Wet Anaerobic Codigestion of Sewage Sludge and OFMSW in Pilot-Scale Continuously Stirred Tank Reactors: Focus on the Reactor Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Configuration
2.2. Substrate and Inocula
2.3. Analytical Methods
2.4. Process Performance
2.5. Microbial Community Analysis
2.5.1. DNA Extraction
2.5.2. NGS Data Analysis
3. Results
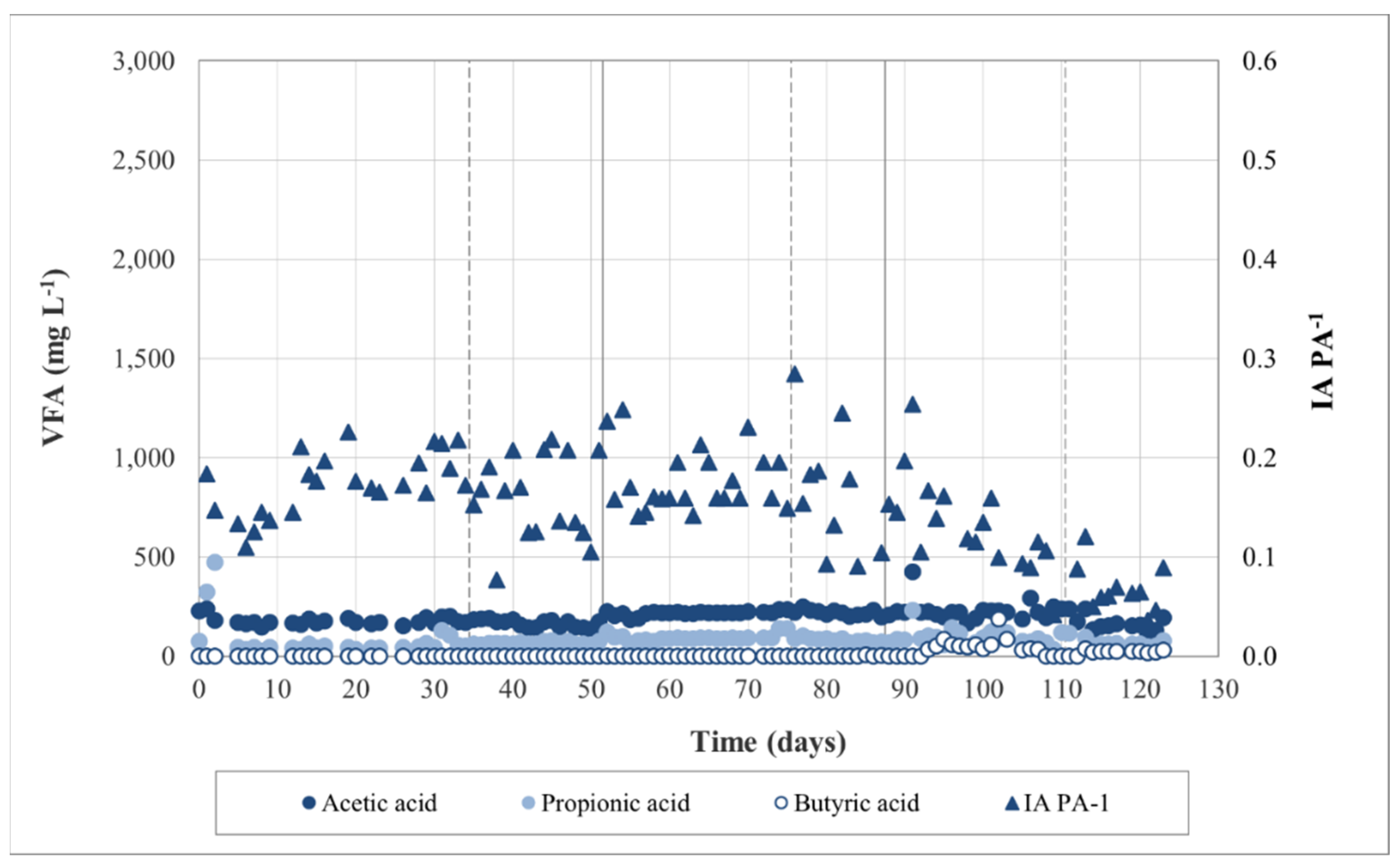
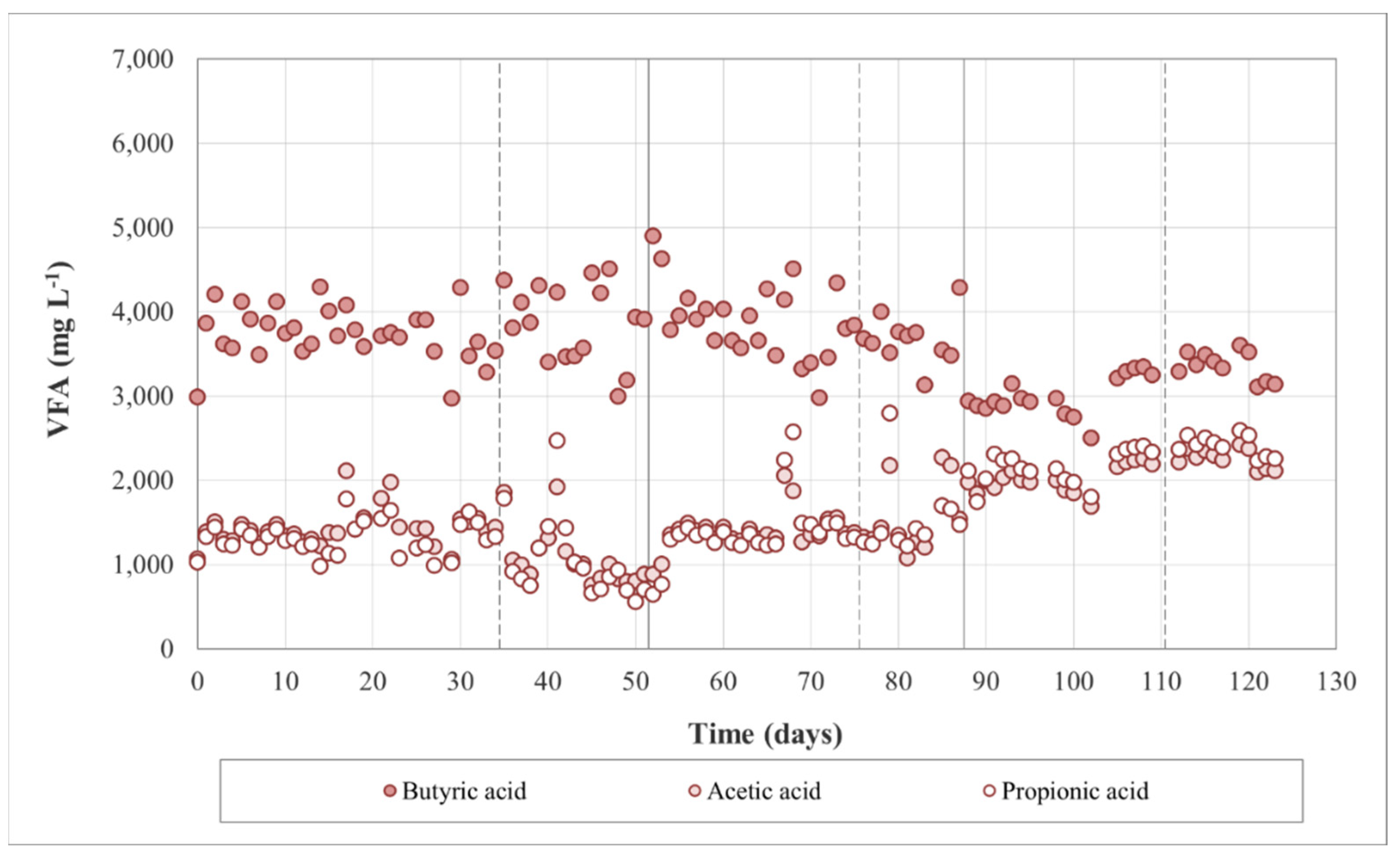
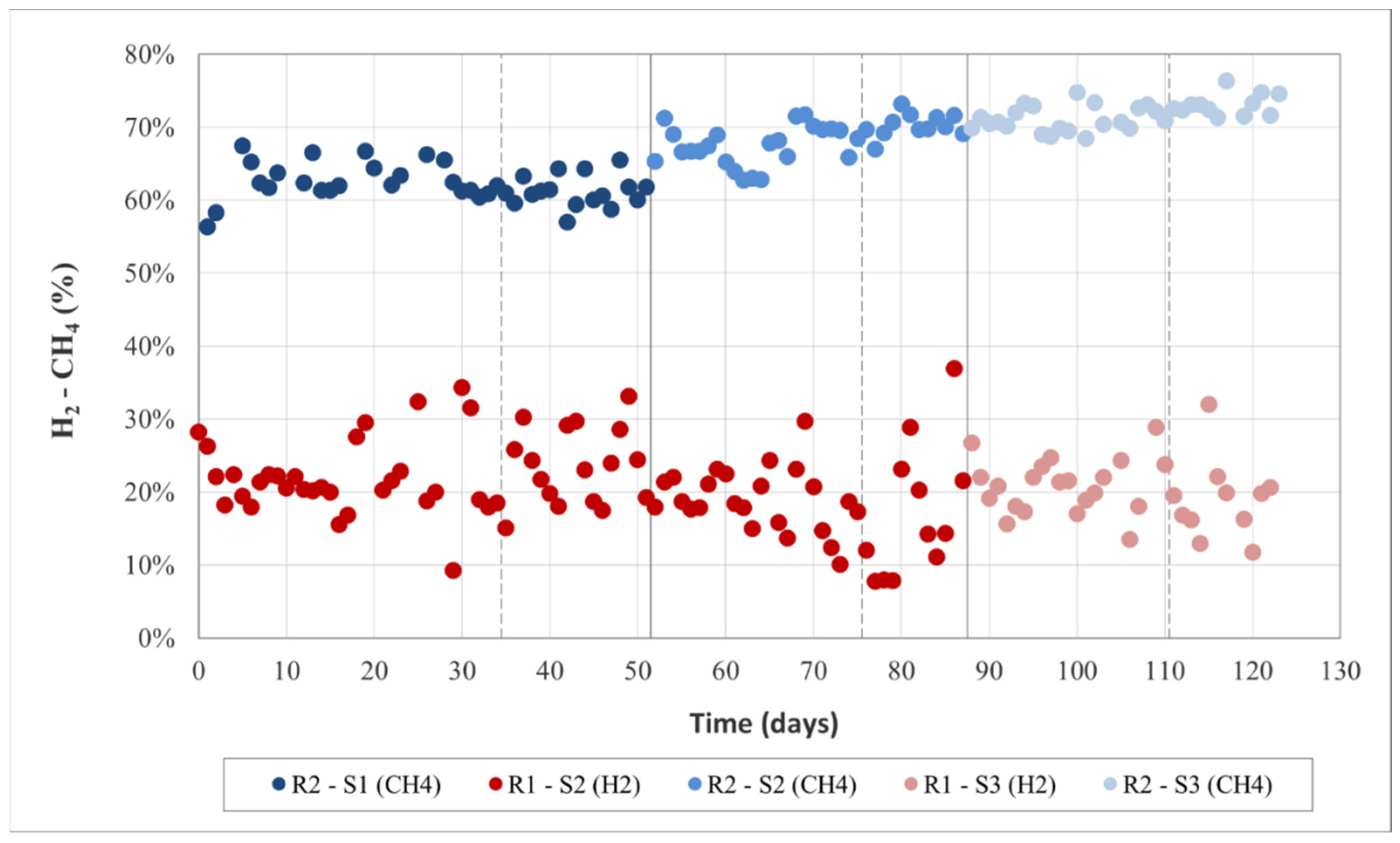
3.1. Anaerobic Digestion Performance
