bio-Diesel Derived Crude glycerol into Added-Value Compounds by Higher Fungi: Production of Biomass, Single Cell Oil and Oxalic Acid. Ind. Crops Prod. 2010, 31, 407–416. [Google Scholar] [CrossRef]
- Pradima, J.; Kulkarni, M.R. Review on Enzymatic Synthesis of Value Added Products of Glycerol, a by-Product Derived from Biodiesel Production. Resour.-Effic. Technol. 2017, 3, 394–405. [Google Scholar] [CrossRef]
- Loureiro-Pinto, M.; González-Benito, G.; Coca, M.; Lucas, S.; García-Cubero, M.T. Valorization of Crude Glycerol from the Biodiesel Industry to 1,3-propanediol by Clostridium Butyricum DSM 10702: Influence of Pretreatment with Ion Exchange Resins. Can. J. Chem. Eng. 2016, 94, 1242–1248. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Papanikolaou, S.; Dietz, D.; Doulgeraki, A.I.; Nychas, G.-J.E.; Zeng, A.-P. Production of 1,3-Propanediol by Clostridium Butyricum Growing on Biodiesel-Derived Crude Glycerol through a Non-Sterilized Fermentation Process. Appl. Microbiol. Biotechnol. 2011, 91, 101–112. [Google Scholar] [CrossRef]
- González-Pajuelo, M.; Andrade, J.; Vasconcelos, I. Production of 1,3-Propanediol by Clostridium Butyricum VPI 3266 in Continuous Cultures with High Yield and Productivity. J. Ind. Microbiol. Biotechnol. 2005, 32, 391–396. [Google Scholar] [CrossRef]
- Wilkens, E.; Ringel, A.K.; Hortig, D.; Willke, T.; Vorlop, K.-D. High-Level Production of 1,3-Propanediol from Crude Glycerol by Clostridium Butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012, 93, 1057–1063. [Google Scholar] [CrossRef]
- Jensen, T.Ø.; Kvist, T.; Mikkelsen, M.J.; Christensen, P.V.; Westermann, P. Fermentation of Crude Glycerol from Biodiesel Production by Clostridium Pasteurianum. J. Ind. Microbiol. Biotechnol. 2012, 39, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.E.; Rehmann, L. The Role of 1,3-Propanediol Production in Fermentation of Glycerol by Clostridium Pasteurianum. Bioresour. Technol. 2016, 209, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.E.; Sokolenko, S.; Liu, X.; Srirangan, K.; Bruder, M.R.; Aucoin, M.G.; Moo-Young, M.; Chung, D.A.; Chou, C.P. Disruption of the Reductive 1,3-Propanediol Pathway Triggers Production of 1, 2-Propanediol for Sustained Glycerol Fermentation by Clostridium Pasteurianum. Appl. Environ. Microbiol. 2016, 82, 5375–5388. [Google Scholar] [CrossRef] [PubMed]
- Metsoviti, M.; Paraskevaidi, K.; Koutinas, A.; Zeng, A.-P.; Papanikolaou, S. Production of 1,3-Propanediol, 2,3-Butanediol and Ethanol by a Newly Isolated Klebsiella Oxytoca Strain Growing on Biodiesel-Derived Glycerol Based Media. Process Biochem. 2012, 47, 1872–1882. [Google Scholar] [CrossRef]
- Yang, G.; Tian, J.; Li, J. Fermentation of 1,3-Propanediol by a Lactate Deficient Mutant of Klebsiella Oxytoca under Microaerobic Conditions. Appl. Microbiol. Biotechnol. 2007, 73, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-K.; Zhang, J.-A.; Liu, D.-H.; Sun, Y.; Liu, H.-J.; Yang, M.-D.; Xu, J.-M. Pilot-Scale Production of 1,3-Propanediol Using Klebsiella Pneumoniae. Process Biochem. 2007, 42, 740–744. [Google Scholar] [CrossRef]
- Oh, B.-R.; Hong, W.-K.; Heo, S.-Y.; Luo, L.H.; Kondo, A.; Seo, J.-W.; Kim, C.H. The Production of 1,3-Propanediol from Mixtures of Glycerol and Glucose by a Klebsiella Pneumoniae Mutant Deficient in Carbon Catabolite Repression. Bioresour. Technol. 2013, 130, 719–724. [Google Scholar] [CrossRef]
- Yang, X.; Kim, D.S.; Choi, H.S.; Kim, C.K.; Thapa, L.P.; Park, C.; Kim, S.W. Repeated Batch Production of 1,3-Propanediol from Biodiesel Derived Crude glycerol by Klebsiella Pneumoniae. Chem. Eng. J. 2017, 314, 660–669. [Google Scholar] [CrossRef]
- Yang, X.; Choi, H.S.; Lee, J.H.; Lee, S.K.; Han, S.O.; Park, C.; Kim, S.W. Improved Production of 1,3-Propanediol from Biodiesel-Derived Crude Glycerol by Klebsiella Pneumoniae in Fed-Batch Fermentation. Chem. Eng. J. 2018, 349, 25–36. [Google Scholar] [CrossRef]
- Metsoviti, M.; Zeng, A.-P.; Koutinas, A.A.; Papanikolaou, S. Enhanced 1,3-Propanediol Production by a Newly Isolated Citrobacter Freundii Strain Cultivated on Biodiesel-Derived Crude glycerol through Sterile and Non-Sterile Bioprocesses. J. Biotechnol. 2013, 163, 408–418. [Google Scholar] [CrossRef]
- Celińska, E.; Drożdżyńska, A.; Jankowska, M.; Białas, W.; Czaczyk, K.; Grajek, W. Genetic Engineering to Improve 1,3-Propanediol Production in an Isolated Citrobacter Freundii Strain. Process Biochem. 2015, 50, 48–60. [Google Scholar] [CrossRef]
- Maervoet, V.E.; Beauprez, J.; De Maeseneire, S.L.; Soetaert, W.K.; De Mey, M. Citrobacter Werkmanii, a New Candidate for the Production of 1,3-Propanediol: Strain Selection and Carbon Source Optimization. Green Chem. 2012, 14, 2168–2178. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. Biological Valorization of Pure and Crude Glycerol into 1,3-Propanediol Using a Novel Isolate Lactobacillus Brevis N1E9. 3.3. Bioresour. Technol. 2016, 213, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Clomburg, J.M.; Gonzalez, R. Metabolic Engineering of Escherichia coli for the Production of 1, 2-propanediol from Glycerol. Biotechnol. Bioeng. 2011, 108, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Przystałowska, H.; Lipiński, D.; Słomski, R. Biotechnological Conversion of Glycerol from Biofuels to 1,3-Propanediol Using Escherichia coli. Acta Biochim. Pol. 2015, 62, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tan, Y.; Zhu, H.; Zhao, K.; Shen, W. Microbial Conversion of Glycerol to 1,3-Propanediol by an Engineered Strain of Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 1628–1634. [Google Scholar] [CrossRef]
- Khanna, S.; Goyal, A.; Moholkar, V.S. Production of N-Butanol from Biodiesel Derived Crude Glycerol Using Clostridium Pasteurianum Immobilized on Amberlite. Fuel 2013, 112, 557–561. [Google Scholar] [CrossRef]
- Xin, F.; Chen, T.; Jiang, Y.; Lu, J.; Dong, W.; Zhang, W.; Ma, J.; Zhang, M.; Jiang, M. Enhanced Biobutanol Production with High Yield from Crude Glycerol by Acetone Uncoupled Clostridium Sp. Strain CT7. Bioresour. Technol. 2017, 244, 575–581. [Google Scholar] [CrossRef]
- Oh, B.-R.; Heo, S.-Y.; Lee, S.-M.; Hong, W.-K.; Park, J.M.; Jung, Y.R.; Kim, D.-H.; Sohn, J.-H.; Seo, J.-W.; Kim, C.H. Production of 2-Butanol from Crude Glycerol by a Genetically-Engineered Klebsiella Pneumoniae Strain. Biotechnol. Lett. 2014, 36, 57–62. [Google Scholar] [CrossRef]
- Ju, J.-H.; Wang, D.; Heo, S.-Y.; Kim, M.-S.; Seo, J.-W.; Kim, Y.-M.; Kim, D.-H.; Kang, S.-A.; Kim, C.-H.; Oh, B.-R. Enhancement of 1,3-Propanediol Production from Industrial by-Product by Lactobacillus Reuteri CH53. Microb. Cell Factories 2020, 19, 6. [Google Scholar] [CrossRef]
