Responses of Vegetation, Soil, and Microbes and Carbon and Nitrogen Pools to Semiarid Grassland Land-Use Patterns in Duolun, Inner Mongolia, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Field Sampling and laboratory Analysis
2.3. Chemical Analysis
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. C and N Pool Variability
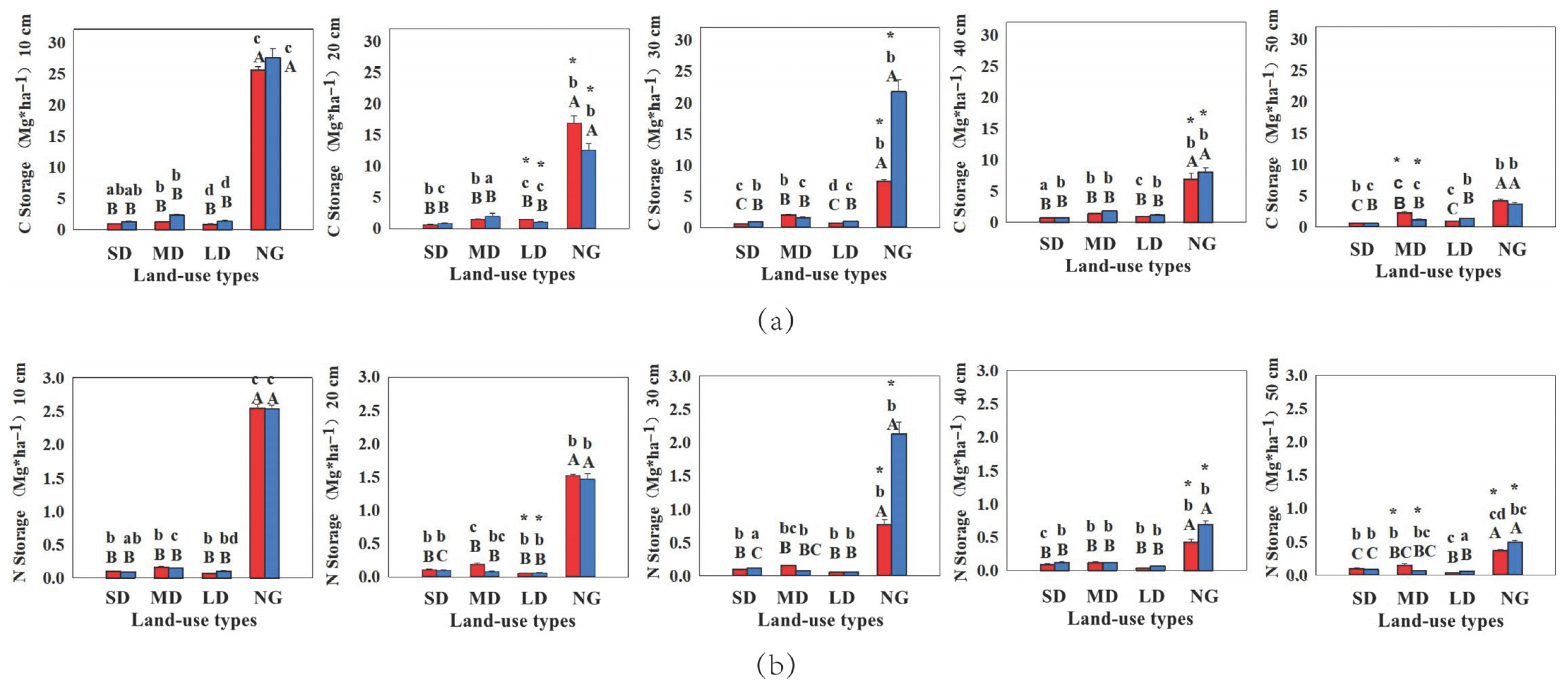
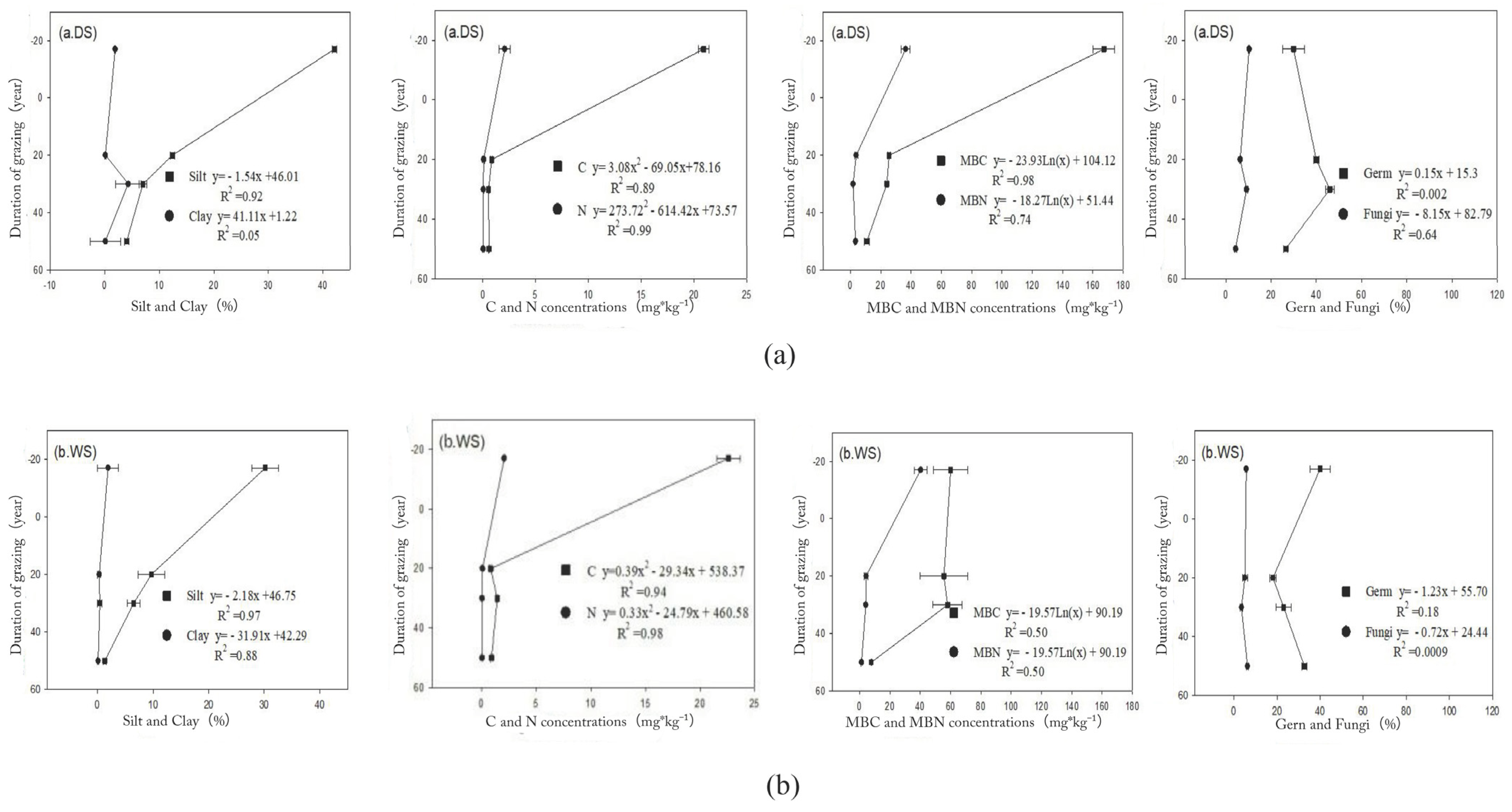
3.1.1. SOC and STN Pools
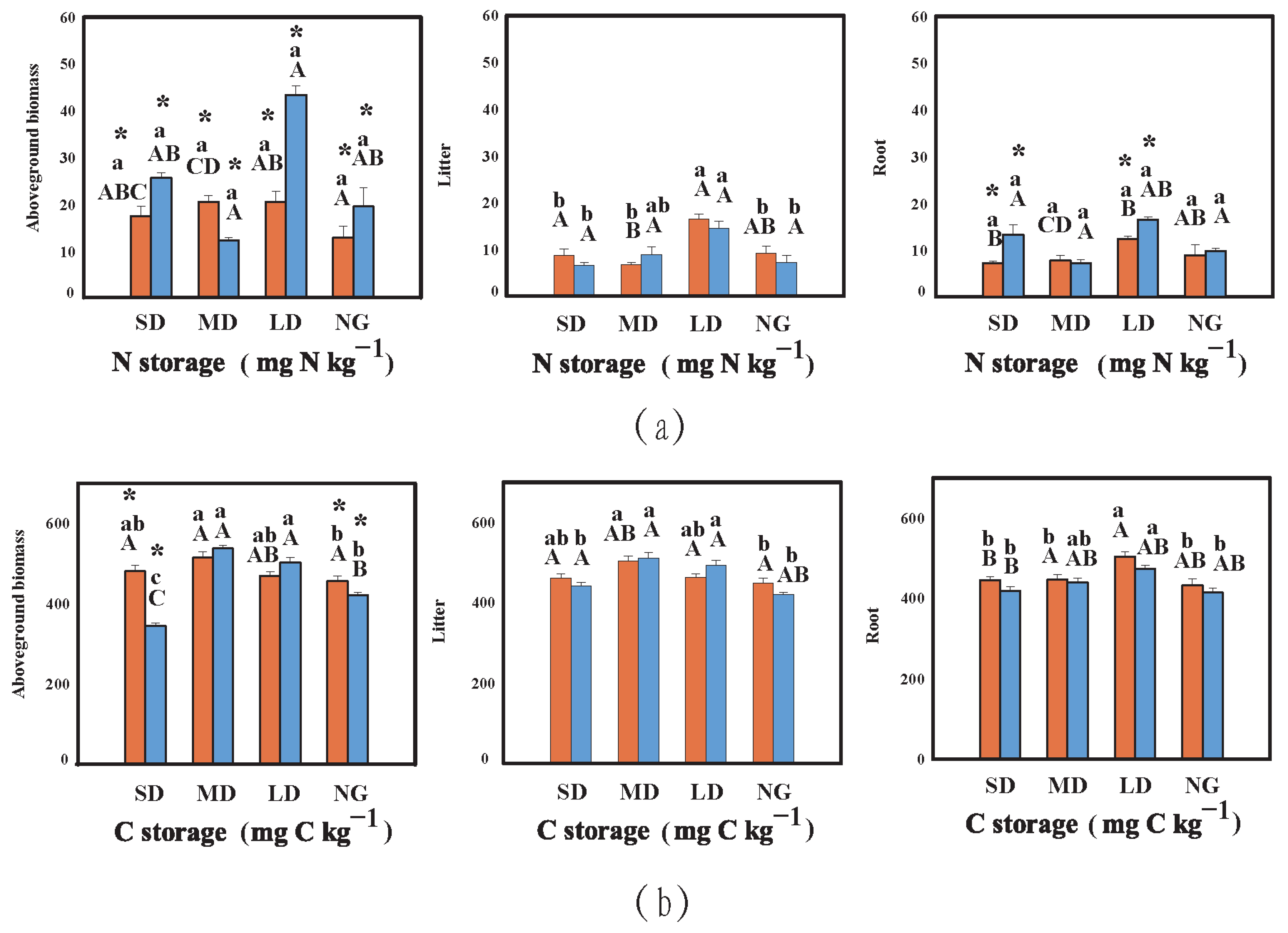
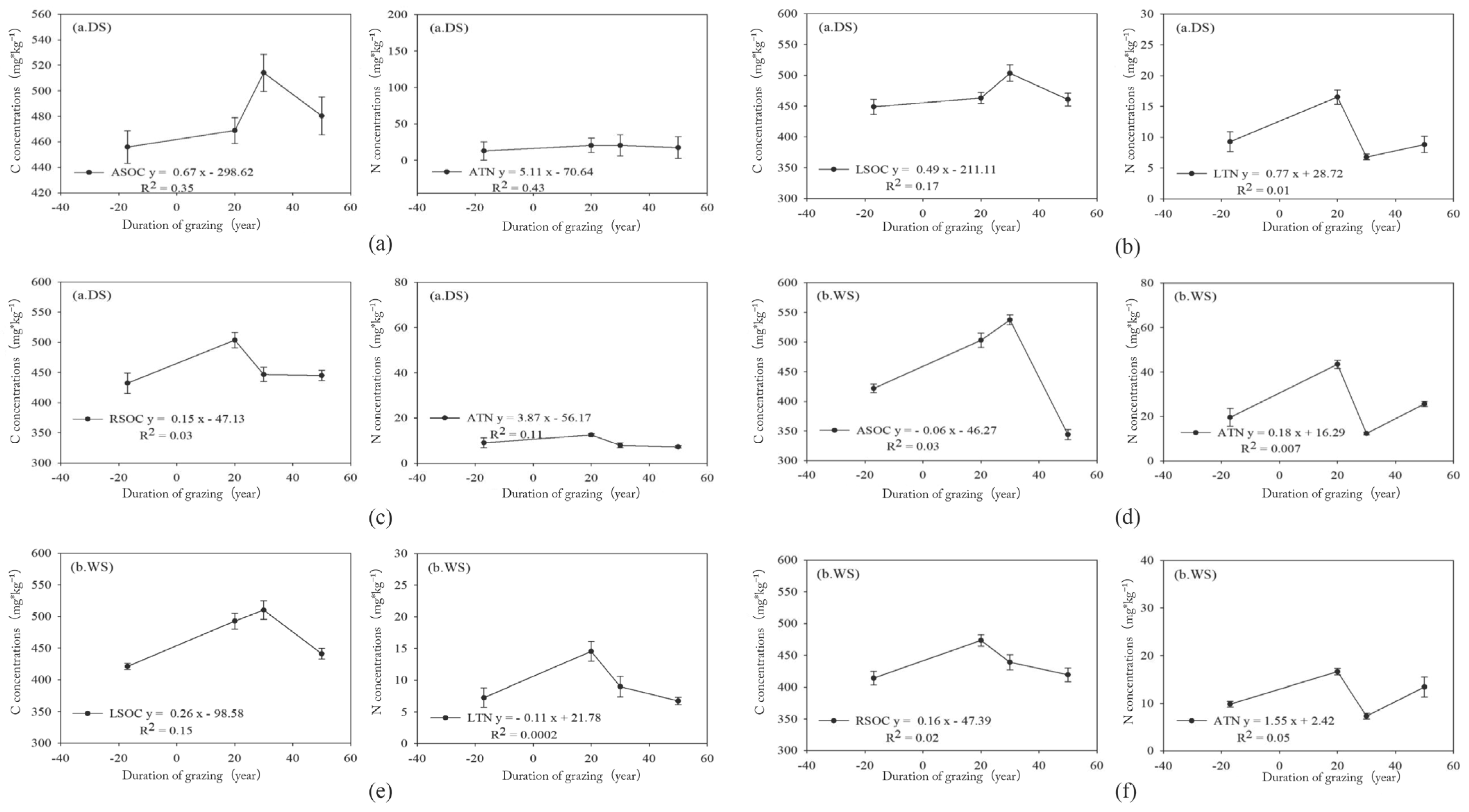
3.1.2. Carbon and Nitrogen in Plants
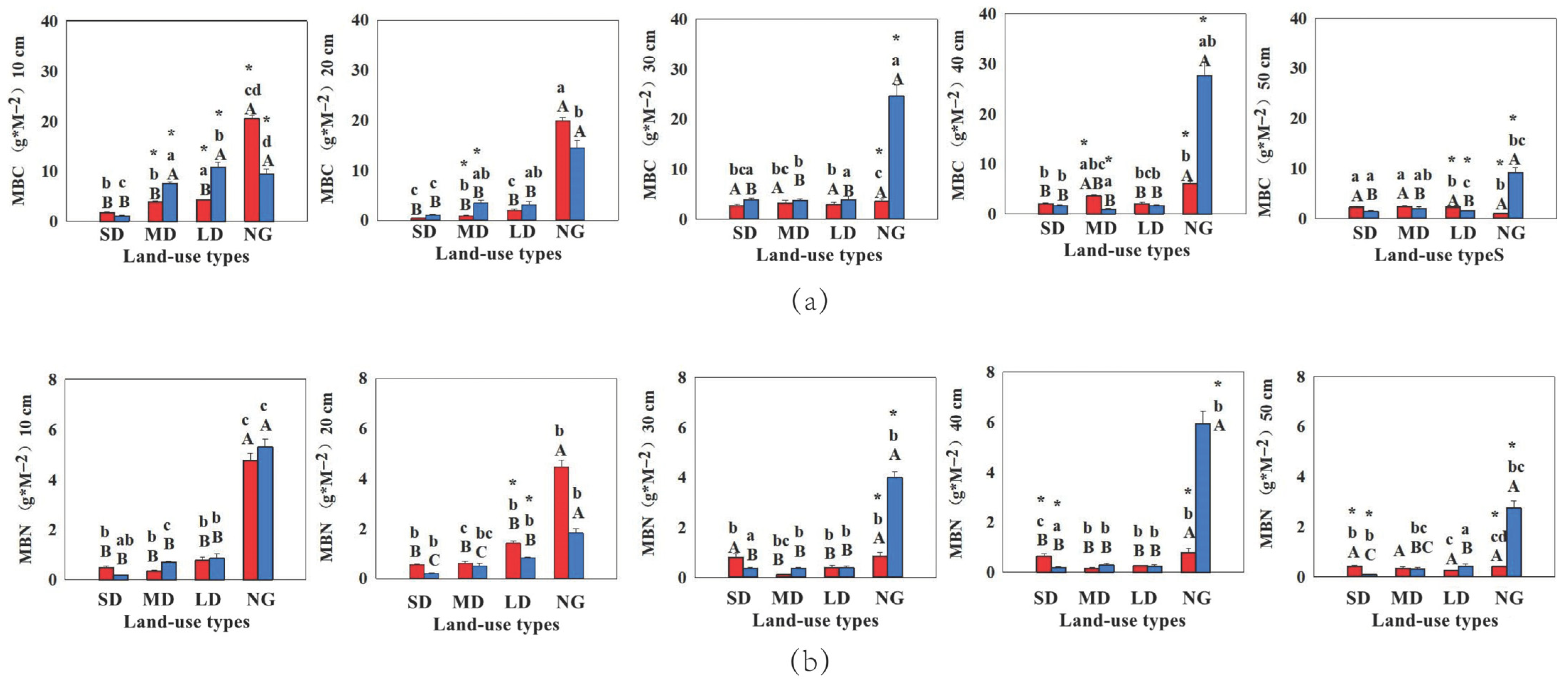
3.1.3. Microbial Biomass Carbon and Nitrogen Pools
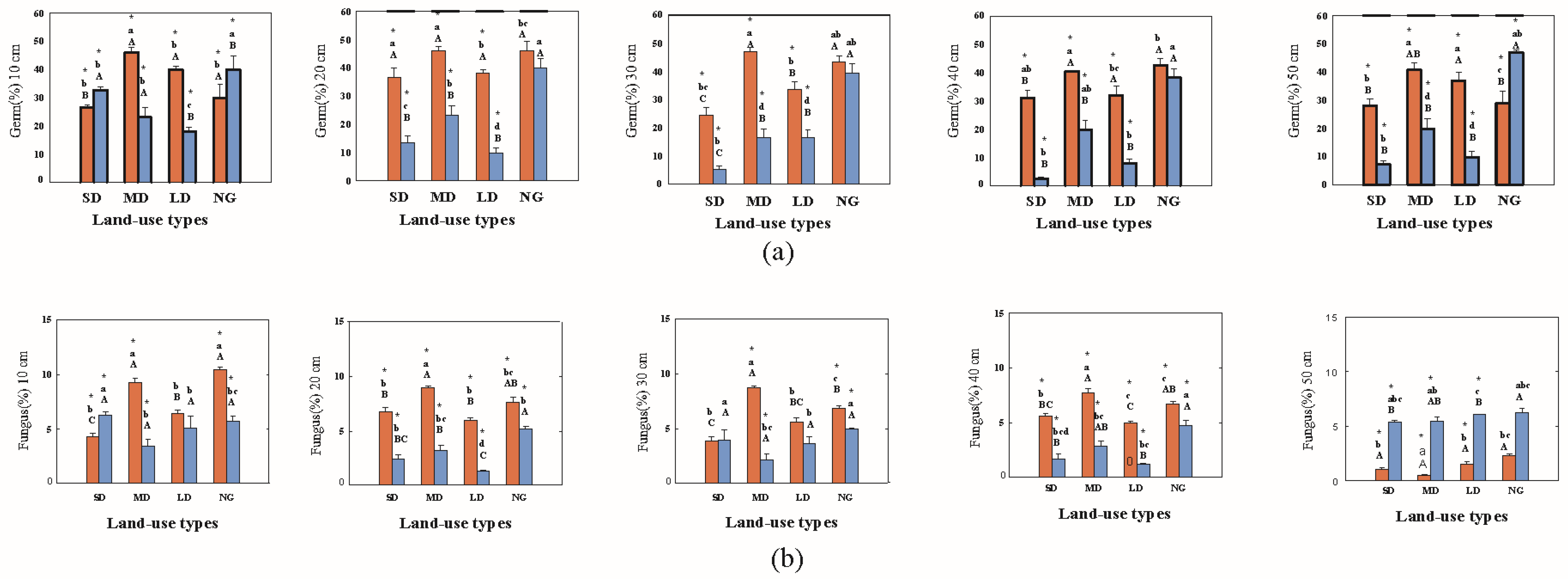
3.1.4. Bacterial and Fungal Abundances
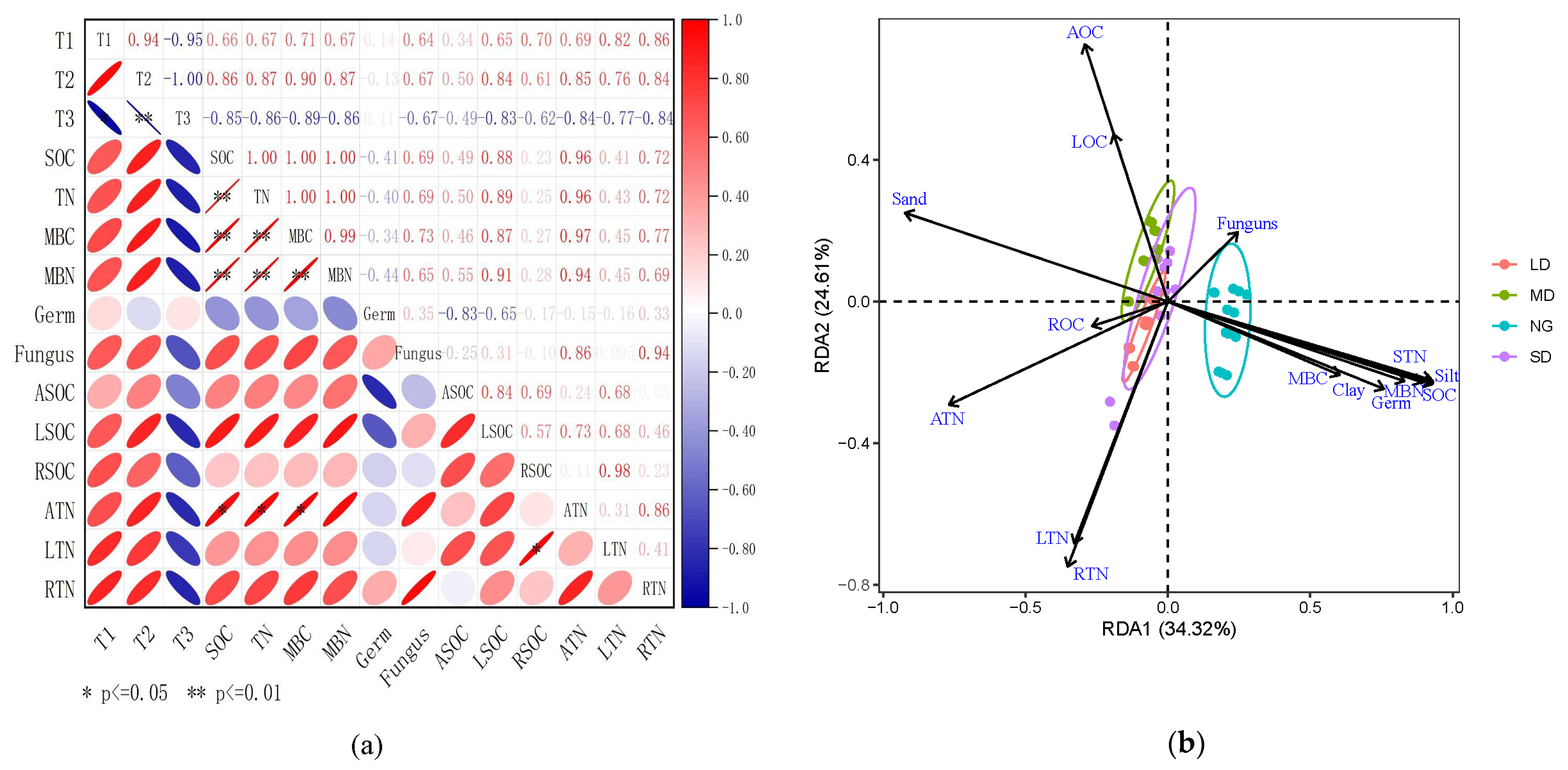
3.2. Relationship between Nutrient Pools and Grazing Intensity
3.3. Analysis of Factors Driving Soil Degradation
4. Discussion
4.1. Responses of Soil Nutrient Pools to Grazing Intensity
4.2. Response of Soil Microbial Abundance to Grazing Intensity
4.3. Effects of Precipitation on Carbon and Nitrogen Pool Cycles
4.4. Response of Plant Nutrient Pools to Grazing Intensity
4.5. Effects of Enclosure on Carbon and Nitrogen Pools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maestre, F.T.; Salguero-Gómez, R.; Quero, J.L. It is getting hotter in here: Determining and projecting the impacts of global environmental change on drylands. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3062–3075. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.R.; Guodong, H.; Xiangyang, H.; Michalk, D.L.; Fujiang, H.; Jianping, W.; Yingjun, Z. Innovative grassland management systems for environmental and livelihood benefits. Proc. Natl. Acad. Sci. USA 2013, 110, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Kansheba, J.M.; Wald, A.E. Entrepreneurial ecosystems quality and productive entrepreneurship: Entrepreneurial attitude as a mediator in early-stage and high-growth activities. J. Small Bus. Enterp. Dev. 2022, 2, 29. [Google Scholar] [CrossRef]
- Davidson, E.A.; Nepstad, D.C.; Klink, C.; Trumbore, S.E. Pasture soils as carbon sink. Nature 1995, 376, 473. [Google Scholar] [CrossRef]
- Scurlock, J.M.O.; Hall, D.O. The global carbon sink: A grassland perspective. Glob. Chang. Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, S.; Johnston, C.A.; Liu, J.; Tieszen, L.L. Analysis of ecosystem controls on soil carbon source-sink relationships in the northwest Great Plains. Glob. Biogeochem. Cycles 2006, 4, 20. [Google Scholar] [CrossRef]
- Lugo, A.E.; Brown, S. Management of tropical soils as sinks or sources of atmospheric carbon. Plant Soil 1993, 149, 27–41. [Google Scholar] [CrossRef]
- Post, W.M.; Emanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil carbon pools and world life zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Donnelly, J.A. Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2. New Phytol. 2010, 164, 423–439. [Google Scholar]
- Billings, S.A. Soil organic matter dynamics and land use change at a grassland/forest ecotone. Soil Biol. Biochem. 2006, 38, 2934–2943. [Google Scholar] [CrossRef]