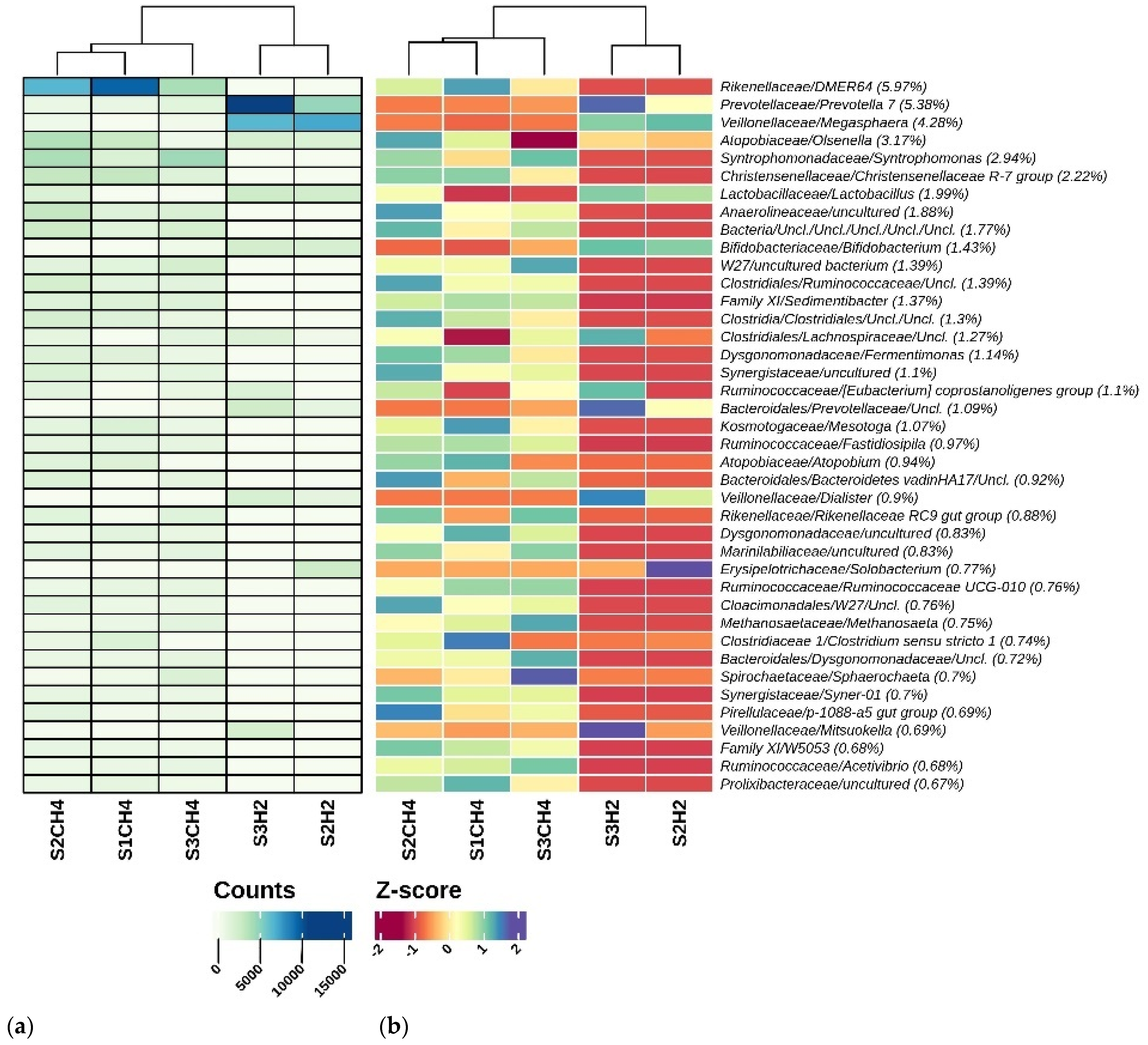
3.2. Microbial Community Analysis
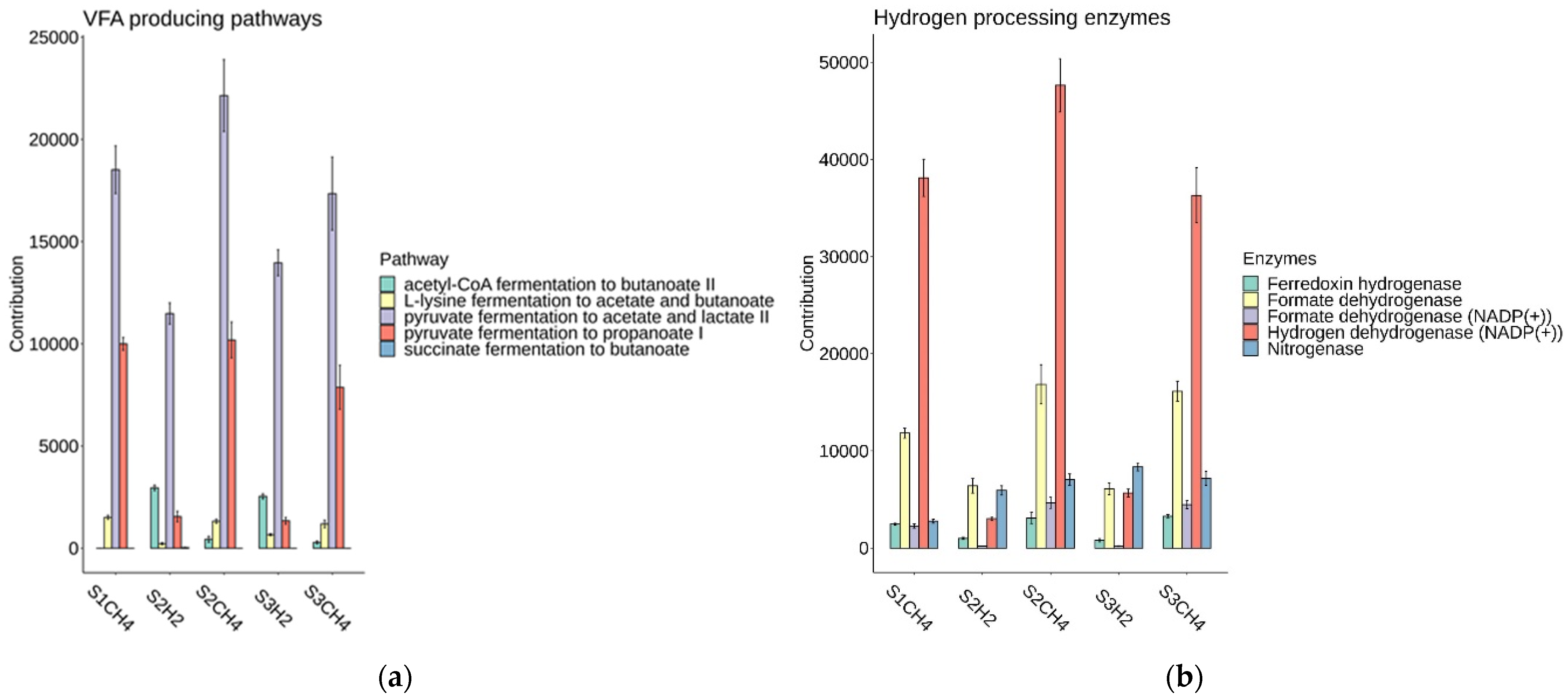
3.2.1. Differences between Hydrogen-Producing Reactors
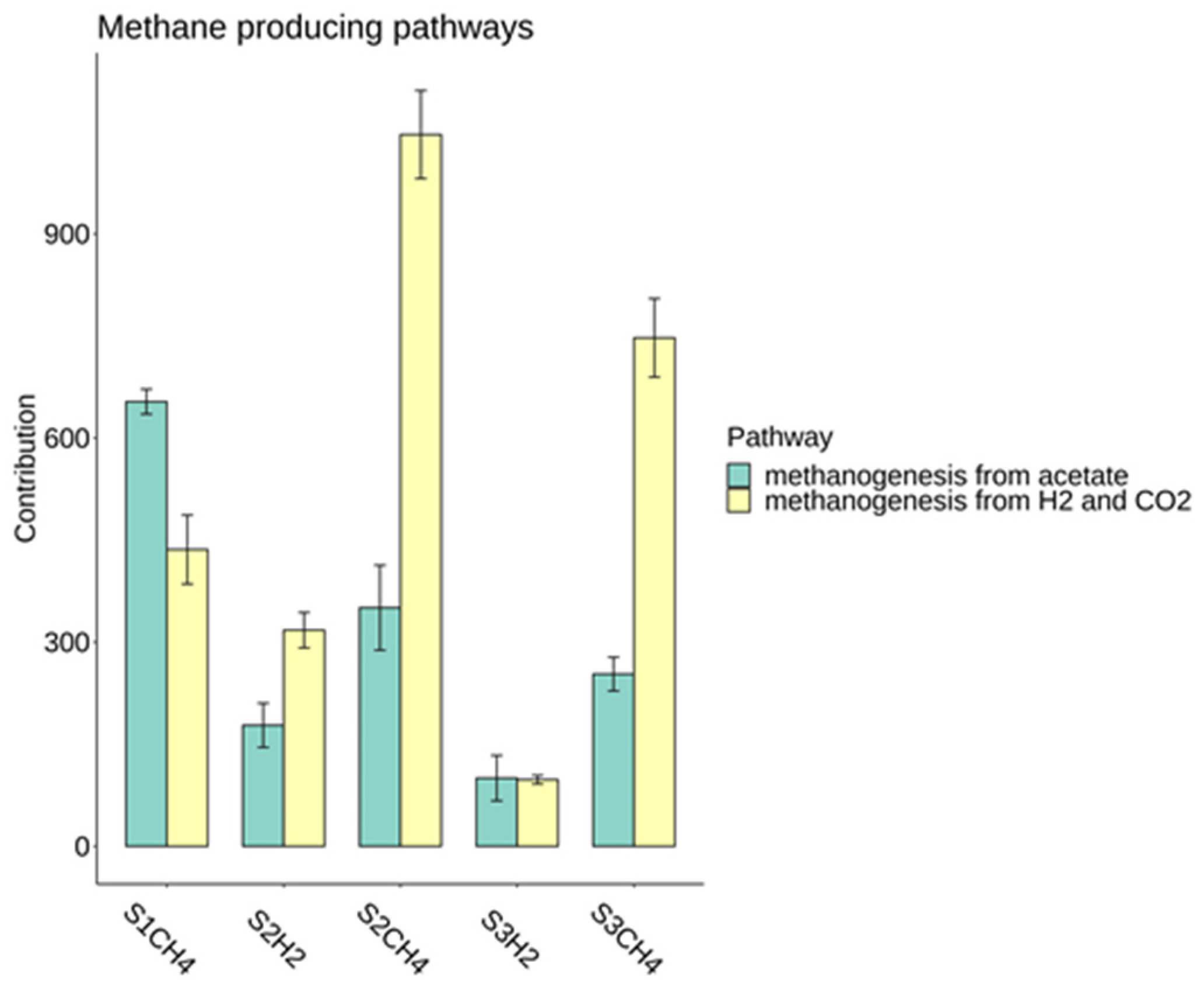
3.2.2. Differences in Methanogenic Rectors
3.2.3. Process Stability and Overall Yield
4. Discussion
Microbial Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Communication from the Commission to the Council and the European Parliament on Future Steps in Bio-Waste Management in the European Union SEC (2010); OPOCE: Bruxelles, Belgium, 2010.
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Coll Lozano, C.; Venus, J. Organic Fraction of Municipal Solid Waste for the Production of L-Lactic Acid with High Optical Purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; European Parliament: Strasbourg, France, 2008; pp. 3–30.
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic Co-Digestion of Organic Fraction of Municipal Solid Waste (OFMSW): Progress and Challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar]
- Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste; European Parliament: Strasbourg, France, 2018; pp. 109–1400.
- Eurostat Generation of Waste by Waste Category, Hazardousness and NACE Rev. 2 Activity. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WASGEN__custom_3110365/default/table?lang=en (accessed on 25 July 2022).
- Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment; European Parliament: Strasbourg, France, 1991; Volume 135.
