Physiological Characteristics and Cold Resistance of Five Woody Plants in Treeline Ecotone of Sygera Mountains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Biochemical Parameters
2.4. Data Analysis
3. Results
3.1. Analysis of the Variation Source for Each Physiological Index between Tree Species and Organs
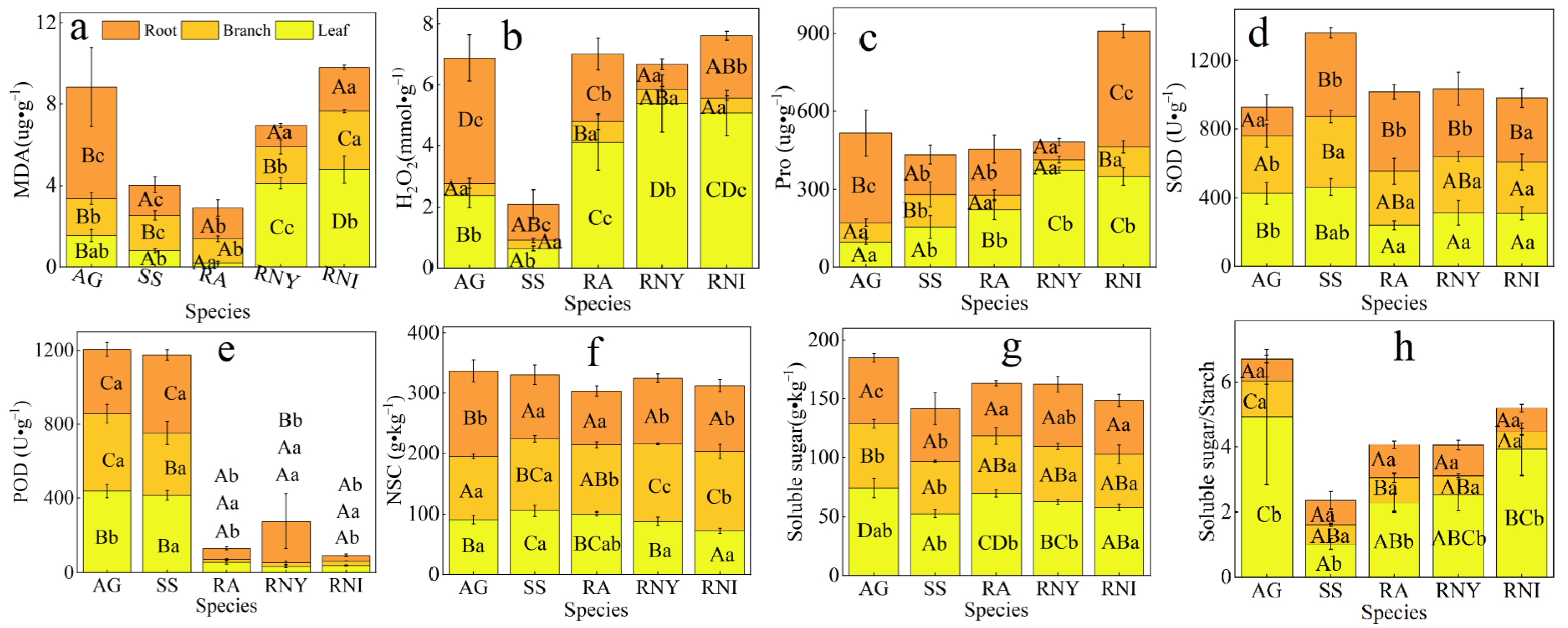
3.2. Physiological Index Distribution Characteristics of Five Dominant Woody Plants in the Treeline Ecotone
3.3. Analysis of the Cold Resistance of Five Dominant Woody Plants in the Treeline Ecotone
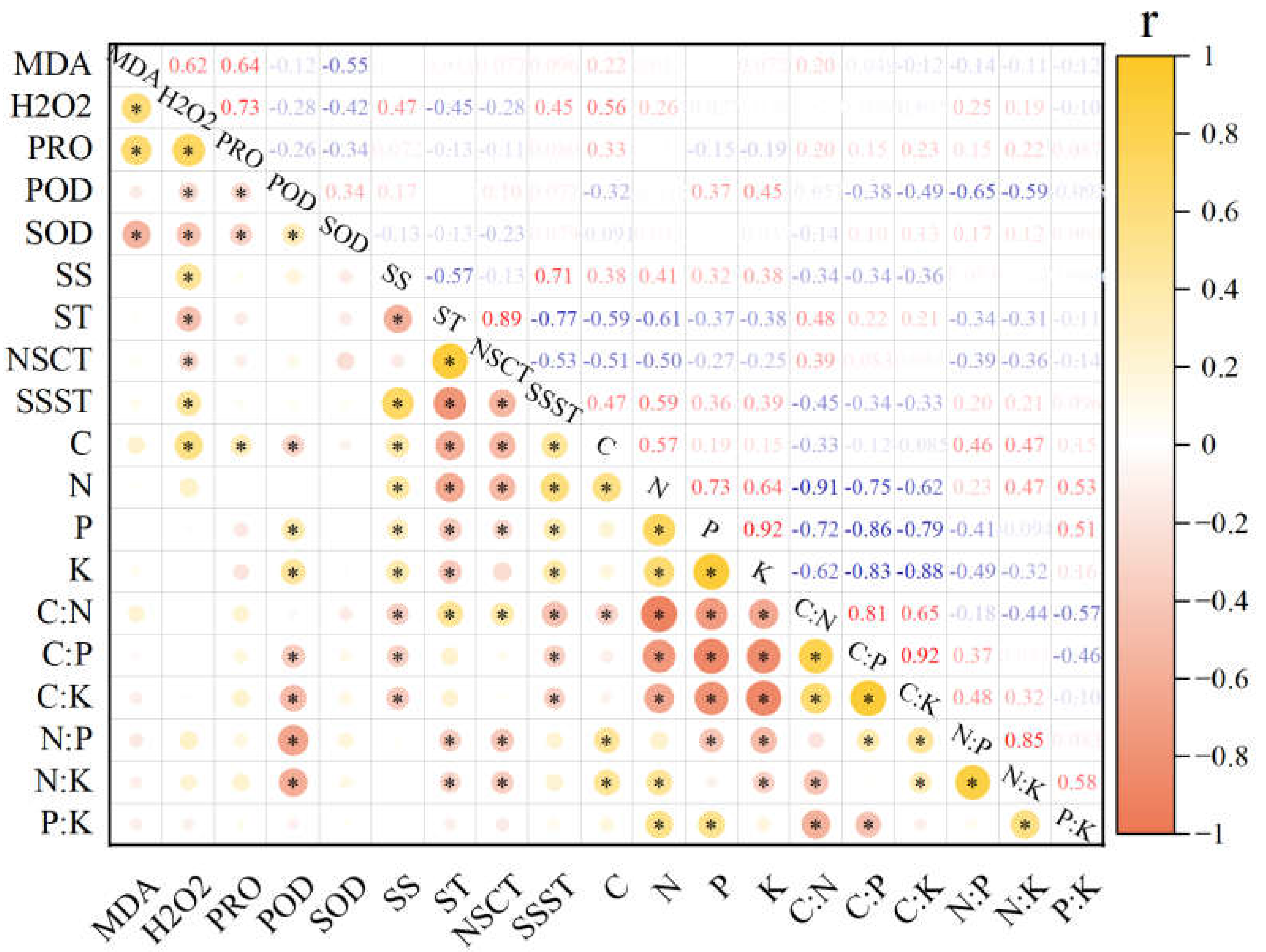
3.4. Analysis of the Correlation between Nutrients and Physiological Indicators of Five Major Woody Plants in the Treeline Ecotone
4. Discussion
4.1. Membrane Metabolites and Cold Resistance of Plants
4.2. Plant Cold Resistance and the Antioxidant System
4.3. Plant Cold Resistance and Osmotic Adjustment Agents
4.4. Plant Nutrition and Frost Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kullman, L. 20th Century Climate Warming and Tree-Limit Rise in the Southern Scandes of Sweden. Ambio 2001, 30, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. A Re-Assessment of High Elevation Treeline Positions and Their Explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cao, B.; Mao, P.; Li, Z.; Hao, M.; Wang, T.; Jiang, F. Fine Root Vertical-Seasonal Distribution of Robinia Pseudoacacia in Relation to Abiotic Factors in a Chronosequence in Coastal Saline Alkali Land of the Yellow River Delta, China. Turk. J. Agric. For. 2021, 45, 750–765. [Google Scholar] [CrossRef]
- Akkemik, Ü.; Genç, S.; Yilmaz, O.Y.; Selvi, E.; Yilmaz, H.; Sevgi, E.; Sevgi, O.; Akarsu, F. Effects of Growing Site Parameters on Vessel Elements of Quercus Ilex through Turkey and Evaluating in Respect of Forestry. Turk. J. Agric. For. 2021, 45, 599–616. [Google Scholar] [CrossRef]
- Chen, W.; Ding, H.; Li, J.; Chen, K.; Wang, H. Alpine Treelines as Ecological Indicators of Global Climate Change: Who Has Studied? What Has Been Studied? Ecol. Inform. 2022, 70, 101691. [Google Scholar] [CrossRef]
- Troll, C. The Upper Timberlines in Different Climatic Zones. Arct. Alp. Res. 1973, 5, A3–A18. [Google Scholar]
- Körner, C.; Paulsen, J. A World-Wide Study of High Altitude Treeline Temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Rossi, S.; Čufar, K.; Ellison, A.M. Critical Minimum Temperature Limits Xylogenesis and Maintains Treelines on the Southeastern Tibetan Plateau. Sci. Bull. 2017, 62, 804–812. [Google Scholar] [CrossRef]
