A High-Permeance Organic Solvent Nanofiltration Membrane via Polymerization of Ether Oxide-Based Polymeric Chains for Sustainable Dye Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane Preparation
2.3. Characterization
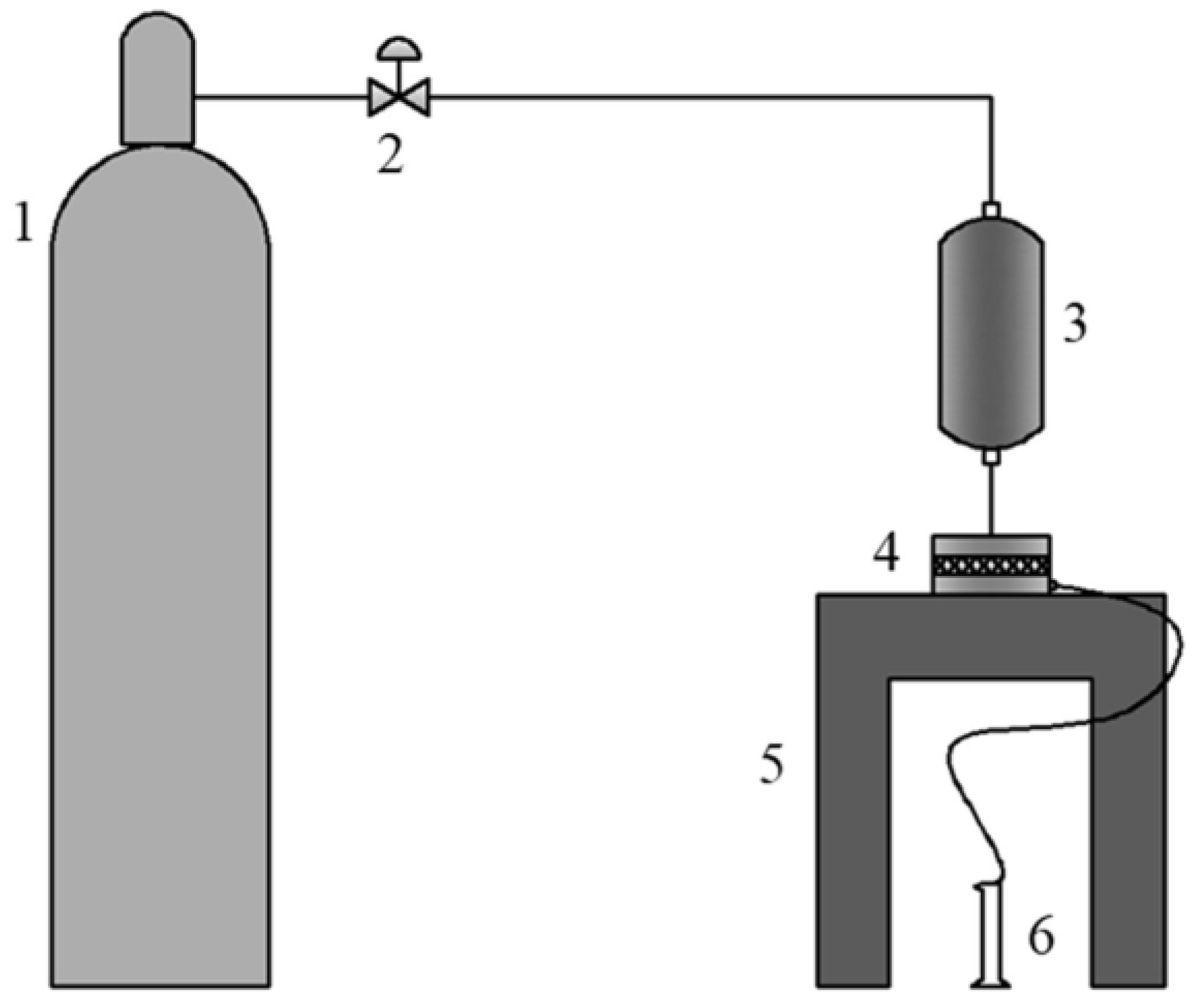
2.4. Membranes Performance Testing
2.5. Molecular Simulation
3. Results
3.1. Characterization Analysis
3.2. Performance Testing
3.2.1. The Separation on Different Dyes
3.2.2. Performance of Different Concentrations
3.2.3. Filtration Performance versus Operation Pressure
3.2.4. Swelling Degree and Solvent Resistance of Nanofiltration Membrane
3.2.5. Long Time Stability Test
3.2.6. Comparison of the Separation Performance with Reported Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| 6FDA | 4,4′-(Hexafluoroisopropylidene) diphthalic anhydride |
| OSN | Organic solvent nanofiltration |
| PEEK | Poly (ether-ether-ketone) |
| PAA | Polyamide acid |
| PEG | Polyethylene glycol |
| PI | Polyimide |
| Matrimid@5218 | 3,3′,4,4′-Benzophenone tetracarboxylic dianhydride and diaminophenylindane |
| BAP | 1,4-Bis (4-aminophenoxy) benzene |
| PEI | Poly(ethylene imine) |
| P84 | Polyimide 84 |
| PDI | Polydispersity |
| poly-TaDb | Poly(butyl acrylamide-co-divinylbenzene) |
| PPy | Polypyrrole |
| DDM | 4,4′-Diaminodiphenylmethane |
| TMC | Trimesoyl chloride |
| DHAQ | 2,6-Dihydroxyanthraquinone |
| RES | 1,3-Benzenediol, resorcinol monomer |
| POSS | Polyhedral oligomeric silsesquioxane |
| PAN | Polyacrylonitrile |
| PIP | Piperazine |
| XP84 | Cross-linked polyimide |
| CA | Cellulose acetate |
| PDA | Polydopamine |
References
- Yun, J.; Wang, Y.; Liu, Z.; Li, Y.; Yang, H.; Xu, Z.L. High efficient dye removal with hydrolyzed ethanolamine-Polyacrylonitrile UF membrane: Rejection of anionic dye and selective adsorption of cationic dye. Chemosphere 2020, 259, 127390. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-food industry waste as resource of chemicals: The role of membrane technology in their sustainable recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Membrane processes based on complexation reactions of pollutants as sustainable wastewater treatments. Sustainability 2009, 1, 978–993. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Gao, H.-W. Preparation of calcium oxalate-bromopyrogallol red inclusion sorbent and application to treatment of cationic dye and heavy metal wastewaters. Environ. Sci. Pollut. Res. 2009, 16, 339–347. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Januszewski, B.; Liu, Y.; Li, D.; Fu, R.; Elimelech, M.; Huang, X. Tailored design of nanofiltration membranes for water treatment based on synthesis-property-performance relationships. Chem. Soc. Rev. 2022, 51, 672–719. [Google Scholar] [CrossRef]