- Vea, E.B.; Romeo, D.; Thomsen, M. Biowaste Valorisation in a Future Circular Bioeconomy. Procedia CIRP 2018, 69, 591–596. [Google Scholar] [CrossRef]
- Di Matteo, U.; Nastasi, B.; Albo, A.; Astiaso Garcia, D. Energy Contribution of OFMSW (Organic Fraction of Municipal Solid Waste) to Energy-Environmental Sustainability in Urban Areas at Small Scale. Energies 2017, 10, 229. [Google Scholar] [CrossRef]
- Baccioli, A.; Ferrari, L.; Pecorini, I.; Marchionni, A.; Susini, C.; Desideri, U. Feasibility Analysis of a Biogas-Fuelled Trigeneration Plant Operating with a MGT. In Proceedings of the 31st International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Guimarães, Portugal, 17–22 June 2018. [Google Scholar]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food Wastes and Sewage Sludge as Feedstock for an Urban Biorefinery Producing Biofuels and Added-Value Bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Alibardi, L.; Astrup, T.F.; Asunis, F.; Clarke, W.P.; De Gioannis, G.; Dessì, P.; Lens, P.N.L.; Lavagnolo, M.C.; Lombardi, L.; Muntoni, A.; et al. Organic Waste Biorefineries: Looking towards Implementation. Waste Manag. 2020, 114, 274–286. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.-R.; Bonk, F.; Schmidt, J.; Thomsen, M. Dark Fermentation Biorefinery in the Present and Future (Bio)Chemical Industry. Rev. Environ. Sci. Bio/Technol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment Methods to Enhance Anaerobic Digestion of Organic Solid Waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Rossi, E.; Pecorini, I.; Panico, A.; Iannelli, R. Impact of Reactor Configuration and Relative Operating Conditions on Volatile Fatty Acids Production from Organic Waste. Environ. Technol. Rev. 2022, 11, 156–186. [Google Scholar] [CrossRef]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon Recovery from Wastewater through Bioconversion into Biodegradable Polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Micolucci, F.; Gottardo, M.; Pavan, P.; Cavinato, C.; Bolzonella, D. Pilot Scale Comparison of Single and Double-Stage Thermophilic Anaerobic Digestion of Food Waste. J. Clean. Prod. 2018, 171, 1376–1385. [Google Scholar] [CrossRef]
- Micolucci, F.; Gottardo, M.; Bolzonella, D.; Pavan, P.; Majone, M.; Valentino, F. Pilot-Scale Multi-Purposes Approach for Volatile Fatty Acid Production, Hydrogen and Methane from an Automatic Controlled Anaerobic Process. J. Clean. Prod. 2020, 277, 124297. [Google Scholar] [CrossRef]
- Xie, S.; Wickham, R.; Nghiem, L.D. Synergistic Effect from Anaerobic Co-Digestion of Sewage Sludge and Organic Wastes. Int. Biodeterior. Biodegrad. 2017, 116, 191–197. [Google Scholar] [CrossRef]
- Francini, G.; Lombardi, L.; Freire, F.; Pecorini, I.; Marques, P. Environmental and Cost Life Cycle Analysis of Different Recovery Processes of Organic Fraction of Municipal Solid Waste and Sewage Sludge. Waste Biomass. Valor. 2019, 10, 3613–3634. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Ye, H.; Wang, Y.; Luo, W.; Chang, J.-S.; Li, Q.; He, N. Anaerobic Co-Digestion of Sewage Sludge and Food Waste for Hydrogen and VFA Production with Microbial Community Analysis. Waste Manag. 2018, 78, 789–799. [Google Scholar] [CrossRef]
- Pecorini, I.; Olivieri, T.; Bacchi, D.; Paradisi, A.; Corti, A.; Carnevale, E. Evaluation of Gas Production in a Industrial Anaerobic Digester by Means of Biochemical Methane Potential of Organic Municipal Solid Waste Components. In Proceedings of the 25th International Conference on Efficiency, Cost, Optimization and Simulation of Energy Conversion Systems and Processes, Perugia, Italy, 26–29 June 2012; pp. 173–184. [Google Scholar]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Long-Term Alkaline Volatile Fatty Acids Production from Waste Streams: Impact of PH and Dominance of Dysgonomonadaceae. Bioresour. Technol. 2022, 346, 126621. [Google Scholar] [CrossRef]
- Pecorini, I.; Baldi, F.; Iannelli, R. Biochemical Hydrogen Potential Tests Using Different Inocula. Sustainability 2019, 11, 622. [Google Scholar] [CrossRef]
- Nabaterega, R.; Kumar, V.; Khoei, S.; Eskicioglu, C. A Review on Two-Stage Anaerobic Digestion Options for Optimizing Municipal Wastewater Sludge Treatment Process. J. Environ. Chem. Eng. 2021, 9, 105502. [Google Scholar] [CrossRef]
- Baldi, F.; Pecorini, I.; Iannelli, R. Comparison of Single-Stage and Two-Stage Anaerobic Co-Digestion of Food Waste and Activated Sludge for Hydrogen and Methane Production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- APAT APAT, Digestione Anaerobica Della Frazione Organica Dei Rifiuti Solidi e Aspetti Fondamentali, Progettuali, Gestionali, Di Impatto Ambientale Ed Integrazione Con La Depurazione Delle Acque Reflue 2005. Available online: https://www.isprambiente.gov.it/contentfiles/00003400/3482-manuali-linee-guida-2005.pdf (accessed on 11 January 2023).
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-Hythane Production from Food Waste by Dark Fermentation Coupled with Anaerobic Digestion Process: A Long-Term Pilot Scale Experience. Int. J. Hydrogen Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- Micolucci, F.; Gottardo, M.; Bolzonella, D.; Pavan, P. Automatic Process Control for Stable Bio-Hythane Production in Two-Phase Thermophilic Anaerobic Digestion of Food Waste. Int. J. Hydrogen Energy 2014, 39, 17563–17572. [Google Scholar] [CrossRef]
- Chinellato, G.; Cavinato, C.; Bolzonella, D.; Heaven, S.; Banks, C.J. Biohydrogen Production from Food Waste in Batch and Semi-Continuous Conditions: Evaluation of a Two-Phase Approach with Digestate Recirculation for PH Control. Int. J. Hydrogen Energy 2013, 38, 4351–4360. [Google Scholar] [CrossRef]
- Rossi, E.; Becarelli, S.; Pecorini, I.; Di Gregorio, S.; Iannelli, R. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Plug-Flow Reactors: Focus on Bacterial Community Metabolic Pathways. Water 2022, 14, 195. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Cappai, G.; De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. An Experimental Study on Fermentative H2 Production from Food Waste as Affected by PH. Waste Manag. 2014, 34, 1510–1519. [Google Scholar] [CrossRef]
- Baldi, F.; Iannelli, R.; Pecorini, I.; Polettini, A.; Pomi, R.; Rossi, A. Influence of the PH Control Strategy and Reactor Volume on Batch Fermentative Hydrogen Production from the Organic Fraction of Municipal Solid Waste. Waste Manag. Res. 2019, 37, 478–485. [Google Scholar] [CrossRef]
- Standard Methods. Available online: https://www.standardmethods.org/ (accessed on 14 April 2020).