- Piper, F.I.; Viñegla, B.; Linares, J.C.; Camarero, J.J.; Cavieres, L.A.; Fajardo, A. Mediterranean and Temperate Treelines Are Controlled by Different Environmental Drivers. J. Ecol. 2016, 104, 691–702. [Google Scholar] [CrossRef]
- Li, X.; Rossi, S.; Sigdel, S.R.; Dawadi, B.; Liang, E. Warming Menaces High-Altitude Himalayan Birch Forests: Evidence from Cambial Phenology and Wood Anatomy. Agric. Meteorol. 2021, 308–309, 108577. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Piper, F.I.; Fajardo, A. Wind Exposure and Light Exposure, More than Elevation-Related Temperature, Limit Tree Line Seedling Abundance on Three Continents. J. Ecol. 2016, 104, 1379–1390. [Google Scholar] [CrossRef]
- Bader, M.Y.; van Geloof, I.; Rietkerk, M. High Solar Radiation Hinders Tree Regeneration above the Alpine Treeline in Northern Ecuador. Plant Ecol. 2007, 191, 33–45. [Google Scholar] [CrossRef]
- Müller, M.; Schickhoff, U.; Scholten, T.; Drollinger, S.; Böhner, J.; Chaudhary, R.P. How Do Soil Properties Affect Alpine Treelines? General Principles in a Global Perspective and Novel Findings from Rolwaling Himal, Nepal. Prog. Phys. Geogr. 2016, 40, 135–160. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Piao, S.; Lu, X.; Camarero, J.J.; Zhu, H.; Zhu, L.; Ellison, A.M.; Ciais, P.; Peñuelas, J. Species Interactions Slow Warming-Induced Upward Shifts of Treelines on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2016, 113, 4380–4385. [Google Scholar] [CrossRef] [PubMed]
- Ameztegui, A.; Coll, L.; Brotons, L.; Ninot, J.M. Land-Use Legacies Rather than Climate Change Are Driving the Recent Upward Shift of the Mountain Tree Line in the Pyrenees. Glob. Ecol. Biogeogr. 2016, 25, 263–273. [Google Scholar] [CrossRef]
- Chen, W.; Ding, H.; Li, J.; Fu, F.; Li, Y.; Xiao, S.; Xu, D.; Lu, J.; Fang, J. How Do Montane Plants Manage to Survive? Inferring from Non-Structural Carbohydrates. Trees Struct. Funct. 2022, 1–18. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H.; Sun, G. Altitudinal Variation of Antioxidative System in Leaves of Rhodiola Quadrifida and R.Celida. Acta Phytoecol. Sin. 2005, 29, 331–337. [Google Scholar]
- Molina-Montenegro, M.A.; Gallardo-Cerda, J.; Flores, T.S.M.; Atala, C. The Trade-off between Cold Resistance and Growth Determines the Nothofagus Pumilio Treeline. Plant Ecol. 2013, 213, 133–142. [Google Scholar] [CrossRef]