- Elmore, A.J.; Asner, G.P. Effects of grazing intensity on soil carbon stocks following deforestation of a Hawaiian dry tropical forest. Glob. Chang. Biol. 2010, 12, 1761–1772. [Google Scholar] [CrossRef]
- Liao, J.D.; Boutton, T.W.; Jastrow, J.D. Storage and dynamics of carbon and nitrogen in soil physical fractions following woody plant invasion of grassland. Soil Biol. Biochem. 2006, 38, 3184–3196. [Google Scholar] [CrossRef]
- Hau, L.C.; Lim, Y.S. Proposed method for evaluating controllers of battery-based storage system in maximum demand reductions. J. Energy Storage 2022, 46, 103850. [Google Scholar] [CrossRef]
- Samuel, E. Impacts of Climate Change and Land Management on Carbon Dynamics of British Upland Grassland Soils. Ph.D. Thesis, University of Leeds, Ritz, UK, 2018. [Google Scholar]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B.A. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Chang. Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Houghton, R.A.; Hackler, J.L.; Lawrence, K.T. The U.S. Carbon Budget: Contributions from Land-Use Change. Science 1999, 285, 574–578. [Google Scholar] [CrossRef]
- Dong, X.B.; Zhang, X.S. The Grassland in the Inner Mongolia is Overloaded and Changes of the Production Pattern are Highlighted. Resour. Sci. 2005, 27, 175–179. [Google Scholar]
- Stampfli, A.; Bloor, J.; Fischer, M.; Zeiter, M. High land-use intensity exacerbates shifts in grassland vegetation composition after severe experimental drought. Glob. Chang. Biol. 2018, 24, 2021–2034. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Photosynthetic Recovery of a Perennial Grass Leymus chinensis after Different Periods of Soil Drought. Plant Prod. Sci. 2007, 10, 277–285. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Mackie, K.A.; Zeiter, M.; Bloor, J.M.G.; Stampfli, A. Plant functional groups mediate drought resistance and recovery in a multisite grassland experiment. J. Ecol. 2019, 107, 937–949. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, S.; Ren, H.; Han, X.; Li, M.H.; Cheng, W.; Jiang, Y.; Chen, H.Y.H. Effects of Water and Nitrogen Addition on Species Turnover in Temperate Grasslands in Northern China. PLoS ONE 2012, 7, e3976. [Google Scholar] [CrossRef]
- Morra, M.J.; Blank, R.R.; Freeborn, L.L.; Shafii, B. Size Fractionation of Soil Organo-Mineral Complexes Using Ultrasonic Dispersion. Soil Sci. 1991, 152, 294–303. [Google Scholar] [CrossRef]
- Gallaher, R.N.; Weldon, C.O.; Boswell, F.C. A semiautomated procedure for total nitrogen in plant and soil samples. Soil Sci. Soc. Am. J. 1976, 40, 887–889. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate Utilization Patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Zhang, N.; Wan, S.; Guo, J.; Han, G.; Gutknecht, J.; Schmid, B.; Yu, L.; Liu, W.; Bi, J.; Wang, Z. Precipitation modifies the effects of warming and nitrogen addition on soil microbial communities in northern Chinese grasslands. Soil Biol. Biochem. 2015, 89, 12–23. [Google Scholar] [CrossRef]
- Deforest, J.L.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Atmospheric Nitrate Deposition, Microbial Community Composition, and Enzyme Activity in Northern Hardwood Forests. Soil Sci. Soc. Am. J. 2004, 68, 132–138. [Google Scholar] [CrossRef]
