Adsorption Properties of a Polyamine Special Ion Exchange Resin for Removing Molybdenum from Ammonium Tungstate Solutions
Abstract
:1. Introduction
2. Materials and Methods
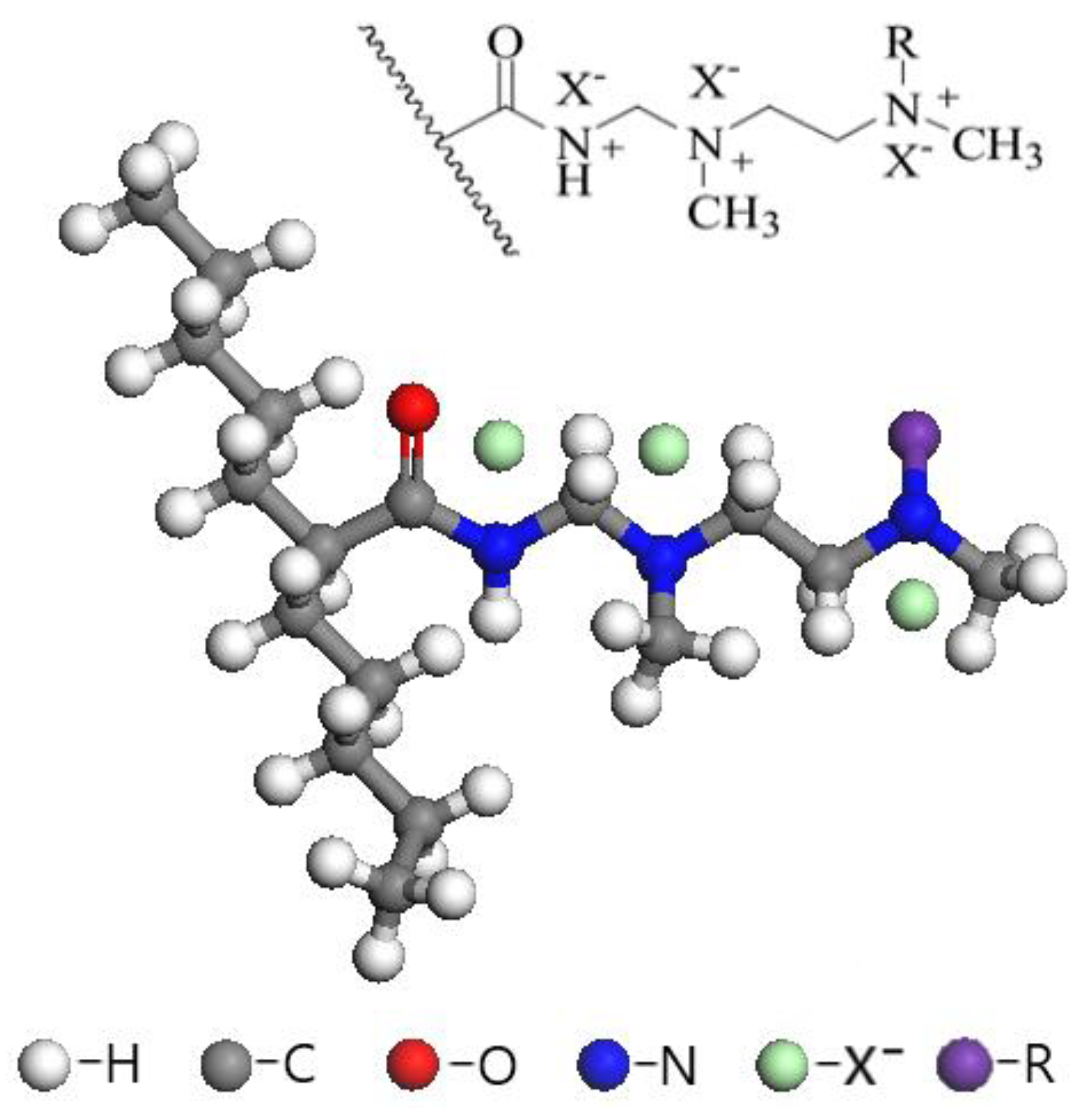
2.1. Materials
2.2. Test Method
2.2.1. Resin Pretreatment
2.2.2. Static Adsorption Tests
2.2.3. Dynamic Adsorption Test
3. Results and Discussion
3.1. Static Adsorption Test
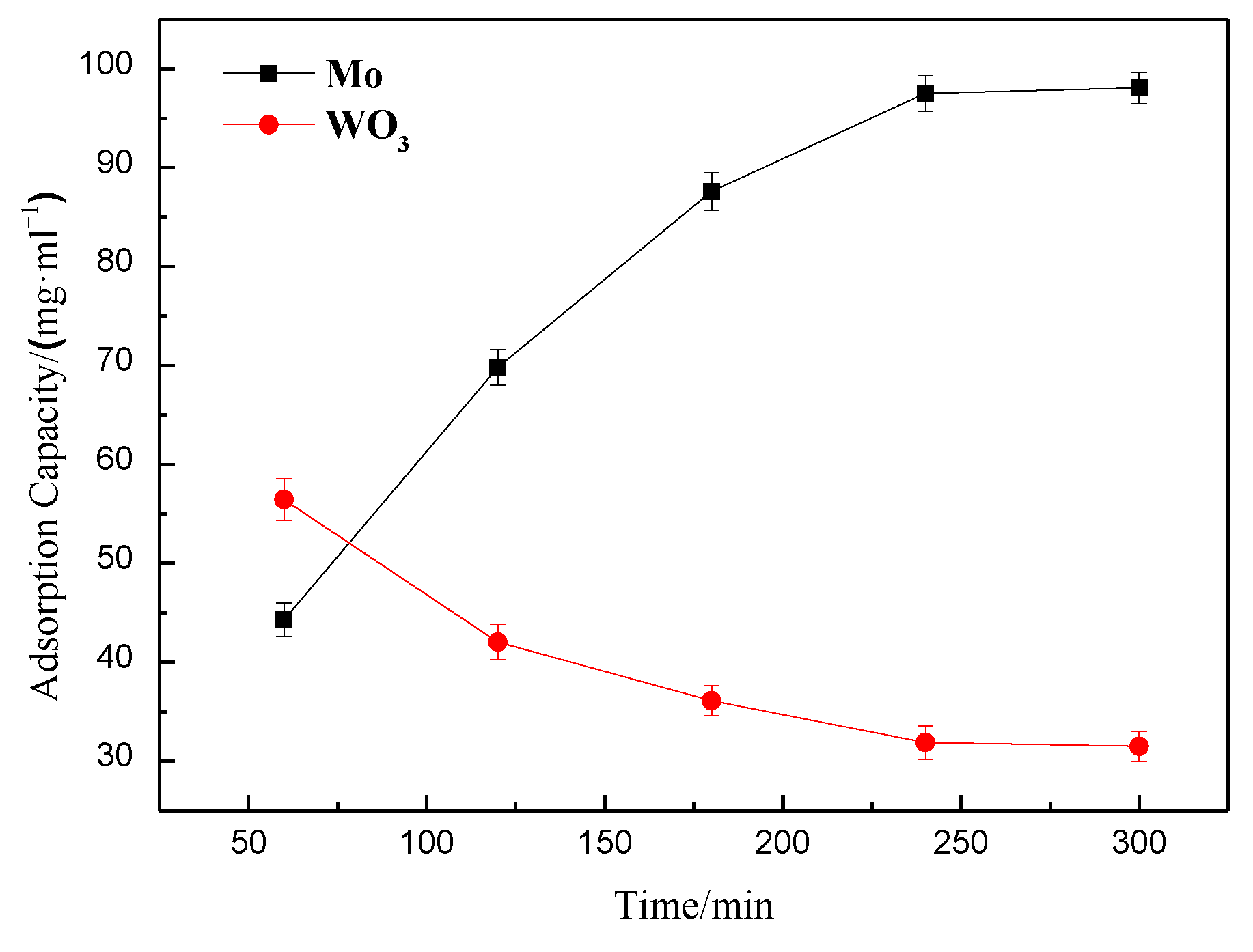
3.1.1. Effect of Adsorption Time on Molybdenum and Tungsten Adsorption Capacities for the Polyamine Special Resin
3.1.2. Effect of S2− Concentration on the Molybdenum and Tungsten Adsorption Capacities of the Polyamine Special Resin
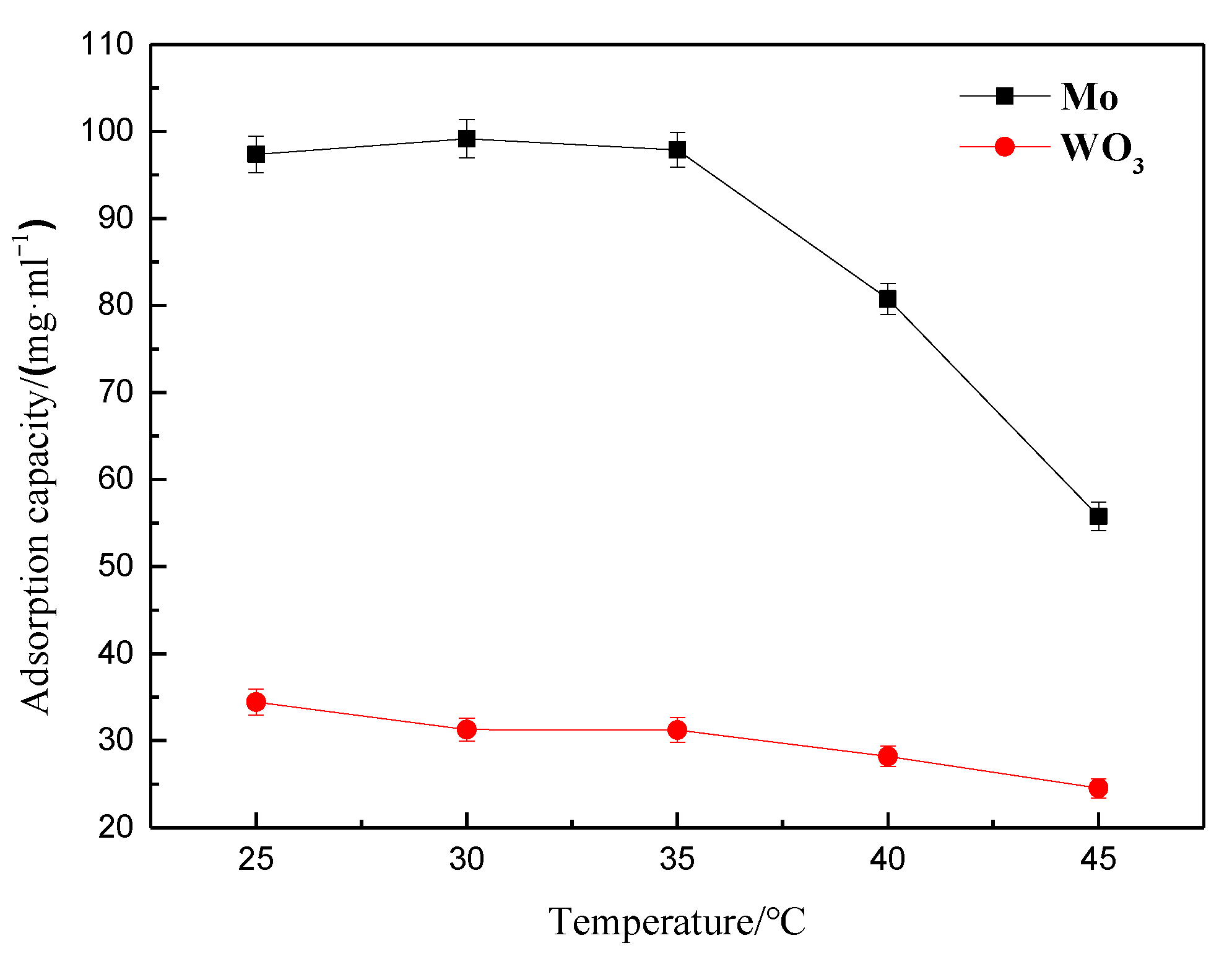
3.1.3. Effect of Temperature on Molybdenum and Tungsten Adsorption by the Polyamine Special Resin
3.1.4. Effect of CO32− Concentration on the Molybdenum and Tungsten Adsorption Capacities of the Polyamine Special Resin
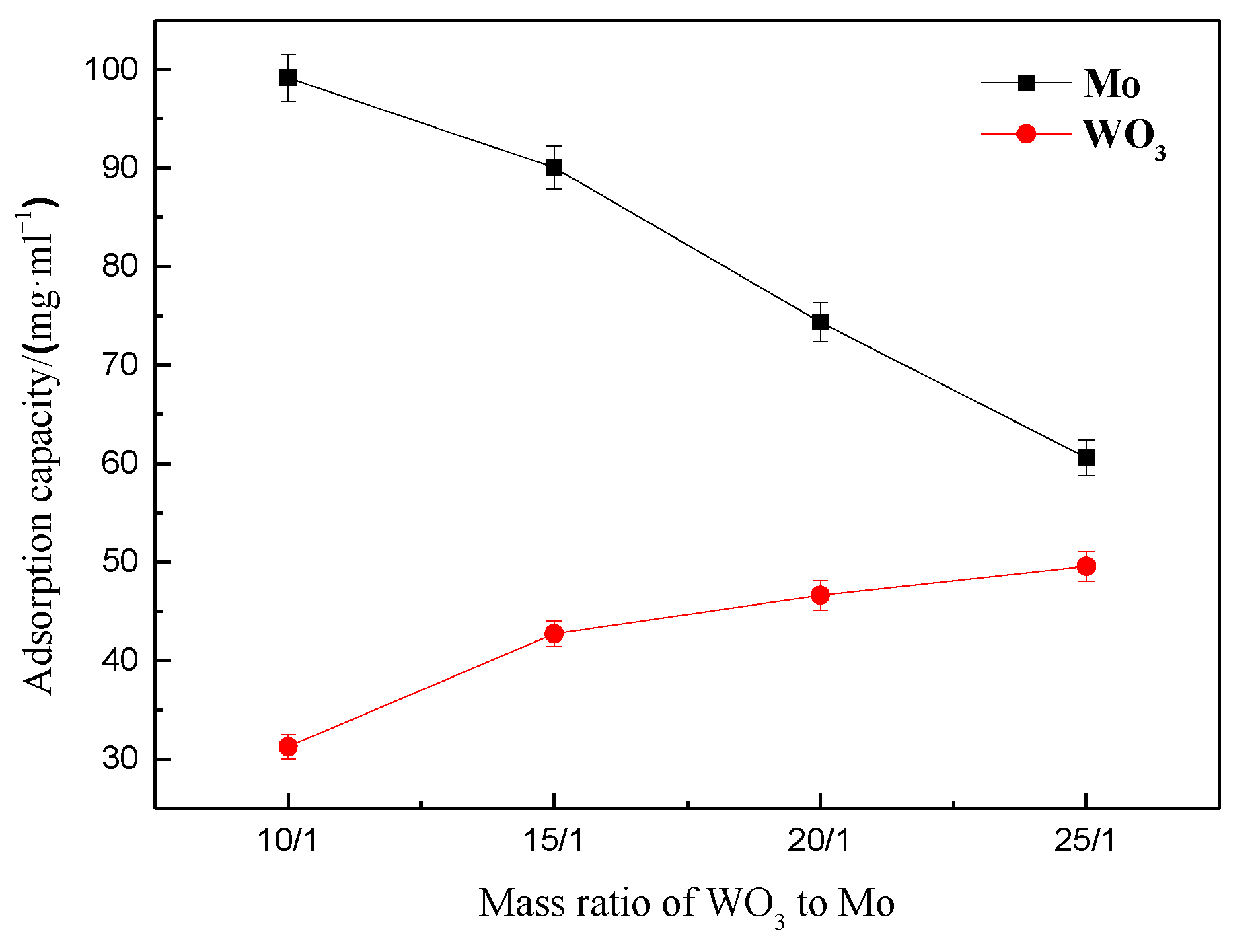