- Ren, J.; Huang, Z.; Zeng, L.; Shi, Z. A Review of Physiological Reaction Mechanism of Plants Exposed to Low Temperature Stress. World For. Res. 2013, 26, 15–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Yu, Q.; Ma, Y.; Gu, W.; Yang, D. Physiological Changes Associated with Enhanced Cold Resistance during Maize (Zea Mays) Germination and Seedling Growth in Response to Exogenous Calcium. Crop Pasture Sci. 2020, 71, 529–538. [Google Scholar] [CrossRef]
- Han, H.; He, H.; Wu, Z.; Cong, Y.; Zong, S.; He, J.; Fu, Y.; Liu, K.; Sun, H.; Li, Y.; et al. Non-Structural Carbohydrate Storage Strategy Explains the Spatial Distribution of Treeline Species. Plants 2020, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Xiao, W.F.; Wang, S.G.; Cheng, G.W.; Cherubini, P.; Cai, X.H.; Liu, X.L.; Wang, X.D.; Zhu, W.Z. Mobile Carbohydrates in Himalayan Treeline Trees I. Evidence for Carbon Gain Limitation but Not for Growth Limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef]
- Chen, L.J.; Xiang, H.Z.; Miao, Y.; Zhang, L.; Guo, Z.F.; Zhao, X.H.; Lin, J.W.; Li, T.L. An Overview of Cold Resistance in Plants. J. Agron. Crop Sci. 2014, 200, 237–245. [Google Scholar] [CrossRef]
- Liang, J.; Niu, Y.; Xie, J.S.; Zhang, J.D. Antioxidase Activities and Photosynthetic Pigment Contents in Larix Principis- Rupp Rech till Leaves along an Altitudinal Gradient. Chin. J. Appl. Ecol. 2007, 18, 1414–1419. [Google Scholar] [CrossRef]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous Brassinolide Enhances the Growth and Cold Resistance of Maize (Zea Mays L.) Seedlings under Chilling Stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Yang, L.T.; Li, Y.R. Physiological and Biochemical Characteristics Related to Cold Resistance in Sugarcane. Sugar Tech. 2015, 17, 49–58. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Si, G.C.; Wang, J.; Han, C.H.; Zhang, G.X. Effects of Microclimate on Soil Bacterial Communities across Two Contrasting Timberline Ecotones in Southeast Tibet. Eur. J. Soil Sci. 2015, 66, 1033–1043. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Wang, H.; Peng, Y.; Fang, Y.; Wang, W.; Ma, Y. Effect of Calcium on Growth and Physiological Characteristics of Maize Seedling under Lead Stress. J. Soil Water Conserv. 2016, 30, 202–207. [Google Scholar]