- Xiao, X.M.; Wang, Y.F.; Chen, Z.Z. Dynamics of primary productivity and soil organic matter of typical steppe in the Xilin River basin of Inner Mongolia and the response to climate change. Acta Bot. Sin. 1996, 38, 45–52. [Google Scholar]
- Bai, Y.; Han, X.; Wu, J.; Chen, Z.; Li, L. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 2004, 431, 181–184. [Google Scholar] [CrossRef]
- Hulbert, L.C. Causes of Fire Effects in Tallgrass Prairie. Ecology 1988, 69, 46–58. [Google Scholar] [CrossRef]
- Collins, S.L.; Smith, M. Scale-dependent interaction of fire and grazing on community heterogeneity in tallgrass prairie. Ecology 2006, 87, 2058–2067. [Google Scholar] [CrossRef]
- Macneil, M.; Haferkamp, M.R.; Vermeire, L.T.; Muscha, J.M. Prescribed fire and grazing effects on carbon dynamics in a northern mixed-grass prairie. Agric. Ecosyst. Environ. 2008, 127, 66–72. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.M.; Naeem, S.; Pan, Q.; Huang, J.; Zhang, L.; Han, X. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Li, Y.; Li, L.; Yu, H.; Qi, M. Nitrogen deposition magnifies the sensitivity of desert steppe plant communities to large changes in precipitation. J. Ecol. 2020, 108, 598–610. [Google Scholar] [CrossRef]
- DeAngelis, D.L.; Mulholland, P.J.; Palumbo, A.V.; Steinman, A.D.; Huston, M.A.; Elwood, J.W. Nutrient Dynamics and Food-Web Stability. Annu. Rev. Ecol. Syst. 1989, 20, 71–95. [Google Scholar] [CrossRef]