- Sumitha, V.; Christy Mathelin, R.; Sivanandham, M. Effect of Major and Minor Nutrients on Lactic Acid Production Using Biodiesel Waste-Derived Crude Glycerol as a Carbon Source by Lactobacillus Casei NCIM 2125. Energy Sources Part Recovery Util. Environ. Eff. 2018, 40, 1322–1331. [Google Scholar] [CrossRef]
- Mazumdar, S.; Clomburg, J.M.; Gonzalez, R. Escherichia coli Strains Engineered for Homofermentative Production of D-Lactic Acid from Glycerol. Appl. Environ. Microbiol. 2010, 76, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Blankschien, M.D.; Clomburg, J.M.; Gonzalez, R. Efficient Synthesis of L-Lactic Acid from Glycerol by Metabolically Engineered Escherichia coli. Microb. Cell Factories 2013, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-K.; Zeng, J.; Jian, J.-H.; Zhu, J.-F.; Zhang, G.-X.; Liu, D.-H. Model-Based Temperature Control for Improving Lactic Acid Production from Glycerol. RSC Adv. 2019, 9, 11614–11620. [Google Scholar] [CrossRef]
- Soo, C.-S.; Yap, W.-S.; Hon, W.-M.; Ramli, N.; Shah, U.K.M.; Phang, L.-Y. Co-Production of Hydrogen and Ethanol by Escherichia coli SS1 and Its Recombinant. Electron. J. Biotechnol. 2017, 30, 64–70. [Google Scholar] [CrossRef]
- Tamires Moreira Melo, N.; Pontes, G.C.; Procópio, D.P.; de Gois e Cunha, G.C.; Eliodório, K.P.; Costa Paes, H.; Basso, T.O.; Parachin, N.S. Evaluation of Product Distribution in Chemostat and Batch Fermentation in Lactic Acid-Producing Komagataella Phaffii Strains Utilizing Glycerol as Substrate. Microorganisms 2020, 8, 781. [Google Scholar] [CrossRef]
- Mabutyana, L.; Pott, R.W. Photo-Fermentative Hydrogen Production by Rhodopseudomonas Palustris CGA009 in the Presence of Inhibitory Compounds. Int. J. Hydrogen Energy 2021, 46, 29088–29099. [Google Scholar] [CrossRef]
- Gupta, A.; Murarka, A.; Campbell, P.; Gonzalez, R. Anaerobic Fermentation of Glycerol in Paenibacillus Macerans: Metabolic Pathways and Environmental Determinants. Appl. Environ. Microbiol. 2009, 75, 5871–5883. [Google Scholar] [CrossRef]
- Varrone, C.; Giussani, B.; Izzo, G.; Massini, G.; Marone, A.; Signorini, A.; Wang, A. Statistical Optimization of Biohydrogen and Ethanol Production from Crude Glycerol by Microbial Mixed Culture. Int. J. Hydrogen Energy 2012, 37, 16479–16488. [Google Scholar] [CrossRef]
- Marchand, K.; Lubitz, W.; Nicol, R. Utilization of Biodiesel Derived Crude Glycerol by Fungal Isolates for Biomass and Single Cell Oil Production. J. Biobased Mater. Bioenergy 2013, 7, 415–419. [Google Scholar] [CrossRef]
- Yu, K.O.; Kim, S.W.; Han, S.O. Engineering of Glycerol Utilization Pathway for Ethanol Production by Saccharomyces Cerevisiae. Bioresour. Technol. 2010, 101, 4157–4161. [Google Scholar] [CrossRef] [PubMed]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The Citric Acid Production from Raw Glycerol by Yarrowia Lipolytica Yeast and Its Regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef] [PubMed]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric Acid Production by Yarrowia Lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I.G. Citric Acid Production from Glycerol-Containing Waste of Biodiesel Industry by Yarrowia Lipolytica in Batch, Repeated Batch, and Cell Recycle Regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, S.; Yang, X.; Lin, C.S.K. Green and Sustainable Succinic Acid Production from Crude Glycerol by Engineered Yarrowia Lipolytica via Agricultural Residue Based in Situ Fibrous Bed Bioreactor. Bioresour. Technol. 2018, 249, 612–619. [Google Scholar] [CrossRef]
- Lung, Y.-T.; Tan, C.H.; Show, P.L.; Ling, T.C.; Lan, J.C.-W.; Lam, H.L.; Chang, J.-S. Docosahexaenoic Acid Production from Crude Glycerol by Schizochytrium Limacinum SR21. Clean Technol. Environ. Policy 2016, 18, 2209–2216. [Google Scholar] [CrossRef]
- Hong, A.; Cheng, K.; Peng, F.; Zhou, S.; Sun, Y.; Liu, C.; Liu, D. Strain Isolation and Optimization of Process Parameters for Bioconversion of Glycerol to Lactic Acid. J. Chem. Technol. Biotechnol. 2009, 84, 1576–1581. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.-L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483. [Google Scholar] [CrossRef]
- Iyyappan, J.; Bharathiraja, B.; Baskar, G.; Kamalanaban, E. Process Optimization and Kinetic Analysis of Malic Acid Production from Crude Glycerol Using Aspergillus Niger. Bioresour. Technol. 2019, 281, 18–25. [Google Scholar] [CrossRef]
- Kuenz, A.; Hoffmann, L.; Goy, K.; Bromann, S.; Prüße, U. High-Level Production of Succinic Acid from Crude Glycerol by a Wild Type Organism. Catalysts 2020, 10, 470. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Freguia, S.; Rabaey, K.; Keller, J. Carbon and Electron Fluxes during the Electricity Driven 1,3-Propanediol Biosynthesis from Glycerol. Environ. Sci. Technol. 2013, 47, 11199–11205. [Google Scholar] [CrossRef] [PubMed]
- Selembo, P.A.; Perez, J.M.; Lloyd, W.A.; Logan, B.E. High Hydrogen Production from Glycerol or Glucose by Electrohydrogenesis Using Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2009, 34, 5373–5381. [Google Scholar] [CrossRef]
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-Driven Metabolic Shift through Direct Electron Uptake by Electroactive Heterotroph Clostridiumpasteurianum. Sci. Rep. 2014, 4, 6961. [Google Scholar] [CrossRef] [PubMed]
- Harussani, M.; Sapuan, S.; Rashid, U.; Khalina, A.; Ilyas, R. Pyrolysis of Polypropylene Plastic Waste into Carbonaceous Char: Priority of Plastic Waste Management amidst COVID-19 Pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Zhou, Q.; Pan, S.-X.; He, Y.; Chang, F. A Review of Catalytic Upgrading of Biodiesel Crude glycerol to Valuable Products. Curr. Green Chem. 2020, 7, 259–266. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Hisham, M.W.; Yarmo, M.A.; Hin, T.Y. A Review on Bio-Oil Production from Biomass by Using Pyrolysis Method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Khosravanipour Mostafazadeh, A.; Solomatnikova, O.; Drogui, P.; Tyagi, R.D. A Review of Recent Research and Developments in Fast Pyrolysis and Bio-Oil Upgrading. Biomass Convers. Biorefin. 2018, 8, 739–773. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Jusoh, A.; Chong, C.T.; Ani, F.N.; Chase, H.A. Progress in Waste Oil to Sustainable Energy, with Emphasis on Pyrolysis Techniques. Renew. Sustain. Energy Rev. 2016, 53, 741–753. [Google Scholar] [CrossRef]
- Harussani, M.M.; Rashid, U.; Sapuan, S.M.; Abdan, K. Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char. Polymers 2021, 13, 3980. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-Oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Shahnazari, A. Catalytic Co-Conversion of Glycerol and Proton-Donor Species to Gasoline-Range Aromatics over Alumina. Ph.D. Thesis, University of New Brunswick, Fredericton, NB, Canada, 2016; p. 113. Available online: https://unbscholar.lib.unb.ca/islandora/object/unbscholar%3A8006/datastream/PDF/download/citation.pdf (accessed on 1 February 2023).