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved Alkalimetric Monitoring for Anaerobic Digestion of High-Strength Wastes. J. Water Pollut. Control Fed. 1986, 58, 406–411. [Google Scholar]
- Drosg, B. Process Monitoring in Biogas Plants; IEA Bioenergy: Paris, France, 2013. [Google Scholar]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R. A Review of Dark Fermentative Hydrogen Production from Biodegradable Municipal Waste Fractions. Waste Manag. 2013, 33, 1345–1361. [Google Scholar] [CrossRef]
- Emerson, E.L.; Weimer, P.J. Fermentation of Model Hemicelluloses by Prevotella Strains and Butyrivibrio Fibrisolvens in Pure Culture and in Ruminal Enrichment Cultures. Appl. Microbiol. Biotechnol. 2017, 101, 4269–4278. [Google Scholar] [CrossRef]
- Feng, K.; Li, H.; Zheng, C. Shifting Product Spectrum by PH Adjustment during Long-Term Continuous Anaerobic Fermentation of Food Waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ikeyama, N.; Toyoda, A.; Murakami, T.; Mori, H.; Iino, T.; Ohkuma, M. Dialister hominis sp. Nov., Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2020, 70, 589–595. [Google Scholar] [CrossRef]
- Bergey’s Manual of Systematics of Archaea and Bacteria (BMSAB); Wiley: Hoboken, NJ, USA, 2015; ISBN 978-1-118-96060-8.
- Sekoai, P.T.; Ghimire, A.; Ezeokoli, O.T.; Rao, S.; Ngan, W.Y.; Habimana, O.; Yao, Y.; Yang, P.; Yiu Fung, A.H.; Yoro, K.O.; et al. Valorization of Volatile Fatty Acids from the Dark Fermentation Waste Streams-A Promising Pathway for a Biorefinery Concept. Renew. Sustain. Energy Rev. 2021, 143, 110971. [Google Scholar] [CrossRef]
- Higdon, S.M.; Huang, B.C.; Bennett, A.B.; Weimer, B.C. Identification of Nitrogen Fixation Genes in Lactococcus Isolated from Maize Using Population Genomics and Machine Learning. Microorganisms 2020, 8, 2043. [Google Scholar] [CrossRef]
- Pinske, C.; Sawers, R.G. Anaerobic Formate and Hydrogen Metabolism. EcoSal. Plus 2016, 7, 246. [Google Scholar] [CrossRef]
- Sikora, A.; Detman, A.; Mielecki, D.; Chojnacka, A.; Błaszczyk, M. Searching for Metabolic Pathways of Anaerobic Digestion: A Useful List of the Key Enzymes; IntechOpen: London, UK, 2018; ISBN 978-1-83881-850-0. [Google Scholar]
- McInerney, M.J.; Bryant, M.P.; Hespell, R.B.; Costerton, J.W. Syntrophomonas Wolfei Gen. Nov. Sp. Nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl. Environ. Microbiol. 1981, 41, 1029–1039. [Google Scholar]
- Shakeri Yekta, S.; Liu, T.; Axelsson Bjerg, M.; Šafarič, L.; Karlsson, A.; Björn, A.; Schnürer, A. Sulfide Level in Municipal Sludge Digesters Affects Microbial Community Response to Long-Chain Fatty Acid Loads. Biotechnol. Biofuels 2019, 12, 259. [Google Scholar] [CrossRef]
- Pelletier, E.; Kreimeyer, A.; Bocs, S.; Rouy, Z.; Gyapay, G.; Chouari, R.; Rivière, D.; Ganesan, A.; Daegelen, P.; Sghir, A.; et al. “Candidatus Cloacamonas Acidaminovorans”: Genome Sequence Reconstruction Provides a First Glimpse of a New Bacterial Division. J. Bacteriol. 2008, 190, 2572–2579. [Google Scholar] [CrossRef]
- Westerholm, M.; Calusinska, M.; Dolfing, J. Syntrophic Propionate-Oxidizing Bacteria in Methanogenic Systems. FEMS Microbiol. Rev. 2021, 46, fuab057. [Google Scholar] [CrossRef]
- Caro-Quintero, A.; Ritalahti, K.M.; Cusick, K.D.; Löffler, F.E.; Konstantinidis, K.T. The Chimeric Genome of Sphaerochaeta: Nonspiral Spirochetes That Break with the Prevalent Dogma in Spirochete Biology. mBio 2012, 3, e00025-12. [Google Scholar] [CrossRef]
- Dassa, B.; Borovok, I.; Lamed, R.; Henrissat, B.; Coutinho, P.; Hemme, C.L.; Huang, Y.; Zhou, J.; Bayer, E.A. Genome-Wide Analysis of Acetivibrio Cellulolyticus Provides a Blueprint of an Elaborate Cellulosome System. BMC Genom. 2012, 13, 210. [Google Scholar] [CrossRef]
- Kanai, T.; Imanaka, H.; Nakajima, A.; Uwamori, K.; Omori, Y.; Fukui, T.; Atomi, H.; Imanaka, T. Continuous Hydrogen Production by the Hyperthermophilic Archaeon, Thermococcus Kodakaraensis KOD1. J. Biotechnol. 2005, 116, 271–282. [Google Scholar] [CrossRef]
- Taherdanak, M.; Jafari, O.; Vaez, E.; Zilouei, H. The Effects of Heat-Shock Pretreatment Conditions on Dark Hydrogen Fermentation from Glucose. Int. J. Ambient Energy 2017, 38, 627–630. [Google Scholar] [CrossRef]
- Ziara, R.M.M.; Miller, D.N.; Subbiah, J.; Dvorak, B.I. Lactate Wastewater Dark Fermentation: The Effect of Temperature and Initial PH on Biohydrogen Production and Microbial Community. Int. J. Hydrogen Energy 2019, 44, 661–673. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, A.; Chen, J. Chemical Inhibitors of Methanogenesis and Putative Applications. Appl. Microbiol. Biotechnol. 2011, 89, 1333–1340. [Google Scholar] [CrossRef]
- Calt, E.A. Products Produced from Organic Waste Using Managed Ecosystem Fermentation. JSD 2015, 8, 43. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile Fatty Acids Production from Food Wastes for Biorefinery Platforms: A Review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Baumann, I.; Westermann, P. Microbial Production of Short Chain Fatty Acids from Lignocellulosic Biomass: Current Processes and Market. BioMed Res. Int. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From Sewage Sludge and Agri-Food Waste to VFA: Individual Acid Production Potential and up-Scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K. Light Fermentation of Dark Fermentation Effluent for Bio-Hydrogen Production by Different Rhodobacter Species at Different Initial Volatile Fatty Acid (VFA) Concentrations. Int. J. Hydrogen Energy 2008, 33, 7405–7412. [Google Scholar] [CrossRef]
