Physiochemical Properties and Microflora of the Rhizosphere Soil of Tobacco Plants with and without Bacterial Wilt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Experimental Methods
2.4. Data Analysis
3. Results
3.1. Physicochemical Properties of Rhizosphere Soil of Tobacco Plants
3.2. Changes in Culturable Microorganism Quantity in Tobacco Rhizosphere Soil
3.3. Changes in Bacterial Community Structure in Tobacco Rhizosphere Soil
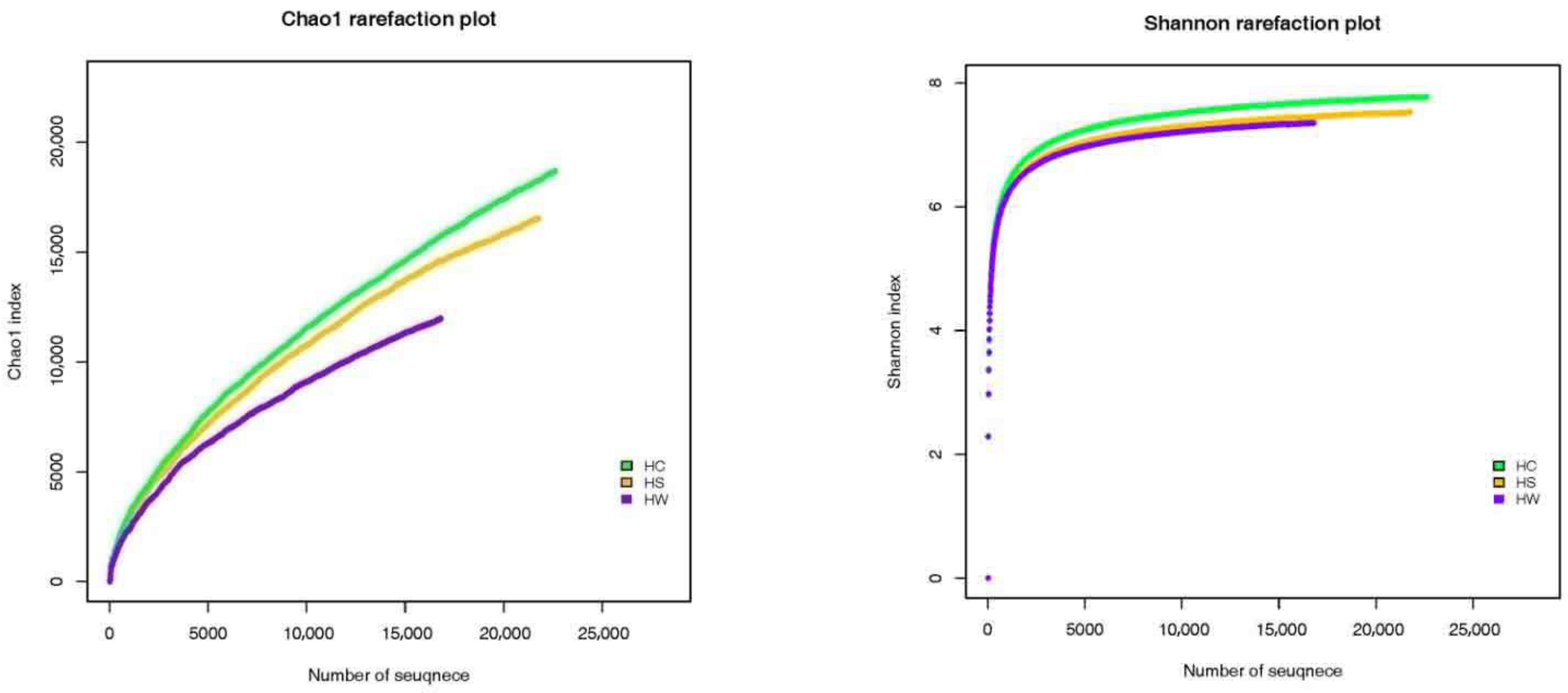
3.4. Changes in Bacterial Diversity in the Rhizosphere Soil of Tobacco Plants
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.J.; Tang, L.; Hu, Z.L.; Li, Y.C.; Huang, Y.B. Application and Prospect of Biochar and Biochar-Based Fertilizer in Tobacco Agriculture. J. Nucl. Agric. Sci. 2021, 35, 0997–1007. [Google Scholar]
- Lvarez, B.; Biosca, E.G.; Lopez, M.M. On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. Curr. Res. 2010, 1, 267–279. [Google Scholar]
- Li, X.S.; Shao, H.; Chen, J.; Huang, R.R.; Wei, L.G.; Hua, J.L. Root colonization of Ralstonia solanacearum in different crops and crop rotation systems for plant disease control. Chin. J. Oil Crop Sci. 2020, 42, 667–673. [Google Scholar]
- Li, Y.Y.; Qin, G.J.; Wang, L.; Yu, J.; Xu, R.B.; Peng, W.X.; Rao, X.F. Soil nutrient elements affecting the occurrence of tobacco bacterial wilt in Qingjiang River Basin. J. South. Agric. 2018, 49, 656–661. [Google Scholar]
- Li, J.Q.; Chen, J.B.; Yuan, Q.H.; Peng, W.; Zhang, Z.; Ma, Z.W.; Xie, R.H.; Li, S.L. Effects of Alien Earth Soil-improving on Soil Nutrient Status and Tobacco Bacterial Wilt. Chin. Tob. Sci. 2017, 38, 48–52. [Google Scholar]
- Shi, H.L.; Xiang, B.K.; Tan, J.; Peng, W.; Sun, Y.; Wang, R.; Wu, W.; Wei, G.; Ding, C. Analysis of bacterial community in rhizosphere soil of tobacco plant infected by bacterial wilt disease. Acta Table Sin. 2018, 24, 57–65. [Google Scholar]
- Li, Y.Y.; Peng, W.X.; Zhang, T.; Sun, Y.X.; Li, X.H. Effects of marigold straw compost on alleviating continuous cropping obstacles of tobacco. J. South. Agric. 2022, 53, 451–459. [Google Scholar]
- Xu, D.Y.; Sun, J.W.; Jiao, Z.H.; Sun, W.S.; Xu, F.H.; Wang, X.; Hu, Y.; Shi, H. Effects of Different Typical Soil Textures on Quality of Tobacco Leaves. Southwest China J. Agric. Sci. 2015, 28, 1295–1299. [Google Scholar]
