Evaluation of Tolerant to CO2 Excess Microalgae for the Production of Multiple Biochemicals in a 3G Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Species and Cultivation in Flasks
2.2. Cultivation in Photobioreactors
2.3. Analytical Measurements
3. Results and Discussion
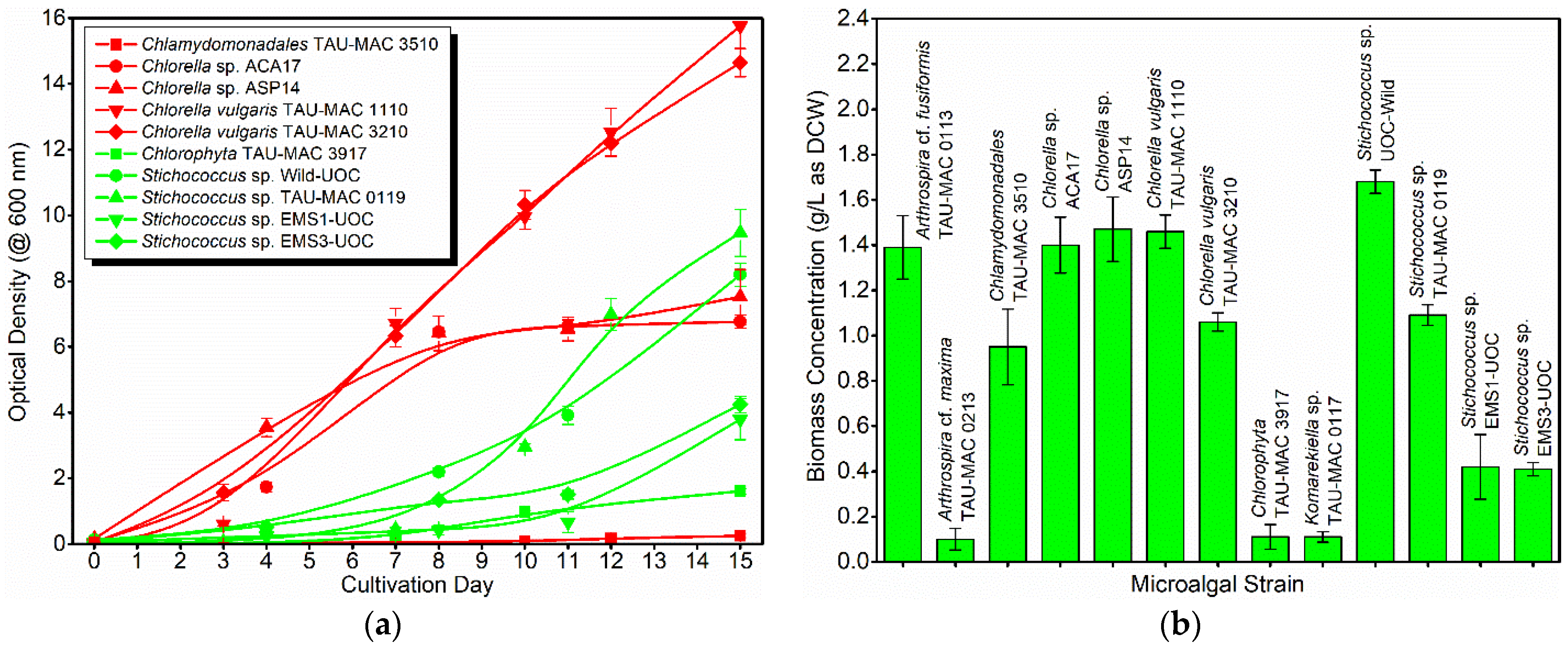
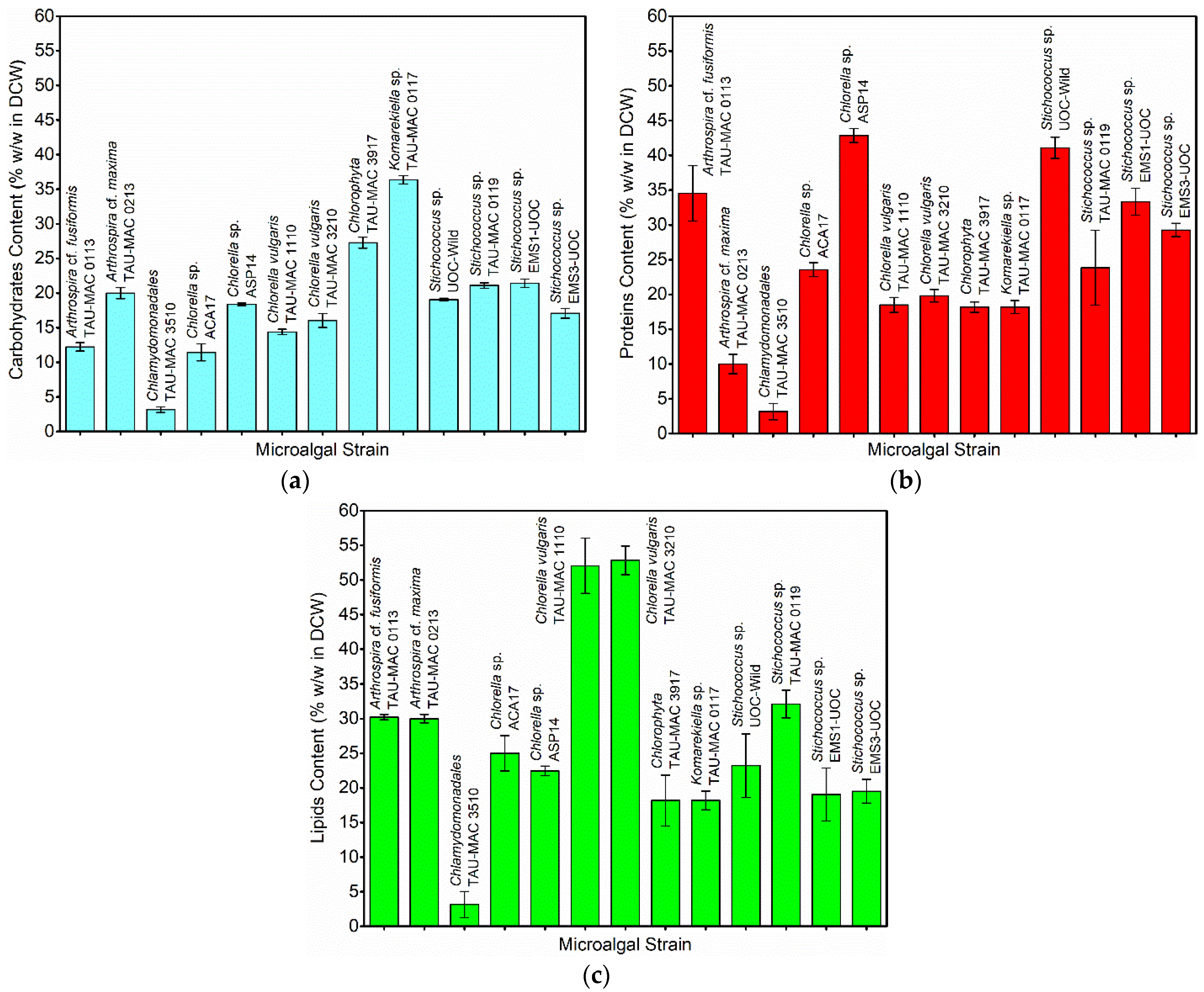
3.1. Microalgal Species’ Screening and Selection
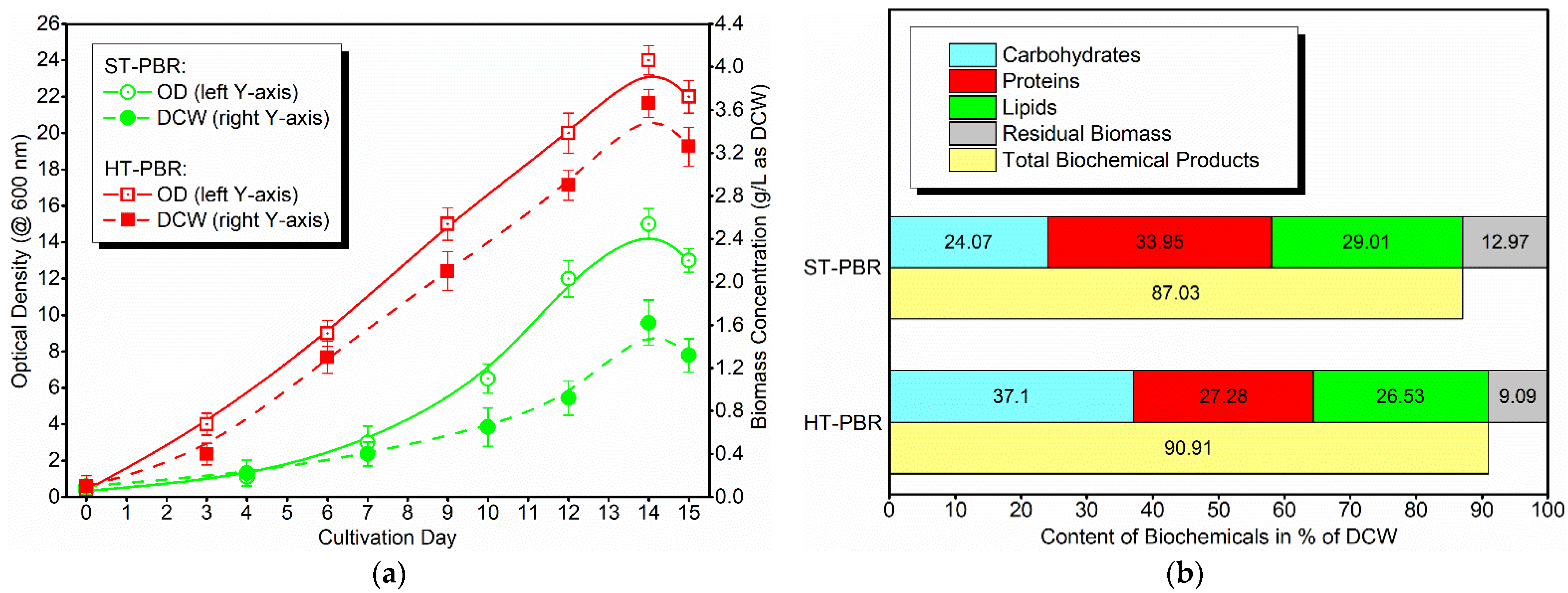
3.2. Cultivation of Stichococcus sp. Wild-TUC in Photobioreactors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Mendonça, H.V.; Assemany, P.; Abreu, M.; Couto, E.; Maciel, A.M.; Duarte, R.L.; dos Santos, M.G.B.; Reis, A. Microalgae in a global world: New solutions for old problems? Renew. Energy 2021, 165, 842–862. [Google Scholar] [CrossRef]
- Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Quadir, M.A.; Annamalai, S.N.; Al Jabri, H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation 2022, 8, 316. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N. Industrial and Biotechnological Applications of Algae: A Review. J. Adv. Plant Biol. 2017, 1, 1–25. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total. Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Chhandama, M.V.L.; Satyan, K.B.; Changmai, B.; Vanlalveni, C.; Rokhum, S.L. Microalgae as a feedstock for the production of biodiesel: A review. Bioresour. Technol. Rep. 2021, 15, 100771. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Talebi, S.; Edalatpour, A.; Tavakoli, O. Algal biorefinery: A potential solution to the food–energy–water–environment nexus. Sustain. Energy Fuels 2022, 6, 2623–2664. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; Pereira, A.S.A.D.P.