- Ghiasian, M. Biophotolysis-Based Hydrogen Production by Cyanobacteria. In Prospects of Renewable Bioprocessing in Future Energy Systems. In Biofuel and Biorefinery Technologies; Rastegari, A.A., Yadav, A.N., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 161–184. ISBN 978-3-030-14463-0. [Google Scholar]
- Uyar, B. Bioreactor Design for Photofermentative Hydrogen Production. Bioprocess Biosyst. Eng. 2016, 39, 1331–1340. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhang, Z.; Lee, D.-J.; Zhou, X.; Jing, Y.; Ge, X.; Jiang, D.; Hu, J.; He, C. Photo-Fermentative Hydrogen Production from Crop Residue: A Mini Review. Bioresour. Technol. 2017, 229, 222–230. [Google Scholar] [CrossRef]
- Chorbadzhiyska, E.; Hubenova, Y.; Hristov, G.; Mitov, M. Microbial Electrolysis Cells as Innovative Technology for Hydrogen Production. Nat. Sci. 2011, 6, 422. [Google Scholar]
- Dange, P.; Pandit, S.; Jadhav, D.; Shanmugam, P.; Gupta, P.K.; Kumar, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K. Recent Developments in Microbial Electrolysis Cell-Based Biohydrogen Production Utilizing Wastewater as a Feedstock. Sustainability 2021, 13, 8796. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Kim, T.-H.; Lee, M.; Kim, S.; Kim, S.-W.; Lee, J. Effects of Various Pretreatments for Enhanced Anaerobic Digestion with Waste Activated Sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; McCabe, M.; Cormican, P.; Zhan, X.; Gardiner, G.E. Inhibition of Volatile Fatty Acids on Methane Production Kinetics during Dry Co-Digestion of Food Waste and Pig Manure. Waste Manag. 2018, 79, 302–311. [Google Scholar] [CrossRef]
- Schink, B.; Stams, A.J.M. Syntrophism among Prokaryotes. In The Prokaryotes: Volume 2: Ecophysiology and Biochemistry; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 309–335. ISBN 978-0-387-30742-8. [Google Scholar]
- Amani, T.; Nosrati, M.; Mousavi, S.M.; Kermanshahi, R.K. Study of Syntrophic Anaerobic Digestion of Volatile Fatty Acids Using Enriched Cultures at Mesophilic Conditions. Int. J. Environ. Sci. Technol. 2011, 8, 83–96. [Google Scholar] [CrossRef]
- Boone, D.R.; Xun, L. Effects of PH, Temperature, and Nutrients on Propionate Degradation by a Methanogenic Enrichment Culture. Appl. Environ. Microbiol. 1987, 53, 1589–1592. [Google Scholar]
- Staley, B.F.; de los Reyes, F.L.; Barlaz, M.A. Effect of Spatial Differences in Microbial Activity, PH, and Substrate Levels on Methanogenesis Initiation in Refuse. Appl. Environ. Microbiol. 2011, 77, 2381–2391. [Google Scholar] [CrossRef]
- Inhibitors of the Methane Fermentation Process with Particular Emphasis on the Microbiological Aspect: A Review—Czatzkowska—2020—Energy Science & Engineering—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/ese3.609 (accessed on 12 July 2022).
- Wang, H.; Fotidis, I.A.; Angelidaki, I. Ammonia Effect on Hydrogenotrophic Methanogens and Syntrophic Acetate-Oxidizing Bacteria. FEMS Microbiol. Ecol. 2015, 91, fiv130. [Google Scholar] [CrossRef]
- Cong, S.; Xu, Y.; Lu, Y. Growth Coordination Between Butyrate-Oxidizing Syntrophs and Hydrogenotrophic Methanogens. Front. Microbiol. 2021, 12, 742531. [Google Scholar] [CrossRef]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse Hydrogen Production and Consumption Pathways Influence Methane Production in Ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef]
- Conrad, R. Contribution of Hydrogen to Methane Production and Control of Hydrogen Concentrations in Methanogenic Soils and Sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an Enhancer in Methanogenic Degradation of Volatile Fatty Acids under Ammonia-Stressed Condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef]
- Silva-Illanes, F.; Tapia-Venegas, E.; Schiappacasse, M.C.; Trably, E.; Ruiz-Filippi, G. Impact of Hydraulic Retention Time (HRT) and PH on Dark Fermentative Hydrogen Production from Glycerol. Energy 2017, 141, 358–367. [Google Scholar] [CrossRef]
- Guyot, J.-P.; Brauman, A. Methane Production from Formate by Syntrophic Association of Methanobacterium Bryantii and Desulfovibrio vulgaris JJ. Appl. Environ. Microbiol. 1986, 52, 1436–1437. [Google Scholar] [CrossRef]
- Belay, N.; Sparling, R.; Daniels, L. Relationship of Formate to Growth and Methanogenesis by Methanococcus Thermolithotrophicus. Appl. Environ. Microbiol. 1986, 52, 1080–1085. [Google Scholar] [CrossRef] [Green Version]
- Guyot, J.P. Role of Formate in Methanogenesis from Xylan by Cellulomonas sp. Associated with Methanogens and Desulfovibrio vulgaris: Inhbition of the Aceticlastic Reaction. 5. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_5/b_fdi_18-19/24195.pdf (accessed on 11 January 2023).