- Li, C.J.; Ahmed, W.; Li, D.F.; Yu, L.J.; Xu, L.J.; Xu, T.; Zhao, Z. Biochar suppresses bacterial wilt disease of flue-cured tobacco by improving soil health and functional diversity of rhizosphere microorganisms. Appl. Soil Ecol. 2022, 171, 104314. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhao, Q.Q.; Wuriyanghan, H.; Yang, C. Biocontrol bacteria strains Y4 and Y8 alleviate tobacco bacterial wilt disease by altering their rhizosphere soil bacteria community. Rhizosphere 2021, 19, 100390. [Google Scholar] [CrossRef]
- Chao, A. Estimating the population-size for capture recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; pp. 20–95. [Google Scholar]
- Chen, Y.; Zheng, H.; Shi, J.X.; Huang, J.G. Effects of Chemical Fertilizer and Rapeseed Meal on Microorganisms in the Rhizosphere of Flue-cured Tobacco Seedlings. Acta Pedol. Sin. 2012, 49, 208–213. [Google Scholar]
- Leclercq-Dransart, J.; Demuynck, S.; Bidar, G.; Douay, F.; Grumiaux, F.; Louvel, B.; Pernin, C.; Leprêtre, A. Does adding fly ash to metal-contaminated soils play a role in soil functionality regarding metal availability, litter quality, microbial activity and the community structure of Diptera larvae. Appl. Soil Ecol. 2019, 138, 99–111. [Google Scholar] [CrossRef]
- Liu, H.; Du, R.W.; Zhao, J.; Wu, Y.K.; Wang, Y.; Yuan, L. Effects of fertilization on enzyme activities and bacterial community structures in rhizosphere soil of flue-cured tobacco. Tob. Sci. Technol. 2016, 49, 1–8. [Google Scholar]
- Ding, Y.; Chen, Y.L.; Lin, Z.Q.; Tuo, Y.Y.; Li, B.; Li, H.L.; Wang, Y. Study on Microbial Community Composition in Rhizosphere Soil of Tobacco Fields with Different Disease Incidence Rates. Chin. Tob. Sci. 2020, 41, 67–73. [Google Scholar]
- Wang, Y.H. Effects of pH Value on Growth and Root Colonization of Ralstonia solanacearum; Chinese Academy of Agricultural Sciences: Beijing, China, 2017; pp. 126–130. [Google Scholar]
- Wang, L.L.; Shi, J.X.; Yuan, S.F.; Wu, K.; Cai, L.T.; Liu, Y.; Yang, X.; Feng, Y.; Shen, B.; Shen, Q. Control of tobacco bacterial wilt with biomanure plus soil amendments. Acta Pedol. Sin. 2013, 50, 150. [Google Scholar]
- Zheng, S.Y.; Chen, D.J.; Ding, W. Nutritional status of rhizosphere soil around bacterial wilt diseased tobacco plant. Acta Table Sin. 2014, 20, 57–60. [Google Scholar]
- Liu, X.Z.; Ma, Y.Y.; Manevski, K. Biochar and alternate wetting-drying cycles improving rhizosphere soil nutrients availability and tobacco growth by altering root growth strategy in Ferralsol and Anthrosol. Sci. Total Envilon. 2022, 806, 150513. [Google Scholar] [CrossRef]