3.1.5. Effect of the Tungsten to Molybdenum Mass Ratio in the Ammonium Tungstate Solution on the Molybdenum and Tungsten Adsorption Capacities of the Resin
3.1.6. Effect of Molybdenum Concentration in Ammonium Tungstate Solution on Molybdenum and Tungsten Adsorption Capacity of Resin
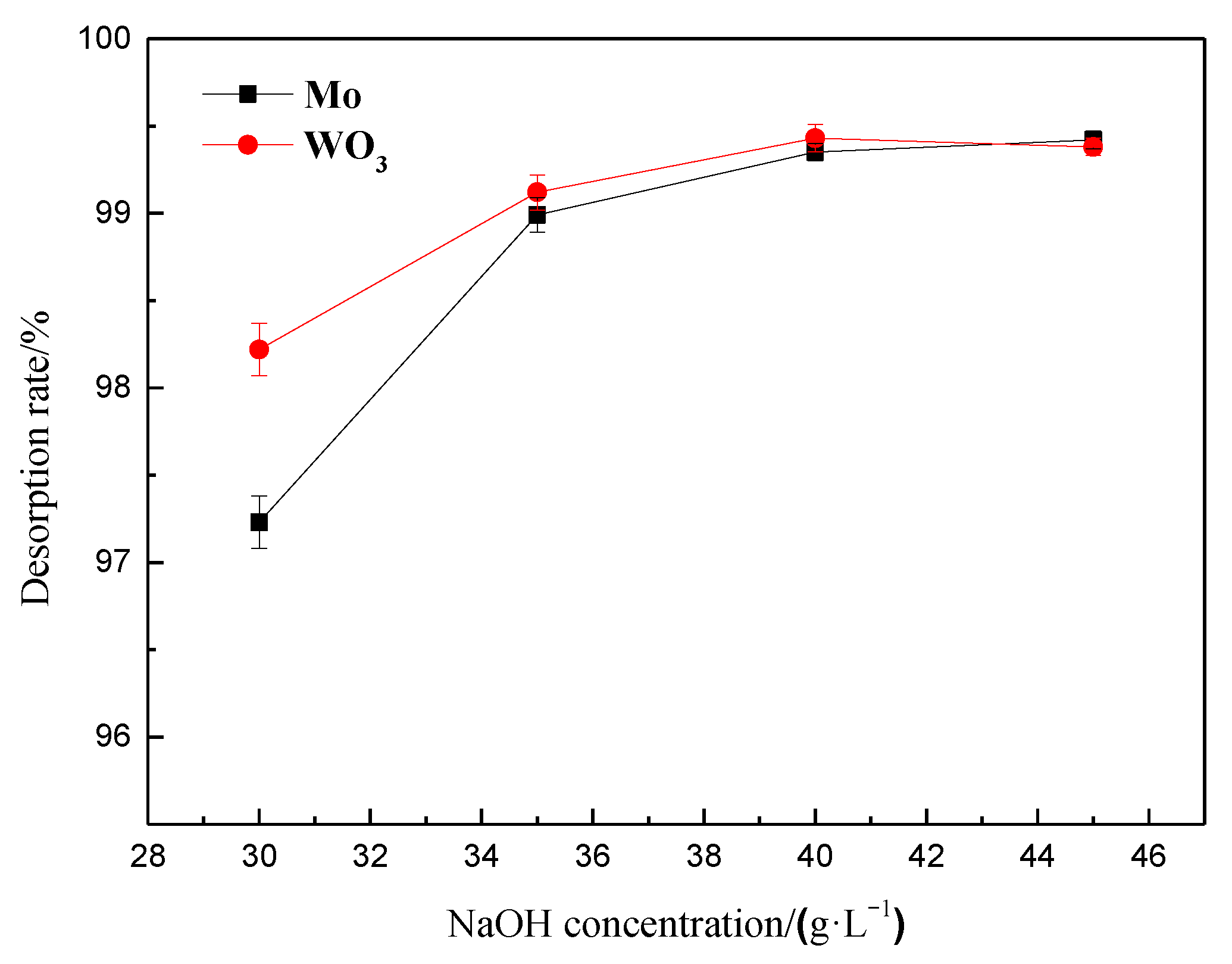
3.1.7. Static Desorption
3.2. Dynamic Adsorption Tests
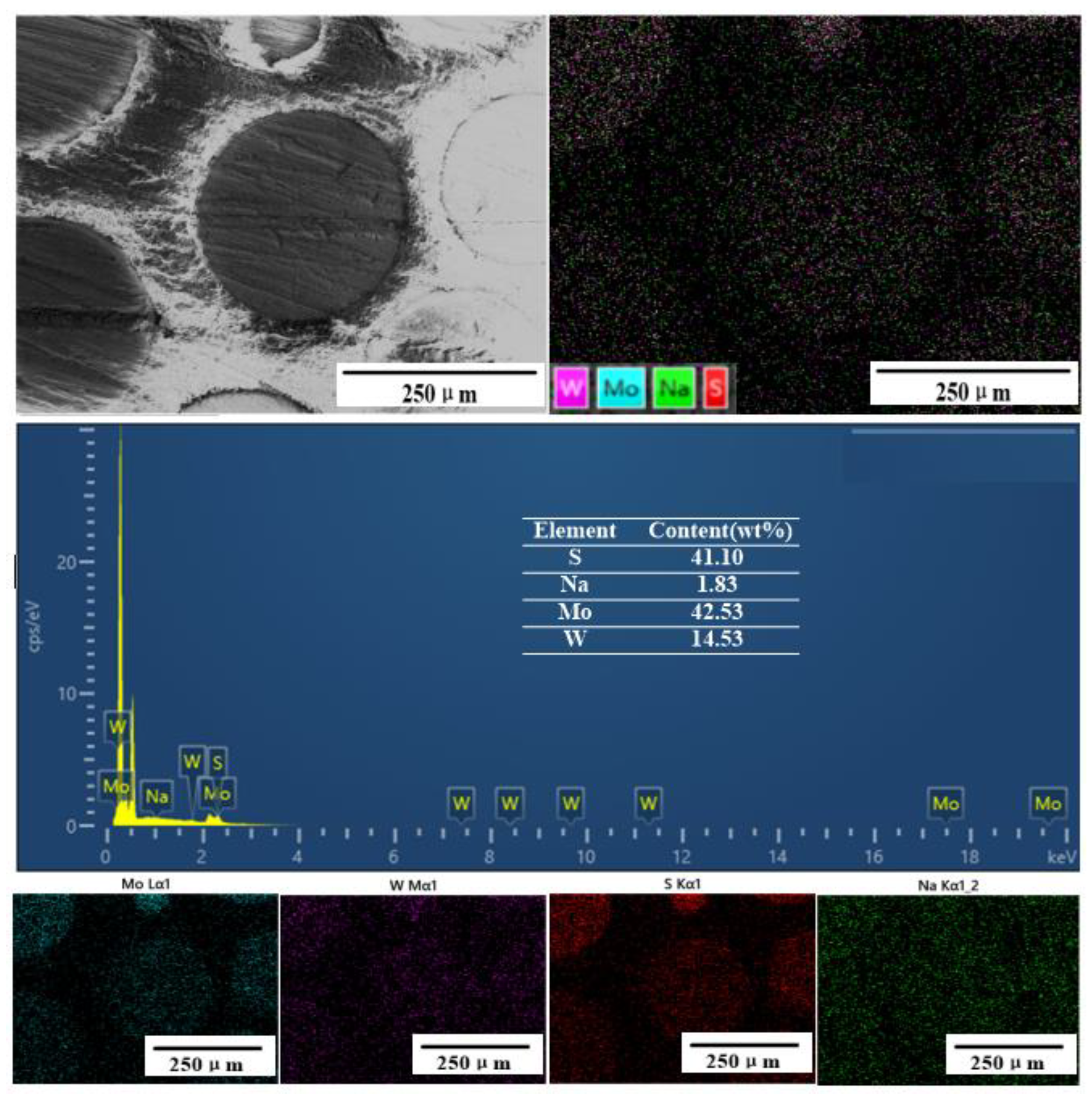
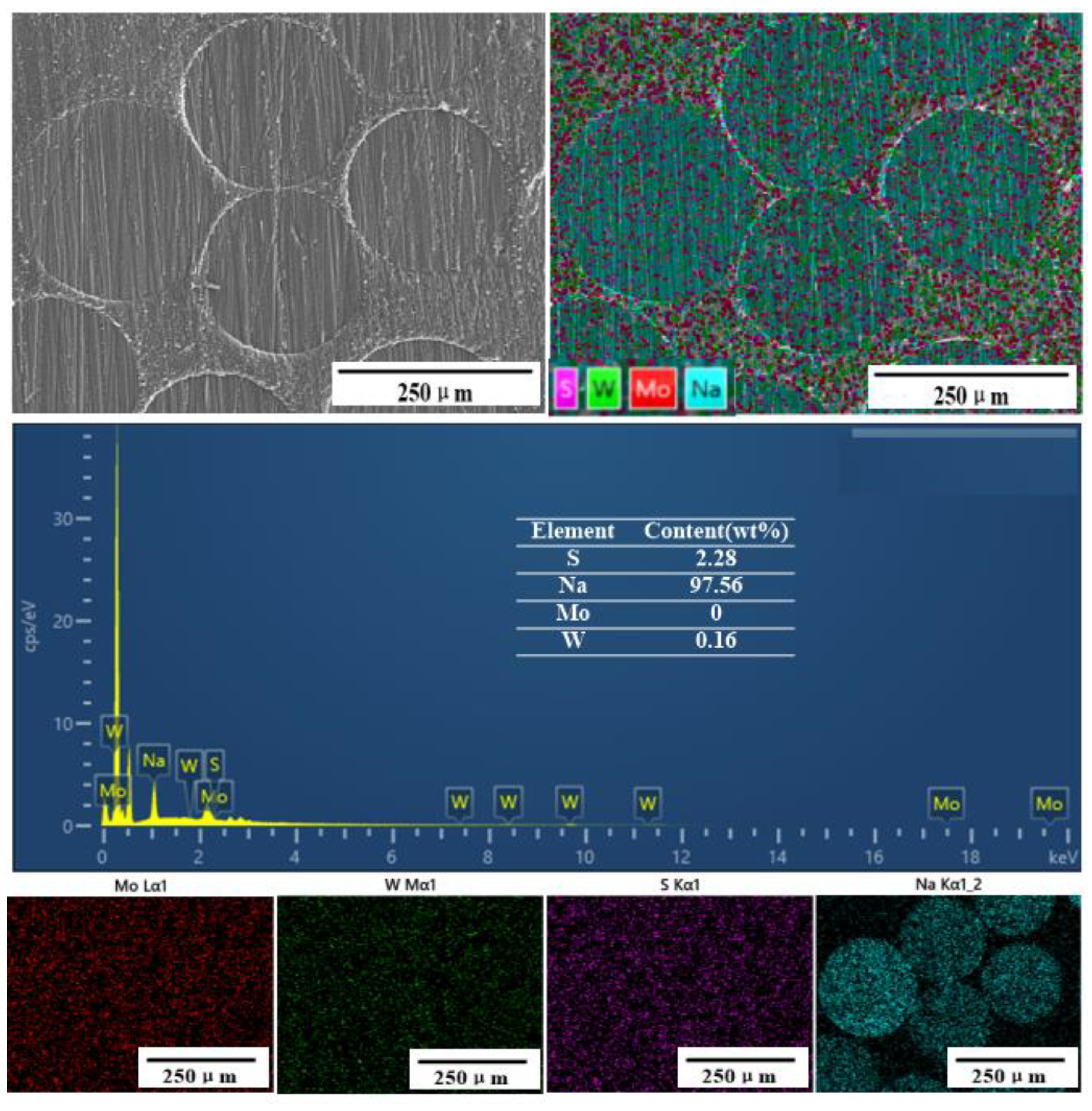
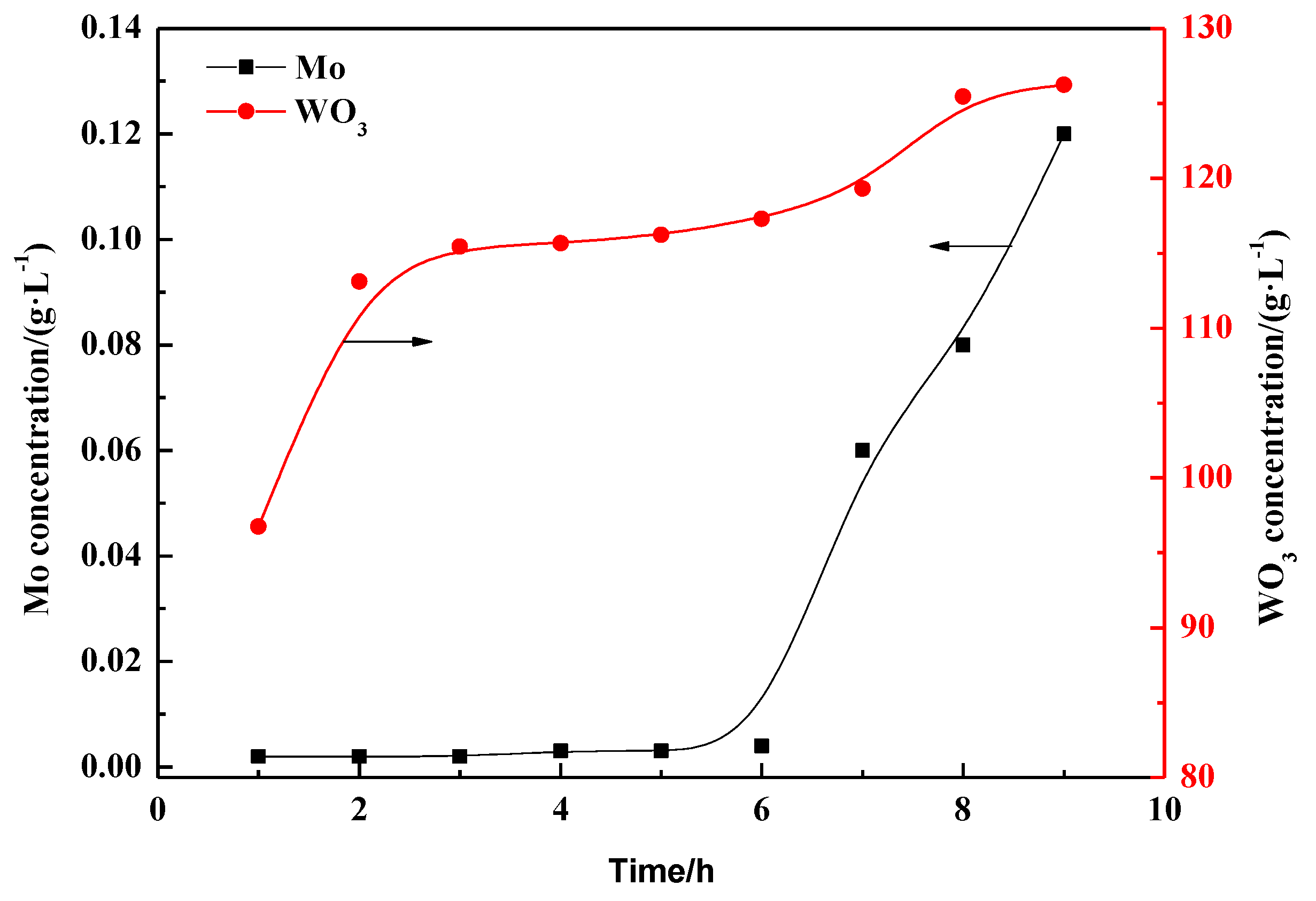
3.2.1. Dynamic Adsorption
3.2.2. Dynamic Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsujisaka, M.; Takano, S.; Murayama, M.; Sohrin, Y. Precise analysis of the concentrations and isotopic compositions of molybdenum and tungsten in geochemical reference materials. Anal. Chim. Acta 2019, 1091, 146–159. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Liu, X.; Zhao, Z.; Wang, W.; Peng, W. Separating W(VI) and Mo(VI) by two-step acid decomposition. Hydrometallurgy 2018, 179, 20–24. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, G.; Guan, W.; Zhou, Q.; Zeng, L.; Wu, S.; Li, Q.; Cao, Z.; Fang, K.; Shi, C. A novel method for preparing tungsten and molybdenum peroxy complex solution and its application to tungsten—Molybdenum separation. Hydrometallurgy 2023, 215, 105974. [Google Scholar] [CrossRef]
- Du, J.; Li, J.; He, D.; Xu, M.; Zhang, G.; Cao, Z.; Wu, S. Green separation and recovery of molybdenum from tungstate solution achieved by using a recyclable vulcanizing agent. J. Clean. Prod. 2021, 278, 123930. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Zeng, L.; Zhou, Q.; Li, Z.; Zhang, D.; Cao, Z.; Guan, W.; Li, Q.; Xiao, L. Study on removal of molybdenum from ammonium tungstate solutions using solvent extraction with quaternary ammonium salt extractant. Hydrometallurgy 2019, 186, 218–225. [Google Scholar] [CrossRef]
- Cao, F.; Wang, W.; Wei, D.-Z.; Liu, W.-G. Separation of tungsten and molybdenum with solvent extraction using functionalized ionic liquid tri-caprylmethylammonium bis(2,4,4-trimethylpentyl)phosphinate. Int. J. Miner. Metall. Mater. 2021, 28, 1769–1776. [Google Scholar] [CrossRef]