- Suib, S.L. New and Future Developments in Catalysis: Catalytic Biomass Conversion; Newnes: Oxford, UK, 2013; ISBN 0-444-53879-8. [Google Scholar]
- Hoang, T.Q.; Zhu, X.; Danuthai, T.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Conversion of Glycerol to Alkyl-Aromatics over Zeolites. Energy Fuels 2010, 24, 3804–3809. [Google Scholar] [CrossRef]
- Suh, Y.-W.; Jang, H.-S.; Bae, K.-B. Method for Producing Bio-Aromatics from Glycerol. U.S. Patent 9,834,489, 5 December 2017. [Google Scholar]
- Müller, J.B.; Vogel, F. Tar and Coke Formation during Hydrothermal Processing of Glycerol and Glucose. Influence of Temperature, Residence Time and Feed Concentration. J. Supercrit. Fluids 2012, 70, 126–136. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Hydrothermal Reforming of Bio-Diesel Plant Waste: Products Distribution and Characterization. Fuel 2010, 89, 501–509. [Google Scholar] [CrossRef]
- Van Bennekom, J.; Venderbosch, R.; Assink, D.; Heeres, H. Reforming of Methanol and Glycerol in Supercritical Water. J. Supercrit. Fluids 2011, 58, 99–113. [Google Scholar] [CrossRef]
- Markočič, E.; Kramberger, B.; van Bennekom, J.G.; Heeres, H.J.; Vos, J.; Knez, Ž. Glycerol Reforming in Supercritical Water; a Short Review. Renew. Sustain. Energy Rev. 2013, 23, 40–48. [Google Scholar] [CrossRef]
- Pavlovic, I.; Knez, Z.; Skerget, M. Hydrothermal Reactions of Agricultural and Food Processing Wastes in Sub-and Supercritical Water: A Review of Fundamentals, Mechanisms, and State of Research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef]
- Remón, J.; Zhu, G.; Budarin, V.L.; Clark, J.H. Analysis and Optimisation of a Microwave-Assisted Hydrothermal Process for the Production of Value-Added Chemicals from Glycerol. Green Chem. 2018, 20, 2624–2636. [Google Scholar] [CrossRef]
- Cui, Z.; Cheng, F.; Jarvis, J.M.; Jena, U.; Brewer, C.E. Integrated Extraction and Catalytic Upgrading of Biocrude Oil from Co-Hydrothermal Liquefaction of Crude Glycerol and Algae. Energy Fuels 2021, 35, 12165–12174. [Google Scholar] [CrossRef]
- Krammer, P.; Mittelstädt, S.; Vogel, H. Investigating the Synthesis Potential in Supercritical Water. Chem. Eng. Technol. Ind. Chem. Equip.-Process Eng. 1999, 22, 126–130. [Google Scholar] [CrossRef]
- El Doukkali, M.; Iriondo, A.; Gandarias, I. Enhanced Catalytic Upgrading of Glycerol into High Value-Added H2 and Propanediols: Recent Developments and Future Perspectives. Mol. Catal. 2020, 490, 110928. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3–ZrO2 Catalyst for Hydrogen Production through the Glycerol Steam Reforming Reaction. Int. J. Hydrogen Energy 2020, 45, 10442–10460. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Gennequin, C.; Aboukais, A.; Abi-Aad, E.; Aouad, S. Zirconia Supported Nickel Catalysts for Glycerol Steam Reforming: Effect of Zirconia Structure on the Catalytic Performance. Int. J. Hydrogen Energy 2020, 45, 4457–4467. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.; Cambra, J.; Arias, P.; Guemez, M.; Sanchez-Sanchez, M.; Navarro, R.; Fierro, J. Glycerol Steam Reforming over Ni Catalysts Supported on Ceria and Ceria-Promoted Alumina. Int. J. Hydrogen Energy 2010, 35, 11622–11633. [Google Scholar] [CrossRef]