- Herbert, D.A.; Fownes, J.H. Hurricane damage to a Hawaiian forest: Nutrient supply rate affects resistance and resilience. Ecology 1999, 80, 908–920. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 2009, 60, 3737. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Anderegg, W.R.L.; Michalak, A.M.; Fisher, J.B.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Wolf, A. Global patterns of drought recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef]
- Sankaran, M. Droughts and the ecological future of tropical savanna vegetation. J. Ecol. 2019, 107, 1531–1549. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Pennanen, T.; Fritze, H.; Vanhala, P.; Kiikkila, O.; Bååth, E. Structure of a Microbial Community in Soil after Prolonged Addition of Low Levels of Simulated Acid Rain. Appl. Environ. Microbiol. 1998, 64, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dorodnikov, M.; Dijkstra, F.A.; Yang, S.; Jiang, Y. Sensitivities to nitrogen and water addition vary among microbial groups within soil aggregates in a semiarid grassland. Biol. Fertil. Soils 2016, 53, 129–140. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Ebblewhite, N.; Dobson, G. Soil microbial community structure: Effects of substrate loading rates. Soil Biol. Biochemistry 1998, 31, 145–153. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal:bacterial dominance in soils—Methods, controls, and ecosystem implications—ScienceDirect. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Trappe, J.J. Foliar and fungal 15N: 14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: Testing analytical models. Oecologia 2005, 146, 258–268. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Q.; Bai, E.; Lü, X.; Li, Q.; Xia, J.; Kardol, P.; Liang, W.; Wang, Z.; Han, X. Nitrogen deposition weakens plant-microbe interactions in grassland ecosystems. Glob. Chang. Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2010, 11, 1111–1120. [Google Scholar] [CrossRef]