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from microalgae biomass: Technology, sustainability, challenges and opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.-H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae Bioactive Compounds to Topical Applications Products—A Review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Colla, L.M.; Reinehr, C.O.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.D.O.; Danesi, E.D.G.; de Carvalho, J.C.M.; Sato, S. Chlorophyll production from Spirulina platensis: Cultivation with urea addition by fed-batch process. Bioresour. Technol. 2004, 92, 133–141. [Google Scholar] [CrossRef]
- Sajilata, M.; Singhal, R.; Kamat, M.Y. Supercritical CO2 extraction of γ-linolenic acid (GLA) from Spirulina platensis ARM 740 using response surface methodology. J. Food Eng. 2008, 84, 321–326. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Madadi, R.; Maljaee, H.; Serafim, L.S.; Ventura, S.P.M. Microalgae as Contributors to Produce Biopolymers. Mar. Drugs 2021, 19, 466. [Google Scholar] [CrossRef]
- Severo, I.A.; Dias, R.R.; Nascimento, T.C.D.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae-derived polysaccharides: Potential building blocks for biomedical applications. World J. Microbiol. Biotechnol. 2022, 38, 150. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter environmental stress. Sci. Total. Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Eppink, M.H.M.; Olivieri, G.; Reith, H.; Berg, C.V.D.; Barbosa, M.J.; Wijffels, R.H. From Current Algae Products to Future Biorefinery Practices: A Review. In Biorefineries—Advances in Biochemical Engineering/Biotechnology; Wagemann, K., Tippkötter, N., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 166, pp. 99–123. [Google Scholar] [CrossRef]