- Zamzuri, N.H.; Mat, R.; Amin, N.A.S.; Talebian-Kiakalaieh, A. Hydrogen Production from Catalytic Steam Reforming of Glycerol over Various Supported Nickel Catalysts. Int. J. Hydrogen Energy 2017, 42, 9087–9098. [Google Scholar] [CrossRef]
- El-Bousiffi, M.; Gunn, D. A Dynamic Study of Steam-Methane Reforming. Int. J. Heat Mass Transf. 2007, 50, 723–733. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, S.; Zhang, Y.; Wang, T.; Luo, S.; Chu, W.; Jing, F. The Role of Zr in NiZrAl Oxides Catalyst and the Evaluation on Steam Reforming of Glycerol for Hydrogen Product. Catal. Today 2019, 319, 229–238. [Google Scholar] [CrossRef]
- Davda, R.; Shabaker, J.; Huber, G.; Cortright, R.; Dumesic, J.A. A Review of Catalytic Issues and Process Conditions for Renewable Hydrogen and Alkanes by Aqueous-Phase Reforming of Oxygenated Hydrocarbons over Supported Metal Catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- El Doukkali, M.; Iriondo, A.; Arias, P.; Requies, J.; Gandarías, I.; Jalowiecki-Duhamel, L.; Dumeignil, F. A Comparison of Sol–Gel and Impregnated Pt or/and Ni Based γ-Alumina Catalysts for Bioglycerol Aqueous Phase Reforming. Appl. Catal. B Environ. 2012, 125, 516–529. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Jusoh, A.; Chong, C.T.; Lee, C.L.; Chase, H.A. Pyrolysis Using Microwave Absorbents as Reaction Bed: An Improved Approach to Transform Used Frying Oil into Biofuel Product with Desirable Properties. J. Clean. Prod. 2017, 147, 263–272. [Google Scholar] [CrossRef]
- Dosuna-Rodríguez, I.; Gaigneaux, E.M. Glycerol Acetylation Catalysed by Ion Exchange Resins. Catal. Today 2012, 195, 14–21. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, S.; Li, Y. Graphene Oxide as a Facile Solid Acid Catalyst for the Production of Bioadditives from Glycerol Esterification. Catal. Commun. 2015, 62, 48–51. [Google Scholar] [CrossRef]
- Gonçalves, V.L.; Pinto, B.P.; Silva, J.C.; Mota, C.J. Acetylation of Glycerol Catalyzed by Different Solid Acids. Catal. Today 2008, 133, 673–677. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Galhano dos Santos, R. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals—A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Zhou, L.; Nguyen, T.-H.; Adesina, A.A. The Acetylation of Glycerol over Amberlyst-15: Kinetic and Product Distribution. Fuel Process. Technol. 2012, 104, 310–318. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Hernández, D.L.; Moreno, J.A.; Mondragón, F.; Fernández, J.J. Alternative Carbon Based Acid Catalyst for Selective Esterification of Glycerol to Acetylglycerols. Appl. Catal. Gen. 2011, 405, 55–60. [Google Scholar] [CrossRef]
- Khayoon, M.; Hameed, B. Acetylation of Glycerol to Biofuel Additives over Sulfated Activated Carbon Catalyst. Bioresour. Technol. 2011, 102, 9229–9235. [Google Scholar] [CrossRef]
- Isahak, W.; Ismail, M.; Yarmo, M.; Jahim, J.; Salimon, J. Purification of Crude Glycerol from Transesterification RBD Palm Oil over Homogeneous and Heterogeneous Catalysts for the Biolubricant Preparation. J. Appl. Sci. 2010, 10, 2590–2595. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of Glycerol Carbonate from Glycerol and Dimethyl Carbonate by Transesterification: Catalyst Screening and Reaction Optimization. Appl. Catal. Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Behr, A.; Obendorf, L. Development of a Process for the Acid-catalyzed Etherification of Glycerine and Isobutene Forming Glycerine Tertiary Butyl Ethers. Eng. Life Sci. 2002, 2, 185–189. [Google Scholar] [CrossRef]