- Yang, S.D.; Zhao, J.C.; Guo, Y.J.; Wu, J.; Long, M.H. Characterization of Soils Bacterial Community Structures in Rhizospheres of Tomatoes Infected with Bacterial Wilt and Its Non-infected Plants. China Veget. 2014, 1, 25–29. [Google Scholar]
- Hu, W.; Wei, J.; Di, Q.; Tao, T.; Zhang, J.; Liu, J.; Shi, X. Flue-cured tobacco (Nicotiana tabacum L.) leaf quality can be improved by grafting with potassium-efficient rootstock. Field Crops Res. 2021, 274, 108305. [Google Scholar] [CrossRef]
- Zhang, C.S.; Kong, F.Y. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Wu, L.K.; Lin, X.M.; Lin, W.X. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chin. J. Plant Ecol. 2014, 38, 298–310. [Google Scholar]
- Zhou, W.J.; Lu, D.G.; Qin, S.J. Research Progress in Interaction between Plant and Rhizosphere Microorganism. J. Jilin Agric. Univ. 2016, 38, 253–260. [Google Scholar]


| Treatment | pH | Available K (mg/kg) | Available P (mg/kg) | Available N (mg/kg) | Organic Carbon (g/kg) |
|---|---|---|---|---|---|
| HW | 5.22 ± 0.031 | 196.22 ± 9.33 | 149.59 ± 6.39 | 105.70 ± 3.32 | 27.31 ± 0.89 |
| HC | 5.52 ± 0.030 | 261.07 ± 11.36 | 159.19 ± 2.61 | 152.60 ± 4.01 | 29.20 ± 0.94 |
| HS | 4.77 ± 0.018 | 137.45 ± 6.58 | 102.16 ± 5.05 | 150.50 ± 4.46 | 25.03 ± 1.02 |
| Treatment | Bacteria (×106 cfu/g) | Fungus (×105 cfu/g) | Actinomyces (×105 cfu/g) |
|---|---|---|---|
| HW | 6.73 ± 0.16 | 2.13 ± 0.05 | 3.73 ± 0.09 |
| HC | 6.84 ± 0.15 | 1.53 ± 0.04 | 1.95 ± 0.05 |
| HS | 4.07 ± 0.09 | 2.56 ± 0.05 | 7.06 ± 0.14 |
| Treatment | Shannon | Chao1 | Coverage |
|---|---|---|---|
| HW | 7.43 | 13,413.82 | 0.93 |
| HC | 8.02 | 15,708.13 | 0.91 |
| HS | 7.68 | 15,062.57 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Li, W.; Zhou, Y.; Zhu, Z.; Wu, X. Physiochemical Properties and Microflora of the Rhizosphere Soil of Tobacco Plants with and without Bacterial Wilt. Sustainability 2023, 15, 3661. https://doi.org/10.3390/su15043661
Zheng C, Li W, Zhou Y, Zhu Z, Wu X. Physiochemical Properties and Microflora of the Rhizosphere Soil of Tobacco Plants with and without Bacterial Wilt. Sustainability. 2023; 15(4):3661. https://doi.org/10.3390/su15043661
Chicago/Turabian StyleZheng, Cong, Wei Li, Yang Zhou, Zhiwen Zhu, and Xiaozong Wu. 2023. "Physiochemical Properties and Microflora of the Rhizosphere Soil of Tobacco Plants with and without Bacterial Wilt" Sustainability 15, no. 4: 3661. https://doi.org/10.3390/su15043661