- Hosseinabadi, S.R.; Wyns, K.; Meynen, V.; Buekenhoudt, A.; Van der Bruggen, B. Solvent-membrane-solute interactions in organic solvent nanofiltration (OSN) for Grignard functionalised ceramic membranes: Explanation via Spiegler-Kedem theory. J. Membr. Sci. 2016, 513, 177–185. [Google Scholar] [CrossRef]
- Wei, C.; He, Z.; Lin, L.; Cheng, Q.; Huang, K.; Ma, S.; Chen, L. Negatively charged polyimide nanofiltration membranes with high selectivity and performance stability by optimization of synergistic imidization. J. Membr. Sci. 2018, 563, 752–761. [Google Scholar] [CrossRef]
- Xu, S.; Lu, D.; Wang, P.; Zhao, Y.; Sun, Y.; Qi, J.; Ma, J. Polyphenol engineered membranes with dually charged sandwich structure for low-pressure molecular separation. J. Membr. Sci. 2020, 601, 117885. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, G.M.; Lai, J.-Y.; Chung, T.-S. Braid-reinforced polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration (OSN). Separ. Purif. Technol. 2022, 290, 120811. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Chen, W.; Lü, Z.; Yu, S.; Liu, M.; Gao, C. Efficient removal of anionic dye by constructing thin-film composite membrane with high perm-selectivity and improved anti-dye-deposition property. Desalination 2020, 476, 114228. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, Z.; Jiang, L.; Yang, H.; Xu, Z. Separation of anionic dye mixtures b y Al-metal-organic framework filled polyacrylonitrile-ethanolamine membrane and its modified product. J. Clean. Prod. 2021, 284, 124778. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Han, Y.; Zhang, L.; Li, S.; Tang, B.; Xu, S.; Xu, Z. Ether modified poly(ether ether ketone) nonwoven membrane with excellent wettability and stability as a lithium ion battery separator. J. Power Sources 2018, 378, 176–183. [Google Scholar] [CrossRef]
- Bruggen, B.V.d.; Schaep, J.; Wilms, D.; Vandecasteele, C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Hardian, R.; Szekely, G. Waltzing around the stereochemistry of membrane crosslinkers for precise molecular sieving in organic solvents. J. Membr. Sci. 2021, 638, 119724. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Park, S.-H.; Zhou, Z.; Ladner, D.A.; Szekely, G. Surface engineering of intrinsically microporous poly(ether-ether-ketone) membranes: From flat to honeycomb structures. J. Membr. Sci. 2021, 621, 118997. [Google Scholar] [CrossRef]
- Lu, T.-D.; Chen, B.-Z.; Wang, J.; Jia, T.-Z.; Cao, X.-L.; Wang, Y.; Xing, W.; Lau, C.H.; Sun, S.-P. Electrospun nanofiber substrates that enhance polar solvent separation from organic compounds in thin-film composites. J. Mater. Chem. A 2018, 6, 15047–15056. [Google Scholar] [CrossRef]