- Li, J.; Lin, P.; Dong, Y.; Guo, X.-H. Effect of Morphology and Physiology of Wetland Plants on Plateaus at Different Altitudes. Plant Sci. J. 2013, 31, 370. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Sahoo, M.R.; Devi, T.R.; Dasgupta, M.; Nongdam, P.; Prakash, N. Reactive Oxygen Species Scavenging Mechanisms Associated with Polyethylene Glycol Mediated Osmotic Stress Tolerance in Chinese Potato. Sci. Rep. 2020, 10, 5404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Liu, Z.P.; Hai, B.I. Comparative Study on the Cold-Resistance of Four Species of Color-Leafed Plants under Drop in Temperature. J. Shanxi Agric. Sci. 2009, 37, 44–47. [Google Scholar]
- Wang, Q.; Cheng, T.; Yu, X.; Teixeira da Silva, J.A.; Byrne, D.H. Physiological and Biochemical Responses of Six Herbaceous Peony Cultivars to Cold Stress. South Afr. J. Bot. 2014, 94, 140–148. [Google Scholar] [CrossRef]
- Cui, G.; Ma, C. Research on Leaf Morphology and Cold Resistance of Alfalfa. Acta Agrestia Sin. 2007, 15, 70–75. [Google Scholar]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Gechev, T.; Willekens, H.; van Montagu, M.; Inzé, D.; van Camp, W.; Toneva, V.; Minkov, I. Different Responses of Tobacco Antioxidant Enzymes to Light and Chilling Stress. J. Plant Physiol. 2003, 160, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Clemente, R.M.; Vives, V.; Zandalinas, S.I.; López-Climent, M.F.; Muñoz, V.; Gómez-Cadenas, A. Biotechnological Approaches to Study Plant Responses to Stress. Biomed. Res. Int. 2013, 2013, 654120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.; Schönbeck, L.; et al. Active Summer Carbon Storage for Winter Persistence in Trees at the Cold Alpine Treeline. Tree Physiol. 2018, 38, 1345–1355. [Google Scholar] [CrossRef]
- Pan, Q.; Han, X.; Bai, Y. Advances in Physiology and Ecology Studies on Stored Non-Structure Carbohydrates in Plants. Chin. Bull. Bot. 2002, 19, 30. [Google Scholar]
- Kavi Kishor, P.B.; Hong, Z.; Miao, G.H.; Hu, C.A.A.; Verma, D.P.S. Overexpression of Δ1-Pyrroline-5-Carboxylate Synthetase Increases Proline Production and Confers Osmotolerance in Transgenic Plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, M.; Corcuera, L.J. Cold Acclimation in Plants. Phytochemistry 1991, 30, 3177–3184. [Google Scholar]
- Charrier, G.; Ngao, J.; Saudreau, M.; Améglio, T. Effects of Environmental Factors and Management Practices on Microclimate, Winter Physiology, and Frost Resistance in Trees. Front. Plant Sci. 2015, 6, 259. [Google Scholar] [CrossRef]