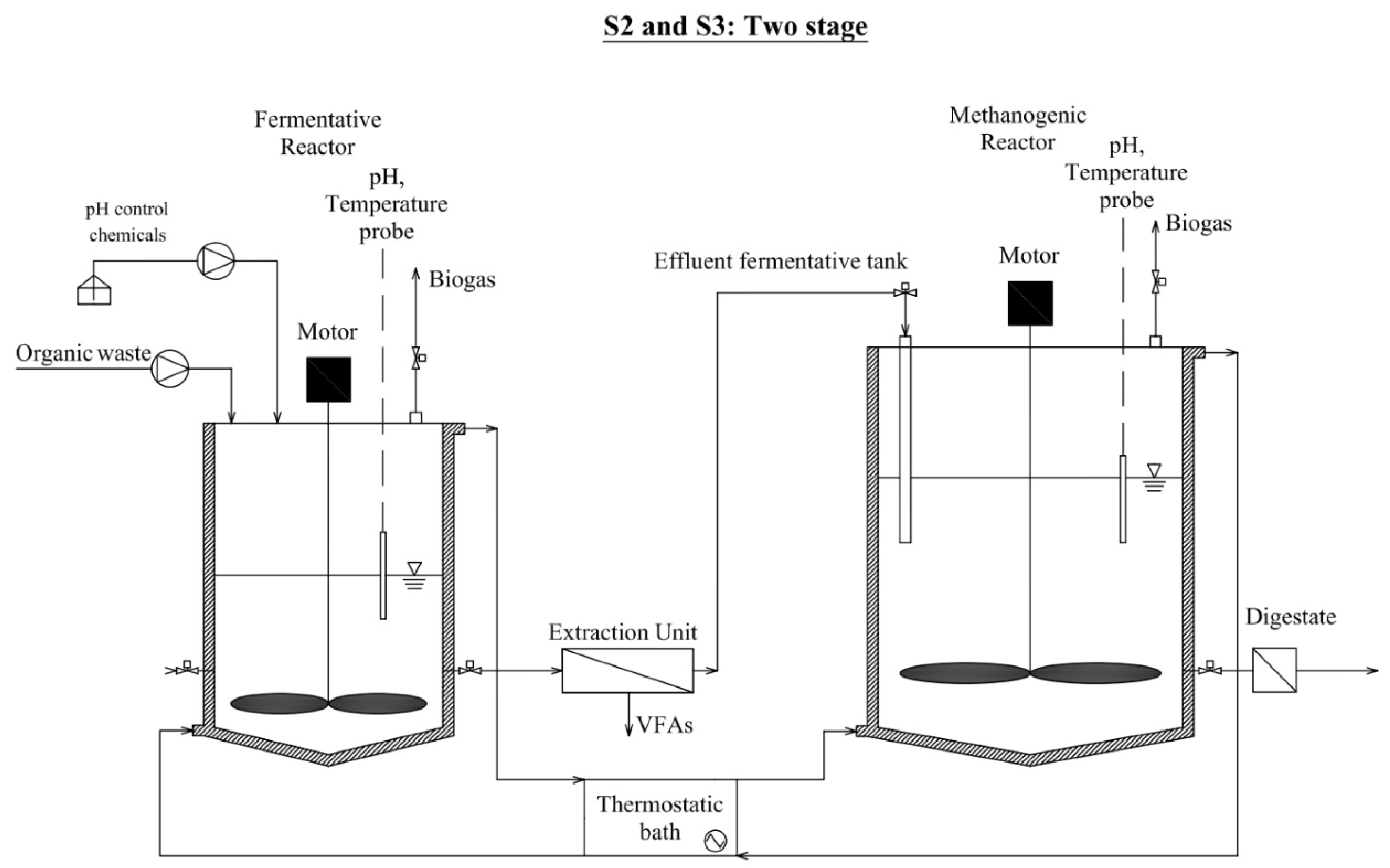







| Parameter | Reference Scenario (S1) S1CH4 | Scenario 2 (S2) S2H2 | Scenario 2 (S2) S2CH4 | Scenario 3 (S3) S3H2 | Scenario 3 (S3) S3CH4 |
|---|---|---|---|---|---|
| Hydraulic Retention Time (HRT) (d) | 17 | 3 | 12 | 1.5 | 12 |
| Organic Loading Rate (ORL) (kgVS/(m3d)) | 2.5 | 14.6 | 2.5 | 27.6 | 2.5 |
| Parameter | Substrate | Inoculum CH4 | Inoculum H2 |
|---|---|---|---|
| TS (%) | 4.9 | 2.49 ± 0.02 | 2.49 ± 0.02 |
| VS/TS (%) | 79.99 | 61.33 ± 0.2 | 61.33 ± 0.2 |
| pH (-) | 4.9 | 8.36 ± 0.02 | 8.36 ± 0.02 |
| Scenario | Archaea (%) | SEM Archaea (%) | V Biogas (NL/Kgtvs) | SEM V Biogas (NL/Kgtvs) | H2 (%) | SEM H2 (%) | CH4(%) | SEM CH4 (%) |
|---|---|---|---|---|---|---|---|---|
| S1CH4 | 3.25 | 0.23 | 519.74 | 19.99 | 0.007 | 0.001 | 59.58 | 0.04 |
| S2H2 | 1.62 | 0.13 | 54.30 | 8.96 | 14.667 | 0.017 | 2.37 | 0.02 |
| S2CH4 | 4.87 | 0.61 | 646.76 | 58.50 | 0.008 | 0.000 | 72.75 | 0.11 |
| S3H2 | 0.80 | 0.04 | 41.49 | 8.76 | 16.545 | 0.066 | 2.39 | 0.01 |
| S3CH4 | 5.45 | 0.17 | 691.32 | 24.23 | 0.010 | 0.001 | 71.09 | 0.08 |
| Scenario | ΔCH4 to S1 (%) | H2 from DF (%) | H2 from DF (NL/KgTVS) | ΔηVS (%) |
|---|---|---|---|---|
| S1 | 0 | - | - | 0 |
| S2 | +52.4 | 14.8 | 7.96 | +16.8 |
| S3 | +59.0 | 16.6 | 6.86 | +20.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecorini, I.; Rossi, E.; Becarelli, S.; Baldi, F.; Di Gregorio, S.; Iannelli, R. Wet Anaerobic Codigestion of Sewage Sludge and OFMSW in Pilot-Scale Continuously Stirred Tank Reactors: Focus on the Reactor Microbial Communities. Sustainability 2023, 15, 3168. https://doi.org/10.3390/su15043168
Pecorini I, Rossi E, Becarelli S, Baldi F, Di Gregorio S, Iannelli R. Wet Anaerobic Codigestion of Sewage Sludge and OFMSW in Pilot-Scale Continuously Stirred Tank Reactors: Focus on the Reactor Microbial Communities. Sustainability. 2023; 15(4):3168. https://doi.org/10.3390/su15043168
Chicago/Turabian StylePecorini, Isabella, Elena Rossi, Simone Becarelli, Francesco Baldi, Simona Di Gregorio, and Renato Iannelli. 2023. "Wet Anaerobic Codigestion of Sewage Sludge and OFMSW in Pilot-Scale Continuously Stirred Tank Reactors: Focus on the Reactor Microbial Communities" Sustainability 15, no. 4: 3168. https://doi.org/10.3390/su15043168