- You, X.; Chong, J.Y.; Goh, K.S.; Tian, M.; Chew, J.W.; Wang, R. Electrospun polyimide-based thin-film composite membranes for organic solvent nanofiltration. J. Membr. Sci. 2021, 640, 119825. [Google Scholar] [CrossRef]
- Manoranjan, N.; Zhang, F.; Wang, Z.; Dong, Y.; Fang, W.; Zhang, Y.; Zhu, Y.; Jin, J. A single-walled carbon nanotube/covalent organic framework nanocomposite ultrathin membrane with high organic solvent resistance for molecule separation. ACS Appl. Mater. Interfaces 2020, 12, 53096–53103. [Google Scholar] [CrossRef]
- Campbell, J.; Burgal, J.D.S.; Szekely, G.; Davies, R.P.; Braddock, D.C.; Livingston, A. Hybrid polymer/MOF membranes for Organic Solvent Nanofiltration (OSN): Chemical modification and the quest for perfection. J. Membr. Sci. 2016, 503, 166–176. [Google Scholar] [CrossRef]
- Lu, T.-D.; Zhao, L.-L.; Yong, W.F.; Wang, Q.; Duan, L.; Sun, S.-P. Highly solvent-durable thin-film molecular sieve membranes with insoluble polyimide nanofibrous substrate. Chem. Eng. J. 2021, 409, 128206. [Google Scholar] [CrossRef]
- Han, C.; Liu, H.; Wang, Y. An ultrapermeable thin film composite membrane supported by “green” nanofibrous polyimide substrate for polar aprotic organic solvent recovery. J. Membr. Sci. 2022, 644, 120192. [Google Scholar] [CrossRef]
- Xu, Y.C.; Cheng, X.Q.; Long, J.; Shao, L. A novel monoamine modification strategy toward high-performance organic solvent nanofiltration (OSN) membrane for sustainable molecular separations. J. Membr. Sci. 2016, 497, 77–89. [Google Scholar] [CrossRef]
- Feng, W.; Li, J.; Fang, C.; Zhang, L.; Zhu, L. Controllable thermal annealing of polyimide membranes for highly-precise organic solvent nanofiltration. J. Membr. Sci. 2022, 643, 120013. [Google Scholar] [CrossRef]
- Davood Abadi Farahani, M.H.; Hua, D.; Chung, T.-S. Cross-linked mixed matrix membranes consisting of carboxyl-functionalized multi-walled carbon nanotubes and P84 polyimide for organic solvent nanofiltration (OSN). Separ. Purif. Technol. 2017, 186, 243–254. [Google Scholar] [CrossRef]
- Davood Abadi Farahani, M.H.; Hua, D.; Chung, T.-S. Cross-linked mixed matrix membranes (MMMs) consisting of amine-functionalized multi-walled carbon nanotubes and P84 polyimide for organic solvent nanofiltration (OSN) with enhanced flux. J. Membr. Sci. 2018, 548, 319–331. [Google Scholar] [CrossRef]
- Gao, Z.F.; Shi, G.M.; Cui, Y.; Chung, T.-S. Organic solvent nanofiltration (OSN) membranes made from plasma grafting of polyethylene glycol on cross-linked polyimide ultrafiltration substrates. J. Membr. Sci. 2018, 565, 169–178. [Google Scholar] [CrossRef]
- Zhang, B.; Qiao, J.; Dong, C.; Yi, C.; Qi, S.; Yang, B. Dibenzo-21-crown-7-ether contained 6FDA-based polyimide membrane with improved gas selectivity. Separ. Purif. Technol. 2021, 264, 118454. [Google Scholar] [CrossRef]