- Peng, X.; Teng, L.; Yan, X.; Zhao, M.; Shen, S. The Cold Responsive Mechanism of the Paper Mulberry: Decreased Photosynthesis Capacity and Increased Starch Accumulation. BMC Genom. 2015, 16, 898. [Google Scholar] [CrossRef]
- He, J.S.; Fang, J.; Wang, Z.; Guo, D.; Flynn, D.F.B.; Geng, Z. Stoichiometry and Large-Scale Patterns of Leaf Carbon and Nitrogen in the Grassland Biomes of China. Oecologia 2006, 149, 115–122. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornellssen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Guo, Q.; Li, J.; Zhang, Y.; Zhang, J.; Lu, D.; Korpelainen, H.; Li, C. Species-Specific Competition and N Fertilization Regulate Non-Structural Carbohydrate Contents in Two Larix Species. For. Ecol. Manag. 2016, 364, 60–69. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of Plant and Soil C:N:P Stoichiometry and Their Relationships to Forest Growth in Subtropical Plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Pandey, G.K.; Mahiwal, S. Role of Potassium in Plants; Springer: Cham, Switzerland, 2020. [Google Scholar]
| Tree Species | Function Type | Altitude/m | Slope/° | CD/% | TH/m | DBH/cm | CW(E-W)/m | CW(N-S)/m |
|---|---|---|---|---|---|---|---|---|
| Abies georgei var. smithii (AG) | E-N-A | 4300 | 25 | 80 | 13.2 ± 0.6 | 134.3 ± 4.3 | 3.4 ± 0.2 | 3.6 ± 0.5 |
| Sabina saltuaria (SS) | E-N-A | 4420 | 25 | 50 | 10.2 ± 0.7 | 90.8 ± 4.5 | 3.2 ± 0.1 | 3.2 ± 0.2 |
| Rhododendron aganniphum (RA) | E-B-S | 4300 | - | - | - | - | - | - |
| Rhododendron nyingchiense (RNY) | E-B-S | 4420 | - | - | - | - | - | - |
| Rhododendron nivale (RNI) | E-B-S | 4420 | - | - | - | - | - | - |
| Parameter | Sources of Variation | Type IIISS | DF | MS | F | p |
|---|---|---|---|---|---|---|
| MDA | Tree species (T) | 43.177 | 4 | 10.794 | 19.317 | 0.000 |
| Organ (O) | 2.343 | 2 | 1.172 | 2.097 | 0.136 | |
| T×O | 73.668 | 8 | 9.208 | 16.479 | 0.000 | |
| H2O2 | T | 30.199 | 4 | 7.550 | 24.381 | 0.000 |
| O | 83.228 | 2 | 41.614 | 134.391 | 0.000 | |
| T×O | 62.436 | 8 | 7.804 | 25.204 | 0.000 | |
| Pro | T | 168,159.362 | 4 | 42,039.840 | 19.249 | 0.000 |
| O | 290,921.055 | 2 | 145,460.527 | 66.604 | 0.000 | |
| T×O | 391,266.440 | 8 | 48,908.305 | 22.394 | 0.000 | |
| POD | T | 1,672,339.493 | 4 | 418,084.873 | 145.484 | 0.000 |
| O | 25,492.132 | 2 | 12,746.066 | 4.435 | 0.018 | |
| T×O | 105,025.950 | 8 | 13,128.244 | 4.568 | 0.000 | |
| SOD | T | 189,144.989 | 4 | 47,286.247 | 10.785 | 0.000 |