- Clacens, J.-M.; Pouilloux, Y.; Barrault, J.; Linares, C.; Goldwasser, M. Mesoporous Basic Catalysts: Comparison with Alkaline Exchange Zeolites (Basicity and Porosity). Application to the Selective Etherification of Glycerol to Polyglycerols. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1998; Volume 118, pp. 895–902. ISBN 0167-2991. [Google Scholar]
- Klepáčová, K.; Mravec, D.; Bajus, M. Tert-Butylation of Glycerol Catalysed by Ion-Exchange Resins. Appl. Catal. Gen. 2005, 294, 141–147. [Google Scholar] [CrossRef]
- Khayoon, M.; Hameed, B. Synthesis of Hybrid SBA-15 Functionalized with Molybdophosphoric Acid as Efficient Catalyst for Glycerol Esterification to Fuel Additives. Appl. Catal. Gen. 2012, 433, 152–161. [Google Scholar] [CrossRef]
- Shi, Y.; Dayoub, W.; Chen, G.-R.; Lemaire, M. Selective Synthesis of 1-O-Alkyl Glycerol and Diglycerol Ethers by Reductive Alkylation of Alcohols. Green Chem. 2010, 12, 2189–2195. [Google Scholar] [CrossRef]
- Karinen, R.; Krause, A. New Biocomponents from Glycerol. Appl. Catal. Gen. 2006, 306, 128–133. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Miquel, S.; Primo, J. Catalysts for the Production of Fine Chemicals: Production of Food Emulsifiers, Monoglycerides, by Glycerolysis of Fats with Solid Base Catalysts. J. Catal. 1998, 173, 315–321. [Google Scholar] [CrossRef]
- Mat, R.; Samsudin, R.A.; Mohamed, M.; Johari, A. Solid Catalysts and Their Application in Biodiesel Production. Bull. Chem. React. Eng. Catal. 2012, 7, 142–149. [Google Scholar] [CrossRef]
- Hunsom, M.; Saila, P. Electrochemical Conversion of Enriched Crude Glycerol: Effect of Operating Parameters. Renew. Energy 2015, 74, 227–236. [Google Scholar] [CrossRef]
- Rahim, S.A.N.M.; Lee, C.S.; Abnisa, F.; Aroua, M.K.; Daud, W.A.W.; Cognet, P.; Pérès, Y. A Review of Recent Developments on Kinetics Parameters for Glycerol Electrochemical Conversion–A by-Product of Biodiesel. Sci. Total Environ. 2020, 705, 135137. [Google Scholar] [CrossRef]
- Li, N.; Xia, W.-Y.; Xu, C.-W.; Chen, S. Pt/C and Pd/C Catalysts Promoted by Au for Glycerol and CO Electrooxidation in Alkaline Medium. J. Energy Inst. 2017, 90, 725–733. [Google Scholar] [CrossRef]
- Liu, C.; Hirohara, M.; Maekawa, T.; Chang, R.; Hayashi, T.; Chiang, C.-Y. Selective Electro-Oxidation of Glycerol to Dihydroxyacetone by a Non-Precious Electrocatalyst–CuO. Appl. Catal. B Environ. 2020, 265, 118543. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Xi, J.; Luo, X. Selective Electro-Oxidation of Glycerol to Dihydroxyacetone by PtAg Skeletons. ACS Appl. Mater. Interfaces 2019, 11, 28953–28959. [Google Scholar] [CrossRef] [PubMed]
- Okada, K. Electrochemical Oxidation of Glycerol in a Proton-Exchange-Membrane Reactor. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2013. Available online: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/97812/kanadian_1.pdf?sequence=1 (accessed on 1 February 2023).