- Zhang, B.; Qiao, J.; Wu, D.; He, X.; Liu, J.; Yi, C.; Qi, S. Enhanced gas separation by free volume tuning in a crown ether-containing polyimide membrane. Separ. Purif. Technol. 2022, 293, 121116. [Google Scholar] [CrossRef]
- Li, M.; Lu, K.J.; Wang, L.; Zhang, X.; Chung, T.-S. Janus membranes with asymmetric wettability via a layer-by-layer coating strategy for robust membrane distillation. J. Membr. Sci. 2020, 603, 118031. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Xie, M.; Huang, M.; Lin, Y.; Tan, W. Membrane distillation of a silver leaching solution: Role of the coexisting aluminum ions on silica scaling. J. Membr. Sci. 2020, 603, 118021. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.; Li, B.; Deng, S.; Zhang, X. Graphene oxide nanofiltration membrane with trimethylamine-N-oxide zwitterions for robust biofouling resistance. J. Membr. Sci. 2021, 640, 119855. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Q.; Chen, G.; Yi, C.; Qi, S.; Yang, B. Fabrication of mixed matrix membranes with zinc ion loaded titanium dioxide for improved CO2 separation. Separ. Purif. Technol. 2021, 254, 117472. [Google Scholar] [CrossRef]
- Wu, D.; Yi, C.; Wang, Y.; Qi, S.; Yang, B. Preparation and gas permeation of crown ether-containing co-polyimide with enhanced CO2 selectivity. J. Membr. Sci. 2018, 551, 191–203. [Google Scholar] [CrossRef]
- Wu, D.; Yi, C.; Doherty, C.M.; Lin, L.; Xie, Z. A crown ether-containing copolyimide membrane with improved free volume for CO2 separation. Ind. Eng. Chem. Res. 2019, 58, 14357–14367. [Google Scholar] [CrossRef]
- Gan, F.; Dong, J.; Xu, X.; Li, M.; Zhao, X.; Zhang, Q. Preparation of thermally rearranged poly(benzoxazole-co-imide) membranes containing heteroaromatic moieties for CO2/CH4 separation. Polymer 2019, 185, 121945. [Google Scholar] [CrossRef]
- Ruan, X.; Zhang, X.; Zhou, Z.; Jiang, X.; Dai, Y.; Yan, X.; He, G. ZIF-8 heterogeneous nucleation and growth mechanism on Zn(II)-doped polydopamine for composite membrane fabrication. Separ. Purif. Technol. 2019, 214, 95–103. [Google Scholar] [CrossRef]
- Li, X.; Vandezande, P.; Vankelecom, I.F.J. Polypyrrole modified solvent resistant nanofiltration membranes. J. Membr. Sci. 2008, 320, 143–150. [Google Scholar] [CrossRef]
- Ahmadiannamini, P.; Li, X.; Goyens, W.; Meesschaert, B.; Vanderlinden, W.; De Feyter, S.; Vankelecom, I.F.J. Influence of polyanion type and cationic counter ion on the SRNF performance of polyelectrolyte membranes. J. Membr. Sci. 2012, 403–404, 216–226. [Google Scholar] [CrossRef]
- Ben Soltane, H.; Roizard, D.; Favre, E. Effect of pressure on the swelling and fluxes of dense PDMS membranes in nanofiltration: An experimental study. J. Membr. Sci. 2013, 435, 110–119. [Google Scholar] [CrossRef]