| O | 17862.737 | 2 | 8931.368 | 2.037 | 0.143 | |
| T×O | 28,3956.021 | 8 | 35,494.503 | 8.095 | 0.000 | |
| SS | T | 1741.269 | 4 | 435.317 | 8.719 | 0.000 |
| O | 2540.121 | 2 | 1270.061 | 25.437 | 0.000 | |
| T×O | 416.552 | 8 | 52.069 | 1.043 | 0.420 | |
| ST | T | 1848.201 | 4 | 462.050 | 5.796 | 0.001 |
| O | 18,258.872 | 2 | 9129.436 | 114.513 | 0.000 | |
| T×O | 9252.173 | 8 | 1156.522 | 14.507 | 0.000 | |
| SS/ST | T | 16.735 | 4 | 4.184 | 5.881 | 0.001 |
| O | 55.921 | 2 | 27.961 | 39.304 | 0.000 | |
| T×O | 27.485 | 8 | 3.436 | 4.830 | 0.000 | |
| NSCT | T | 902.101 | 4 | 225.525 | 1.807 | 0.145 |
| O | 7309.347 | 2 | 3654.674 | 29.288 | 0.000 | |
| T×O | 9188.871 | 8 | 1148.609 | 9.205 | 0.000 |
| Parameter | C1 | C2 | U1 | U2 | D | Comprehensive Evaluation |
|---|---|---|---|---|---|---|
| Rhododendron nivale | 1.98 | 0.59 | 1 | 0.7 | 0.916 | 1 |
| Rhododendron nyingchiense | 1.81 | 0.45 | 0.964 | 0.65 | 0.877 | 2 |
| Rhododendron aganniphum | 0.45 | −0.96 | 0.674 | 0.21 | 0.545 | 3 |
| Sabina saltuaria | −2.72 | 1.55 | 0 | 1 | 0.278 | 4 |
| Abies georgei var. smithii | −1.53 | −1.62 | 0.253 | 0 | 0.183 | 5 |
| Weights | - | - | 0.722 | 0.28 | - | - |
| Parameter | C1 | C2 | U1 | U2 | D | Comprehensive Evaluation |
|---|---|---|---|---|---|---|
| Rhododendron nivale | 3.67 | 0.32 | 1 | 0.713 | 0.928 | 1 |
| Rhododendron nyingchiense | −1.01 | 1.14 | 0.095 | 1 | 0.323 | 2 |
| Rhododendron aganniphum | −1.17 | 1.05 | 0.064 | 0.969 | 0.292 | 3 |
| Sabina saltuaria | 0 | −1.72 | 0.29 | 0 | 0.217 | 4 |
| Abies georgei var. smithii | −1.5 | −0.79 | 0 | 0.325 | 0.082 | 5 |
| Weights | - | - | 0.748 | 0.252 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Chen, W.; Li, J.; Fu, F.; Li, Y.; Xiao, S. Physiological Characteristics and Cold Resistance of Five Woody Plants in Treeline Ecotone of Sygera Mountains. Sustainability 2023, 15, 3040. https://doi.org/10.3390/su15043040
Ding H, Chen W, Li J, Fu F, Li Y, Xiao S. Physiological Characteristics and Cold Resistance of Five Woody Plants in Treeline Ecotone of Sygera Mountains. Sustainability. 2023; 15(4):3040. https://doi.org/10.3390/su15043040
Chicago/Turabian StyleDing, Huihui, Wensheng Chen, Jiangrong Li, Fangwei Fu, Yueyao Li, and Siying Xiao. 2023. "Physiological Characteristics and Cold Resistance of Five Woody Plants in Treeline Ecotone of Sygera Mountains" Sustainability 15, no. 4: 3040. https://doi.org/10.3390/su15043040