- Mostafazadeh, A.K.; De La Torre, M.S.; Padilla, Y.; Drogui, P.; Brar, S.K.; Tyagi, R.D.; Le Bihan, Y.; Buelna, G.; Moroyoqui, P.G. An Insight into an Electro-Catalytic Reactor Concept for High Value-Added Production from Crude Glycerol: Optimization, Electrode Passivation, Product Distribution, and Reaction Pathway Identification. Renew. Energy 2021, 172, 130–144. [Google Scholar] [CrossRef]
- Li, K.; Sun, Y. Electrocatalytic Upgrading of Biomass-derived Intermediate Compounds to Value-added Products. Chem. Eur. J. 2018, 24, 18258–18270. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Kouamé, R.S.B. Selective Electrooxidation of Glycerol into Value-Added Chemicals: A Short Overview. Front. Chem. 2019, 7, 100. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ab Rahim, M.H.; Alqahtani, T.M.; Witoon, T.; Lim, J.-W.; Cheng, C.K. A Review on Advances in Green Treatment of Glycerol Waste with a Focus on Electro-Oxidation Pathway. Chemosphere 2021, 276, 130128. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Shi, Y.; Xia, C.; Huang, Z.; Manzo, M.; Cai, L.; Ma, H.; Zhang, S.; Jiang, J.; Sonne, C. Progress in Pyrolysis Conversion of Waste into Value-Added Liquid Pyro-Oil, with Focus on Heating Source and Machine Learning Analysis. Energy Convers. Manag. 2021, 245, 114638. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Yang, H.; Liu, M.; Xiao, H.; Wu, Z.; Chen, H.; Naqvi, S.R. Prediction of Bio-Oil Yield and Hydrogen Contents Based on Machine Learning Method: Effect of Biomass Compositions and Pyrolysis Conditions. Energy Fuels 2020, 34, 11050–11060. [Google Scholar] [CrossRef]
- Mirkouei, A.; Haapala, K.R.; Sessions, J.; Murthy, G.S. A Review and Future Directions in Techno-Economic Modeling and Optimization of Upstream Forest Biomass to Bio-Oil Supply Chains. Renew. Sustain. Energy Rev. 2017, 67, 15–35. [Google Scholar] [CrossRef]
- Dang, Q.; Wright, M.M.; Li, W. Technoeconomic Analysis of a Hybrid Biomass Thermochemical and Electrochemical Conversion System. Energy Technol. 2018, 6, 178–187. [Google Scholar] [CrossRef]
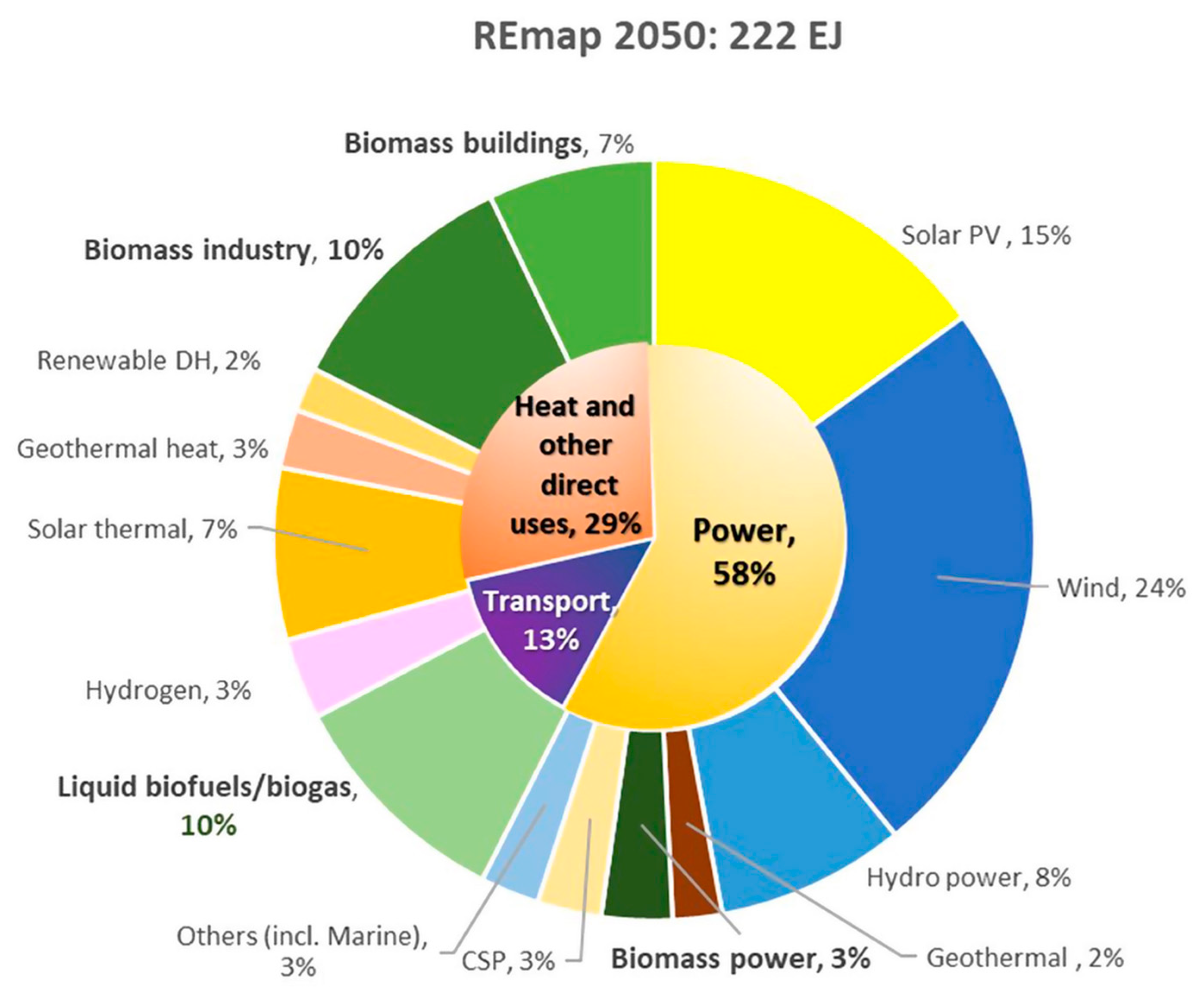
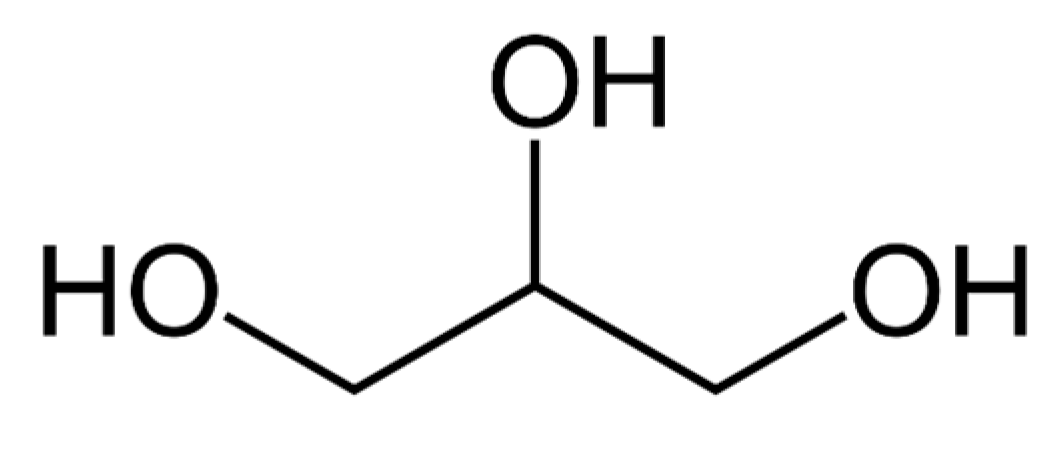
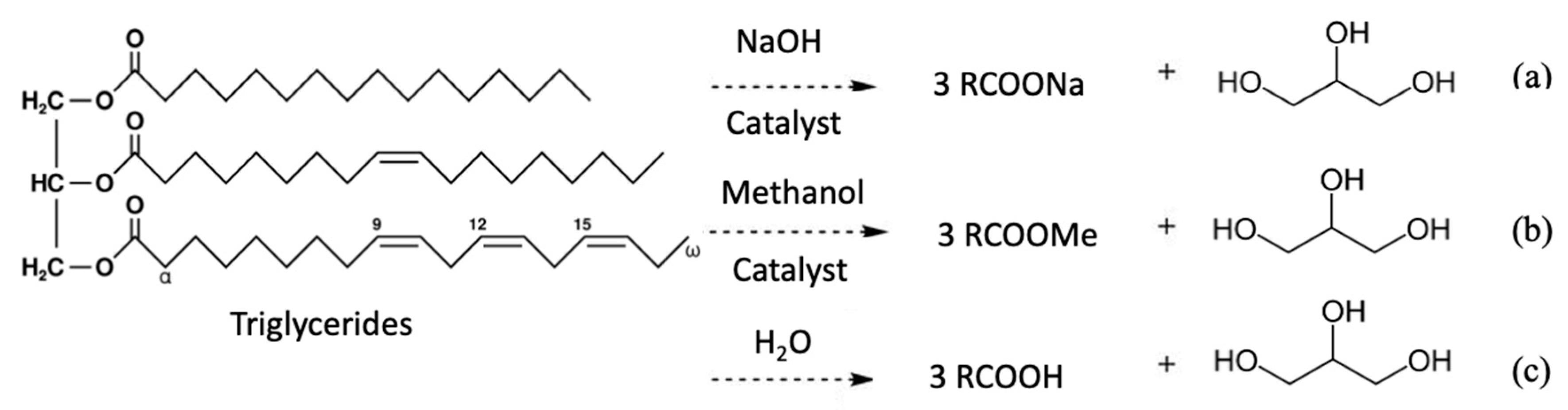
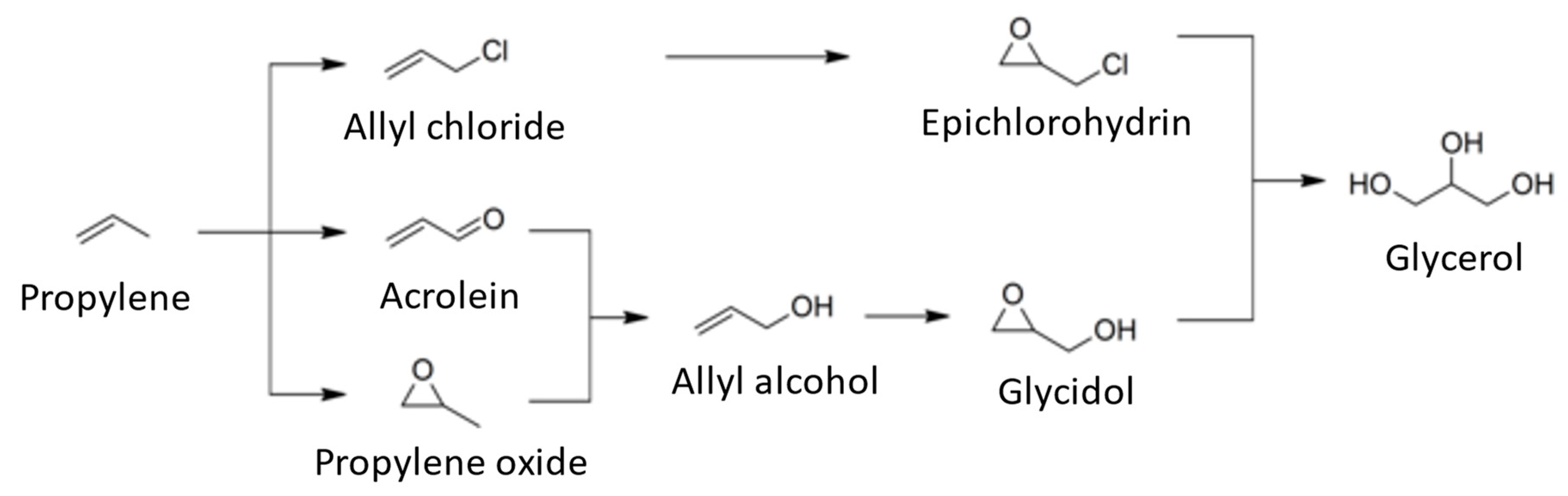
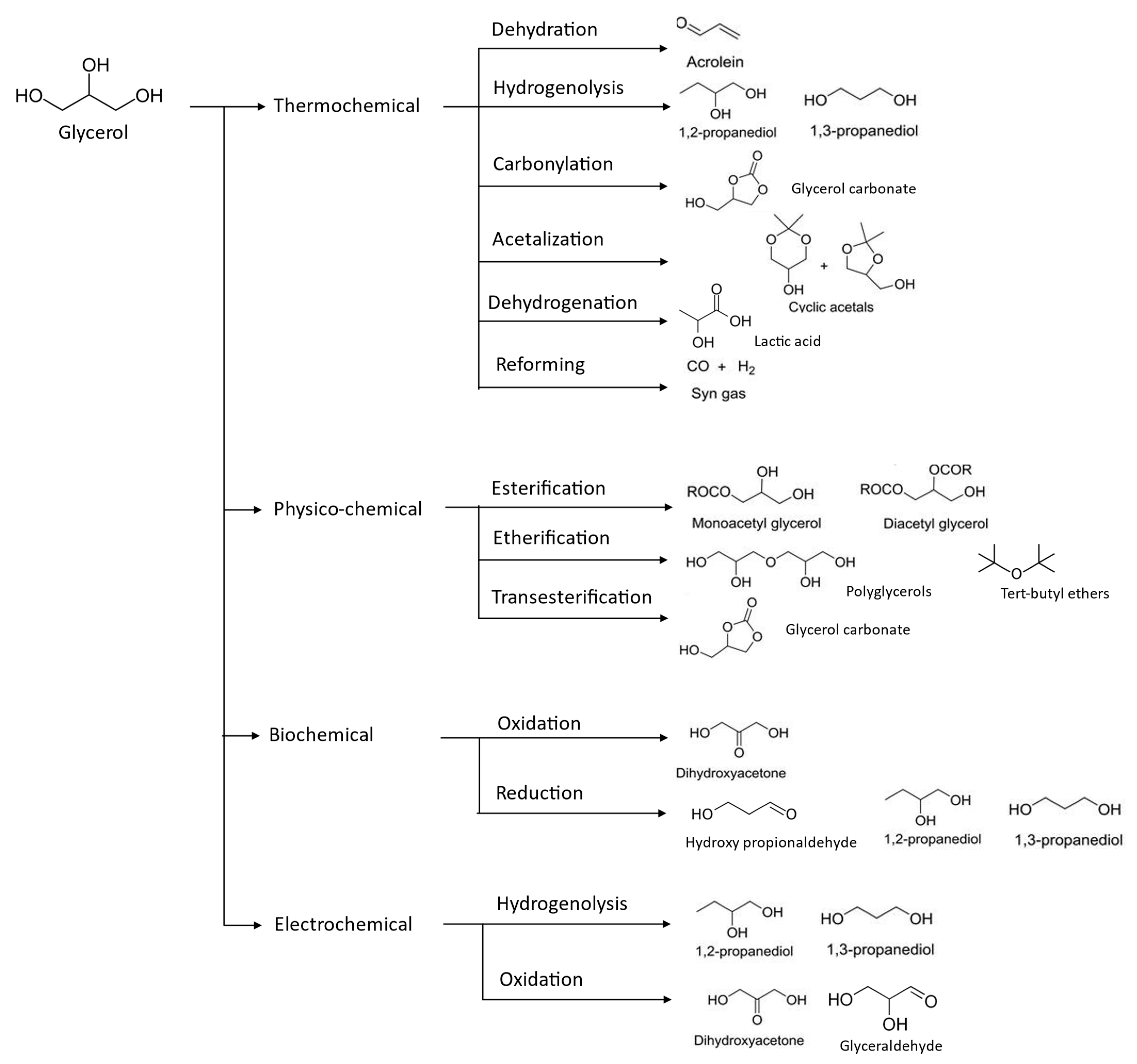

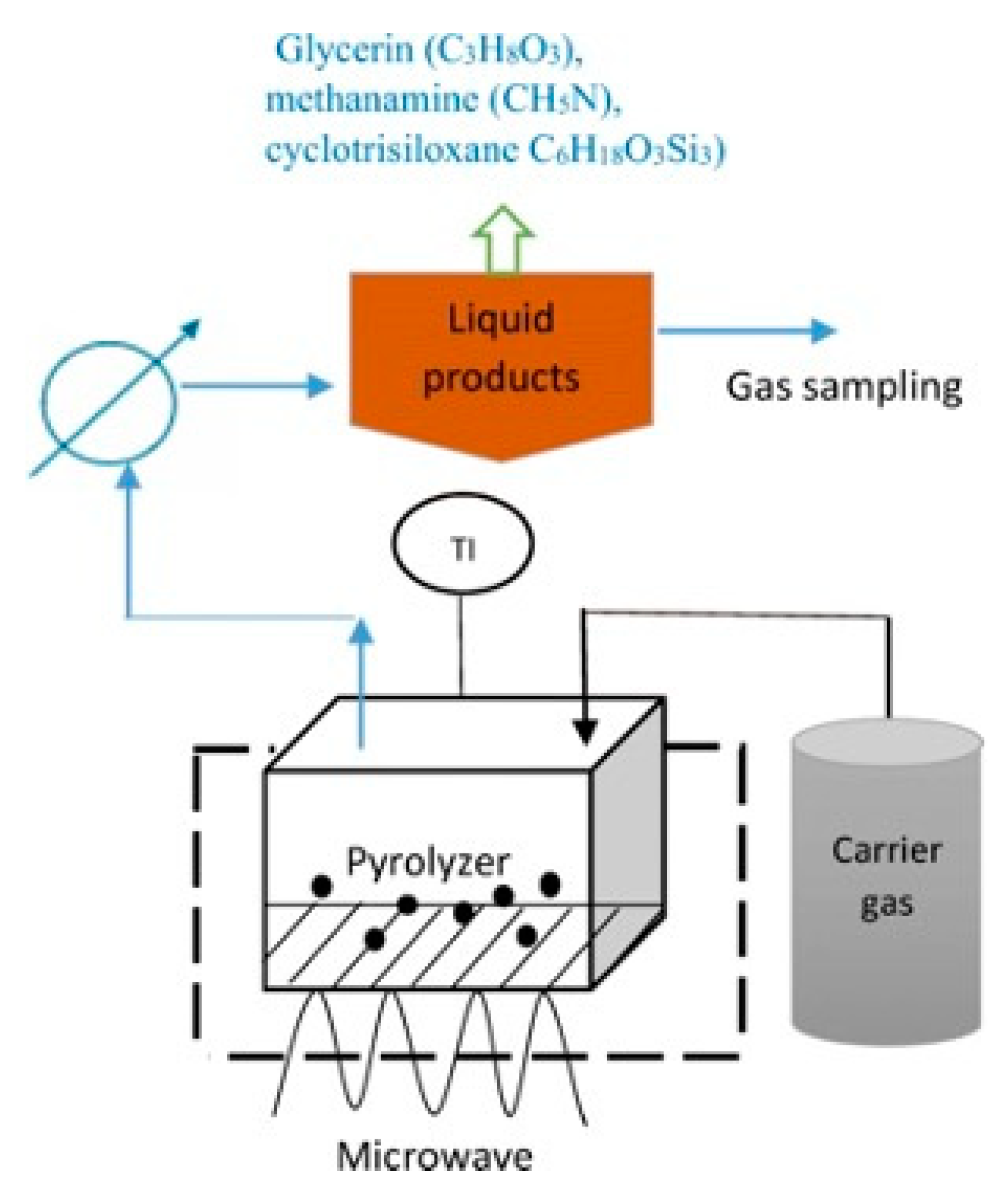

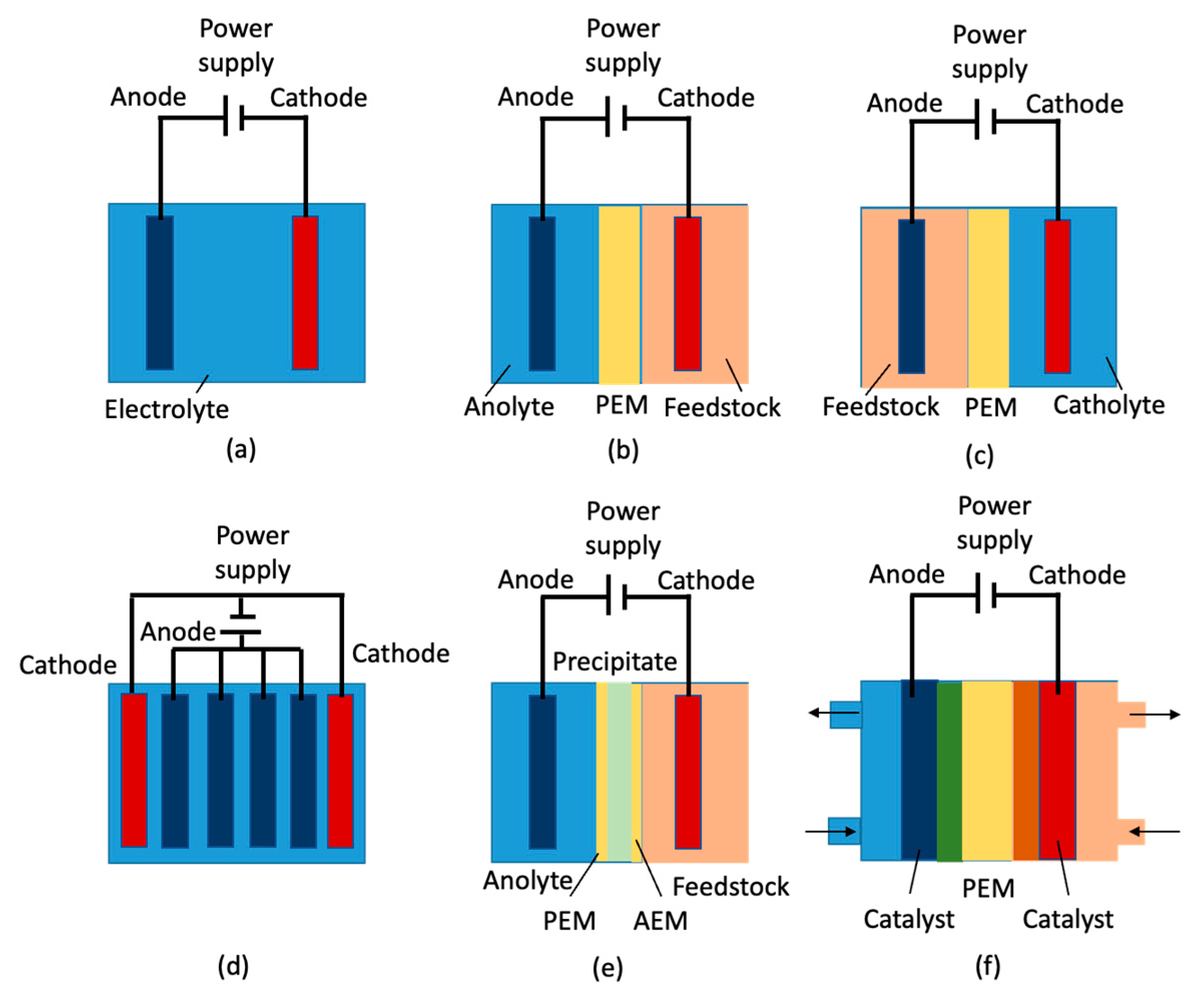
| Properties | Values |
|---|---|
| Form and color | Liquid and colorless |
| Formula weight (amu) | 92.09 |
| Density at 20 °C (g/cm3) | 1.26 |
| Melting point (°C) | 18 |
| Boiling point (°C) | 290 |
| Thermal conductivity (W/m/K) | 0.29 |
| Ignition temperature/flash point (°C) | 177 |
| Calorific value (MJ/kg) | 18 |
| Composition | Commercial Glycerol | Refined Glycerol | Crude Glycerol |
|---|---|---|---|
| Glycerol content (%) | 99.2–99.9 | 99.1–99.8 | 60–80 |
| Moisture content (%) | 0.14–0.29 | 0.1–0.8 | 1.5–6.5 |
| Ash (%) | <0.002 | 0.054 | 1.5–2.5 |
| Soap (%) | 0.04–0.07 | 0.1–0.16 | 3.0–5.0 |
| pH value (acidity level) | 0.04–0.07 | 0.10–0.16 | 0.7–1.3 |
| Color (APHA) | 1.8–10.3 | 34–45 | Dark |
| Upgrading Technique | Reaction Mechanism | End-Products | Process Condition | Process Efficiency | Ref. | |