- Mahto, A.; Halakarni, M.A.; Maraddi, A.; D’Souza, G.; Samage, A.A.; Thummar, U.G.; Mondal, D.; Nataraj, S.K. Upcycling cellulose acetate from discarded cigarette butts: Conversion of contaminated microfibers into loose-nanofiltration membranes for selective separation. Desalination 2022, 535, 115807. [Google Scholar] [CrossRef]
- Mohammed, S.; Hegab, H.M.; Ou, R. Nanofiltration performance of glutaraldehyde crosslinked graphene oxide-cellulose nanofiber membrane. Chem. Eng. Res. Des. 2022, 183, 1–12. [Google Scholar] [CrossRef]
- Jansen, J.C.; Darvishmanesh, S.; Tasselli, F.; Bazzarelli, F.; Bernardo, P.; Tocci, E.; Friess, K.; Randova, A.; Drioli, E.; Van der Bruggen, B. Influence of the blend composition on the properties and separation performance of novel solvent resistant polyphenylsulfone/polyimide nanofiltration membranes. J. Membr. Sci. 2013, 447, 107–118. [Google Scholar] [CrossRef]
- Ben Soltane, H.; Roizard, D.; Favre, E. Study of the rejection of various solutes in OSN by a composite polydimethylsiloxane membrane: Investigation of the role of solute affinity. Separ. Purif. Technol. 2016, 161, 193–201. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Golemme, G.; Jansen, J.C.; Choi, S.H. Asymmetric PEEKWC membranes for treatment of organic solvent solutions. J. Membr. Sci. 2011, 368, 144–149. [Google Scholar] [CrossRef]
- Nie, L.; Chuah, C.Y.; Bae, T.H.; Lee, J.M. Graphene-based advanced membrane applications in organic solvent nanofiltration. Adv. Funct. Mater. 2020, 31, 2006949. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Li, S.; Xu, S.; Tian, L.; Su, B.; Han, L.; Mandal, B. Fundamental understanding on the preparation conditions of high-performance polyimide-based hollow fiber membranes for organic solvent nanofiltration (OSN). Separ. Purif. Technol. 2021, 254, 117600. [Google Scholar] [CrossRef]
- Lai, X.; Wang, C.; Wang, L.; Xiao, C. A novel PPTA/PPy composite organic solvent nanofiltration (OSN) membrane prepared by chemical vapor deposition for organic dye wastewater treatment. J. Water Process Eng. 2022, 45, 102533. [Google Scholar] [CrossRef]
- Jin, L.; Hu, L.; Liang, S.; Wang, Z.; Xu, G.; Yang, X. A novel organic solvent nanofiltration (OSN) membrane fabricated by Poly(m-phenylene isophthalamide) (PMIA) under large-scale and continuous process. J. Membr. Sci. 2022, 647, 120259. [Google Scholar] [CrossRef]
- Alwan Almijbilee, M.M.; Wang, Y.; Peng, M.; Kong, A.; Zhang, J.; Li, W. Ion-binding ameliorates the organic solvents nanofiltration performance of poly (butyl acrylamide-co-divinylbenzene) composites. Separ. Purif. Technol. 2021, 279, 119629. [Google Scholar] [CrossRef]