- Gutknecht, J.L.M.; Field, C.B.; Balser, T.C. Microbial communities and their responses to simulated global change fluctuate greatly over multiple years. Glob. Chang. Biol. 2012, 18, 2256–2269. [Google Scholar] [CrossRef]
| Land-Use Type | Location | Dominant and Subdominant Species | pH | Sand (%) | Silt (%) | Clay (%) | C:N Ratio | Land-Use History | Coverage DS | Coverage WS |
|---|---|---|---|---|---|---|---|---|---|---|
| SD | 116°28′ 42°20′ | L. chinensis, S. grandis, C. squarrosa, Artemisia frigida Willd., Salsola collina Pall., Achnatherum sibiricum Keng | 7.4 ± 0.1 a | 98.7 ± 0.3 a (WS) 96.0 ± 0.4 a (DS) | 1.3 ± 0.3 c (WS) 4.0 ± 0.4 bc (DS) | 0.1 ± 0.1 b (WS) 0.1 ± 2.8 b (DS) | 10.6 ± 0.6 b | Free-grazing, long-term heavy grazing 20-ha plot that has been grazing for more than 50 years | 5–15% | 15–25% |
| MD | 116°27′ 42°10′ | L. chinensis, S. grandis, C. squarrosa, A. frigida, Kochia prostrate Schrad, A. sibiricum | 7.3 ± 0.1 a | 93.5 ± 1.1 a (WS) 93.0 ± 0.7 a (DS) | 6.5 ± 1.1 c (WS) 7.0 ± 0.7 c (DS) | 0.4 ± 0.2 b (WS) 4.3 ± 2.3 b (DS) | 12.49 ± 0.3 b | Free-grazing, long-term heavy grazing 24-ha plot that has been grazing since 1989 (About 30 years) | 30~35% | 40–50% |
| LD | 116°29′ 42°20′ | L. chinensis, S. grandis, C. squarrosa, A. sibiricum, A. frigida, S. collina | 7.2 ± 0.1 a | 90.3 ± 2.4 b (WS) 87.6 ± 0.4 b (DS) | 9.7 ± 2.4 b (WS) 12.4 ± 0.4 b (DS) | 0.3 ± 0.1 b (WS) 0.1 ± 0.1 b (DS) | 32.5 ± 0.6 a | Free-grazing, light grazing 24-ha plot that has been grazing since 1979 (About 20 years) | 45–50% | 50–65% |