|---|---|---|---|---|---|---|
| Advantages | Disadvantages | |||||
| Biochemical | ||||||
| Microbial fermentation | Aerobic and anaerobically digestion | Bioethanol, diols—2,3-BDO, 1,3-PDO, DHA, LA, SA, PA, and H2 | Enzymatic biocatalysts—yeast/fungi/modified strains/bacteria/microalgae, mild temperature (70–160 °C) and pressure, pH 5.5–7, stirring speed (200–400 rpm), process time (0.5–5 days) | High product selectivity (<70%), promotes biocatalyst, cheaper manufacturing cost | Complex microorganisms’ preparation, longer reaction times (1–5 days), lower kinetic reaction, risks of working with pathogenic microbes | [33,34,35,36,37,38,39,40,41,42] |
| Bio-electrochemical fermentation | Anaerobic reaction | 1,3-PDO, 3-HPA, H2 | Fed-batch mode in a H-cell reactor, biocathodes—bacteria/mixed microbial inoculum, mild temperature and pressure, time (1–10 days) | Higher production rate compared to non-EC fermentation | Using pure cultures, higher costs. Longer reaction times | [43,44,45] |
| Thermochemical | ||||||
| Pyrolysis gasification | Dehydration | Acetaldehyde, acrolein, HA, and H2 | Fixed bed reactor, temperature (650–850 °C); atmospheric pressure; catalyst (acid catalysts—metal-assisted zeolites, MMO); residence time (>7 s) | High temperature favors syngas/H2 (80%) Low temperature yields liquid products (70%) Shorter time | Produces CO | [46,47,48] |
| Fast pyrolysis | Hydrogenation, dehydration, decarboxylation, deoxygenation | Bio-BTX, diols, propylene glycol, syngas | Temperature (400–800 °C); absence of oxygen; residence time (0.5–3 s), external H2 supply (for hydrotreating), catalysts (modified zeolites, MMO, metal nitrides/phosphides, bifunctional catalysts) | High yield of HC compound | Coking issues, irreversible deactivation, high cost for hydrotreating process | [49,50] |
| Hydrothermal/supercritical fluids | Hydrocracking, dehydration | C2–4 HCs, H2, CO2, and other syngas | Batch reactor, temperature (>300 °C), time (1–4 h), pressure (10–30 MPa), solvent (deionized water, CO2) | No char formed, high H2 yields at 380 °C (90% of product gas) | Low selectivity (39%) and yield (40%), high cost of organic solvents | [9,51,52] |
| Carbonylation | GC | Temperature (>150 °C), pressure (50 atm), solvent (methanol) | 100% selectivity, high GC (90%), | [53,54] | ||
| Dehydrogenation, keto-enol tautomerization, benzylic acid rearrangement | Lactic acid, propylene glycol | Temperature (>290 °C), pressure (50 atm), solvent (water), time (3 h), catalyst (CaO) | Other alternative for lactic acid production, high glycerol conversion (98%) | Low lactic acid yields | [55,56] | |
| SR | Dehydrogenation, dehydration | H2 and other syngas | Fixed-bed reactor, catalyst (Ni and Pt-supported catalysts, Co, Cu, and Fe), temperature (350–800 °C) | Ni catalysts exhibit good catalytic behavior, high conversion rate (65–95%), high selectivity (50–82%) | Some setups take longer reaction time (8–20 h), requires proper catalysts | [57,58,59,60] |
| APR | Dehydrogenation, hydrogenolysis | H2, PDO, ethylene glycol | Ambient processing parameters (150–265 °C) at pressures (15–70 bar), catalysts (Pt/Ni-based γ-Al2O3), time (1–56 h) | Low energy consumption. High glycerol conversion (70–80%), H2 yield (~20%) | Dramatical deactivation at high temperature. Longer reaction time for high conversion (>25 h) | [61,62,63,64] |