- Shao, L.; Cheng, X.; Wang, Z.; Ma, J.; Guo, Z. Tuning the performance of polypyrrole-based solvent-resistant composite nanofiltration membranes by optimizing polymerization conditions and incorporating graphene oxide. J. Membr. Sci. 2014, 452, 82–89. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, A.; Wang, Y.; He, X.; Zhao, S.; Zhang, J.; Li, W. Modulating hydrophobicity of composite polyamide membranes to enhance the organic solvent nanofiltration. Separ. Purif. Technol. 2019, 223, 211–223. [Google Scholar] [CrossRef]
- Sun, S.-P.; Chan, S.-Y.; Chung, T.-S. A slow-fast phase separation (SFPS) process to fabricate dual-layer hollow fiber substrates for thin-film composite (TFC) organic solvent nanofiltration (OSN) membranes. Chem. Eng. Sci. 2015, 129, 232–242. [Google Scholar] [CrossRef]
- Jimenez-Solomon, M.F.; Song, Q.; Jelfs, K.E.; Munoz-Ibanez, M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–767. [Google Scholar] [CrossRef]
- Jimenez Solomon, M.F.; Bhole, Y.; Livingston, A.G. High flux hydrophobic membranes for organic solvent nanofiltration (OSN)-Interfacial polymerization, surface modification and solvent activation. J. Membr. Sci. 2013, 434, 193–203. [Google Scholar] [CrossRef]
- Xu, Y.C.; Tang, Y.P.; Liu, L.F.; Guo, Z.H.; Shao, L. Nanocomposite organic solvent nanofiltration membranes by a highly-efficient mussel-inspired co-deposition strategy. J. Membr. Sci. 2017, 526, 32–42. [Google Scholar] [CrossRef]
- Xu, Y.C.; Wang, Z.X.; Cheng, X.Q.; Xiao, Y.C.; Shao, L. Positively charged nanofiltration membranes via economically mussel-substance-simulated co-deposition for textile wastewater treatment. Chem. Eng. J. 2016, 303, 555–564. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Xu, Y.; You, F.; Sun, H.; Shao, L. Realizing Mussel-Inspired Polydopamine Selective Layer with Strong Solvent Resistance in Nanofiltration toward Sustainable Reclamation. ACS Sustain. Chem. Eng. 2017, 5, 5520–5528. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, Z.; Wang, N.; An, Q.-F.; Guo, H. Counterion exchanged hydrophobic polyelectrolyte multilayer membrane for organic solvent nanofiltration. J. Membr. Sci. 2021, 620, 118827. [Google Scholar] [CrossRef]











| Code | Molecular Formula | Molecular Weight (g·mol−1) | Molecular Charge [37] | Molar Volume (cm3·mol−1) |
|---|---|---|---|---|
| Rose Bengal | C20H2Cl4I4Na2O5 | 1017.64 | −2 | 272.8 |
| Methyl blue | C37H27N3Na2O9S3 | 799.80 | +1 [38] | 241.9 |
| Victoria blue B | C33H32ClN3 | 506.08 | - | - |
| Crystal violet | C25H30N3Cl | 407.98 | +1 | 231 |
| Solvent | Molecular Formula | Molecular Weight (g·mol−1) | Viscosity at 25 °C (mPa·s) [42,43,44] | Molar Volume (g·mol−1) [42] | Stokes Diameter (nm) | Kinetic Diameter (nm) [45] | Relative Polarity [45] |
|---|---|---|---|---|---|---|---|
| Toluene | C7H8 | 92.15 | 0.55 | 106.3 | 0.91 | 0.55 | 0.099 |