| NG | 116°16′ 42°02′ | L. chinensis, S. grandis, A. michnoi, A. sibiricum, C. squarrosa, Carex korshinskyi | 7.1 ± 0.1 a | 67.3 ± 2.5 c (WS) 56.0 ± 1.5 c (DS) | 30.2 ± 2.4 a (WS) 42.1 ± 0.4 a (DS) | 1.9 ± 0.5 a (WS) 1.9 ± 0.1 a (DS) | 7.8 ± 0.6 c | 40-ha plot fenced since 2000 | 60~65% | 70~85% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Lv, S.; Diao, Z.; Wang, D.; Li, D.; Zheng, Z. Responses of Vegetation, Soil, and Microbes and Carbon and Nitrogen Pools to Semiarid Grassland Land-Use Patterns in Duolun, Inner Mongolia, China. Sustainability 2023, 15, 3434. https://doi.org/10.3390/su15043434
Gao X, Lv S, Diao Z, Wang D, Li D, Zheng Z. Responses of Vegetation, Soil, and Microbes and Carbon and Nitrogen Pools to Semiarid Grassland Land-Use Patterns in Duolun, Inner Mongolia, China. Sustainability. 2023; 15(4):3434. https://doi.org/10.3390/su15043434
Chicago/Turabian StyleGao, Xiuli, Shihai Lv, Zhaoyan Diao, Dewang Wang, Daikui Li, and Zhirong Zheng. 2023. "Responses of Vegetation, Soil, and Microbes and Carbon and Nitrogen Pools to Semiarid Grassland Land-Use Patterns in Duolun, Inner Mongolia, China" Sustainability 15, no. 4: 3434. https://doi.org/10.3390/su15043434
APA StyleGao, X., Lv, S., Diao, Z., Wang, D., Li, D., & Zheng, Z. (2023). Responses of Vegetation, Soil, and Microbes and Carbon and Nitrogen Pools to Semiarid Grassland Land-Use Patterns in Duolun, Inner Mongolia, China. Sustainability, 15(4), 3434. https://doi.org/10.3390/su15043434