| Microwave-assisted pyrolysis | Gasification | Syngas (H2, CO2) | Temperature (300–900 °C), carbonaceous catalysts (activated carbon), gas flow rates of 100–2000 mL/min | Higher production of syngas at low temperature | High-energy consumption | [65,66,67] |
| Physicochemical | ||||||
| Emulsification | Microemulsion | GDM, emulsion fuel | Surfactants (RL, Span 80, Tween 80), cosurfactants (alkanols), temperature (45–65 °C), time (>12 h) | Direct use of fuel, improved pour, and cloud point | Longer reaction time and suitable with low concentration waste | [68,69,70] |
| Trans-esterification | Carbonylation | GC | Alkyl carbonates (DMC, DEC), catalysts (Ca-based catalysts, hydrotalcites, biochar), temperature (90–120 °C), time (1 h), low pressure (30–50 mbar) | High GC yield (90%), water removal, increasing heating value and stability | Low end-product yields | [14,71,72,73] |
| Catalytic etherification | Etherification, glycerol condensation | tert-butyl ethers, alkyl glycerol ethers, polyglycerols | Tert-butyl alcohol/ isobutylene, batch mode, temperature (90–260 °C), catalysts (acid/base catalysts, zeolites, silica), time (2–24 h), pressure (0.1 MPa) | High GC (96%) at longer reaction time (24 h) | Complex system, low conversion (<30%) | [74,75,76] |
| Electrochemical | ||||||
| Electrolysis | Electro oxidation, hydrogenation, and hydro deoxygenation | DHA, CO2, glyceric acid, lactic acid, acetone, 1,2-PDO, isopropanol | Ambient temperature and pressure; electrocatalyst (CoOx, Au, Pt, AuPt NPs, Pt/C-Bi/Sb); electrolyte (alkaline, acidic medium), Ambient T, and t = 4 h | High selectivity (90–50%) and production rate, low processing cost | Low oxidation of secondary alcohol group, long electrolysis time (>5 h) | [77,78,79,80,81,82,83] |
| Parameters | Water Phase or Status | |||
|---|---|---|---|---|
| Ambient | Steam | Subcritical | Supercritical | |
| Temperature, T (°C) | 25 | 100 | 250 | 373 |
| Pressure, P (MPa) | 0.1 | 0.1 | 5.0 | 22.1 |
| Density, ρ (g/cm3) | 1 | 0.0003 | 0.80 | 0.17 |
| Dielectric constant, ε | 78.5 | ~1 | 27.1 | 5.9 |
| Dynamic viscosity, η (mPas) | 0.89 | 0.02 | 0.11 | 0.03 |
| Heat capacity, cp (kJ/kg/K) | 4.22 | 2.1 | 4.86 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moklis, M.H.; Cheng, S.; Cross, J.S. Current and Future Trends for Crude Glycerol Upgrading to High Value-Added Products. Sustainability 2023, 15, 2979. https://doi.org/10.3390/su15042979
Moklis MH, Cheng S, Cross JS. Current and Future Trends for Crude Glycerol Upgrading to High Value-Added Products. Sustainability. 2023; 15(4):2979. https://doi.org/10.3390/su15042979
Chicago/Turabian StyleMoklis, Muhammad Harussani, Shou Cheng, and Jeffrey S. Cross. 2023. "Current and Future Trends for Crude Glycerol Upgrading to High Value-Added Products" Sustainability 15, no. 4: 2979. https://doi.org/10.3390/su15042979
APA StyleMoklis, M. H., Cheng, S., & Cross, J. S. (2023). Current and Future Trends for Crude Glycerol Upgrading to High Value-Added Products. Sustainability, 15(4), 2979. https://doi.org/10.3390/su15042979