| Ethyl acetate | C4H8O2 | 88.12 | 0.42 | 98.5 | 0.86 | 0.52 | 0.228 |
| Ethanol | C2H6O | 46.07 | 1.08 | 58.5 | 0.62 | 0.44 | 0.654 |
| n-Hexane | C6H14 | 86.2 | 0.33 | 130.7 | - | 0.51 | 0.009 |
| Code | Separation Dyes | Performance (L∙m−2∙h−1∙bar−1) | Rejection (%) | Reference |
|---|---|---|---|---|
| Poly-TaDb | Brilliant Blue R (BB, 825.9 g·mol−1), 0.4 MPa | 1.70 | >90 | [49] |
| PPy/H-PAN | Rose Bengal (1017 g·mol−1) 5 bar, room temperature | 1.46 (0.79~1.03) | >90 (96~98) | [50] |
| DDM-TMC/C-PEI | Erythrosin B | 1.05 | >90 | [51] |
| Matrimid 5218 PI dual-layer HF | Remazol brilliant blue R (627 g·mol−1) | 1.5 | 96 | [52] |
| PEEKWC | Rose Bengal (1017 g·mol−1) | 1.6 | 90 | [44] |
| PAR-DHAQ/PI | Crystal violet (CV, 408 g·mol−1) 30 bar, 30 °C | 0.6 | 97.7 | [53] |
| PAR-RES/PI | Crystal violet (CV, 408 g·mol−1) 30 bar, 30 °C | 0.6 | 99.7 | [53] |
| PA/crosslinked P84 PI | Styrene oligomers (236 g·mol−1) | 1.5 | 98 | [54] |
| Catechol/POSS/PI | Rose Bengal | 2.23 | 84.2 | [55] |
| Catechol/PEI/PAN | Bromothymol blue (624 g·mol−1) | 1.8 | 68 | [56] |
| Crosslinked polyimide XP84 PIP-0.1%-10min | Acid fuchsin (585.5 g·mol−1) 30 °C, 10 bar | 1.17 | 99.1 | [57] |
| Crosslinked polyimide XP84 PIP-0.1%-10min-ACT | Acid fuchsin (585.5 g·mol−1) 30 °C, 10 bar | 1.59 | 99.5 | [57] |
| [(PDDA/PAA-CSH)2.5]PFO | Methyl blue, 0.4 MPa, 100 mg/L dye/ethanol solution | 6 | ~68 | [59] |
| CA | Methyl blue, aq. solution, 25 ppm, | 4 | ~82 | [40] |
| PDA/PI | Methyl blue, in ethanol, 5 bar, 25 °C | 0.91 | 99 | [58] |
| BAP/6FDA | Methyl blue (20 ppm) 0.7 MPa | 2.18 | 94.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Yi, C.; Wu, D.; Qiao, J.; Zhang, L. A High-Permeance Organic Solvent Nanofiltration Membrane via Polymerization of Ether Oxide-Based Polymeric Chains for Sustainable Dye Separation. Sustainability 2023, 15, 3446. https://doi.org/10.3390/su15043446
Zhang B, Yi C, Wu D, Qiao J, Zhang L. A High-Permeance Organic Solvent Nanofiltration Membrane via Polymerization of Ether Oxide-Based Polymeric Chains for Sustainable Dye Separation. Sustainability. 2023; 15(4):3446. https://doi.org/10.3390/su15043446
Chicago/Turabian StyleZhang, Beibei, Chunhai Yi, Dongyun Wu, Jie Qiao, and Lihua Zhang. 2023. "A High-Permeance Organic Solvent Nanofiltration Membrane via Polymerization of Ether Oxide-Based Polymeric Chains for Sustainable Dye Separation" Sustainability 15, no. 4: 3446. https://doi.org/10.3390/su15043446
APA StyleZhang, B., Yi, C., Wu, D., Qiao, J., & Zhang, L. (2023). A High-Permeance Organic Solvent Nanofiltration Membrane via Polymerization of Ether Oxide-Based Polymeric Chains for Sustainable Dye Separation. Sustainability, 15(4), 3446. https://doi.org/10.3390/su15043446