- Li, J.; Cao, Z.; Zeng, L.; Xu, M.; Zhang, G.; Li, Q.; Guan, W. A new recyclable sulfurizing reagent for Mo-S complexes and separation of molybdenum from tungstate solutions. Sep. Purif. Technol. 2019, 212, 490–496. [Google Scholar] [CrossRef]
- Su, S.; Huang, Y.; Liu, B.; Han, G.; Xue, Y.; Wang, Y. A Feasible Strategy for Deeply Separating Low Concentrations of Molybdenum from Tungstate Solutions with a High-Efficiency Microbubble Floating-Extraction Concept. ACS Sustain. Chem. Eng. 2021, 10, 146–158. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, G.; Gao, C. Solvent extraction separation of molybdenum and tungsten from ammonium solution by H2O2-complexation. Hydrometallurgy 2012, 127, 84–90. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, Z.; Huo, G.; Wang, X.; Pu, H. Mechanism of Selective Precipitation of Molybdenum from Tungstate Solution. Jom 2019, 72, 800–805. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, Z.; Chen, X. Selective precipitation of tungstate from molybdate-containing solution using divalent ions. Hydrometallurgy 2011, 110, 115–119. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, L.; Qin, Z.; Yin, R.; Weng, D.; Wang, Z.; Luo, D. Separation of vanadium, tungsten and molybdenum from spent SCR catalysts solu-tion by solvent extraction with primary amine N1923. Waste Manag. 2022, 150, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, G.; Zeng, L.; Guan, W.; Xiao, L.; Li, Q.; Cao, Z.; Lu, X. Continuous solvent extraction operations for the separation of W and Mo in high concentrations from ammonium solutions with acidified N1923. Hydrometallurgy 2019, 184, 39–44. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Guan, W.; Zeng, L.; Zhou, Q.; Xia, Y.; Wang, Q.; Li, Q.; Cao, Z. Complete removal of trace vanadium from ammonium tungstate solutions by solvent extraction. Hydrometallurgy 2018, 179, 268–273. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, G.; Xiao, L.; Wang, D. Continuous running test of the solvent extraction separation of Mo and W by H2O2-complexation with TRPO/TBP. Hydrometallurgy 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Zeng, L.; Guan, W.; Wu, S.; Li, Z.; Zhou, Q.; Zhang, D.; Qing, J.; Long, Y.; et al. Continuous solvent extraction operations for the removal of molybdenum from ammonium tungstate solution with quaternary ammonium salt extractant. Hydrometallurgy 2020, 195, 105401. [Google Scholar] [CrossRef]
- Guo, F.; Xi, X.; Ma, L.; Nie, Z. Property and mechanism on sorption of molybdenum from tungstate solution with a porous amine resin. J. Clean. Prod. 2022, 335, 130304. [Google Scholar] [CrossRef]
- Guo, F.; Xi, X.; Ma, L.; Nie, Z.; Nie, Z. Highly efficient sorption of molybdenum from tungstate solution with modified D301 resin. RSC Adv. 2021, 11, 29939–29947. [Google Scholar] [CrossRef]
- Gong, D.; Zhou, K.; Peng, C.; He, D.; Chen, W. Resin-enhanced acid leaching of tungsten from scheelite. Hydrometallurgy 2018, 182, 75–81. [Google Scholar] [CrossRef]
- Jia, L.-P.; Huang, J.-J.; Ma, Z.-L.; Liu, X.-H.; Chen, X.-Y.; Li, J.-T.; He, L.-H.; Zhao, Z.-W. Research and development trends of hydrometallurgy: An overview based on Hydrometallurgy literature from 1975 to 2019. Trans. Nonferrous Met. Soc. China 2020, 30, 3147–3160. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, F.; Hu, Y.; Wang, S.; Sun, P.; Huo, G.; Li, H. Adsorption behaviour of WO42− onto 201×7 resin in highly concentrated tungstate solutions. Int. J. Refract. Met. Hard Mater. 2010, 28, 633–637. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Huo, G.-S.; Liao, C.-H. Separation of macro amounts of tungsten and molybdenum by ion exchange with D309 resin. Trans. Nonferrous Met. Soc. China 2014, 24, 3008–3013. [Google Scholar] [CrossRef]
- Huo, G.S.; Zhao, Z.W.; Li, H.G.; Sun, P.M.; Li, Y.J. Theory and practice of sulfuration of molybdate in tungstate solution. Min. Metall. Enginering 2003, 6, 46–49. [Google Scholar]



| NO. | Volume/mL | Concentration/(g·L−1) | Desorption Rate/% | |||
|---|---|---|---|---|---|---|
| Mo | WO3 | NaOH | Mo | WO3 | ||
| First desorption | 485 | 26.85 | 4.95 | 4.33 | 97.45 | 98.09 |
| Second desorption | 490 | 0.43 | 0.06 | 43.12 | 1.57 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, B.; Zeng, X.; Huang, L.; Huang, W.; Shu, R. Adsorption Properties of a Polyamine Special Ion Exchange Resin for Removing Molybdenum from Ammonium Tungstate Solutions. Sustainability 2023, 15, 3837. https://doi.org/10.3390/su15043837
Zeng B, Zeng X, Huang L, Huang W, Shu R. Adsorption Properties of a Polyamine Special Ion Exchange Resin for Removing Molybdenum from Ammonium Tungstate Solutions. Sustainability. 2023; 15(4):3837. https://doi.org/10.3390/su15043837
Chicago/Turabian StyleZeng, Bin, Xiangrong Zeng, Lijinhong Huang, Wanfu Huang, and Ronghua Shu. 2023. "Adsorption Properties of a Polyamine Special Ion Exchange Resin for Removing Molybdenum from Ammonium Tungstate Solutions" Sustainability 15, no. 4: 3837. https://doi.org/10.3390/su15043837