- Karapatsia, A.; Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. An experimental investigation of Stichococcus sp. cultivation conditions for optimal co-production of carbohydrates, proteins and lipids following a biorefinery concept. Biomass Bioenergy 2016, 89, 123–132. [Google Scholar] [CrossRef]
- Paul, V.; Shekharaiah, P.S.C.; Kushwaha, S.; Sapre, A.; Dasgupta, S.; Sanyal, D. Role of Algae in CO2 Sequestration Addressing Climate Change: A Review. In Renewable Energy and Climate Change—Smart Innovation, Systems and Technologies; Deb, D., Dixit, A., Chandra, L., Eds.; Springer: Singapore, 2020; Volume 161, pp. 257–265. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Costa, J.A.V.; de Freitas, B.C.B.; Lisboa, C.R.; Santos, T.D.; Brusch, L.R.D.F.; de Morais, M.G. Microalgal biorefinery from CO2 and the effects under the Blue Economy. Renew. Sustain. Energy Rev. 2018, 99, 58–65. [Google Scholar] [CrossRef]
- Yadav, G.; Karemore, A.; Dash, S.K.; Sen, R. Performance evaluation of a green process for microalgal CO2 sequestration in closed photobioreactor using flue gas generated in-situ. Bioresour. Technol. 2015, 191, 399–406. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hamasaki, A.; Sioji, N.; Ikuta, Y. Influence of CO2, SO2 and NO in Flue Gas on Microalgae Productivity. J. Chem. Eng. Jpn. 1997, 30, 620–624. [Google Scholar] [CrossRef]
- Oliveira, G.M.; Caetano, N.; Mata, T.M.; Martins, A.A. Biofixation of CO2 emissions from natural gas combined cycle power plant. Energy Rep. 2020, 6, 140–146. [Google Scholar] [CrossRef]
- Molitor, H.R.; Schnoor, J.L. Using Simulated Flue Gas to Rapidly Grow Nutritious Microalgae with Enhanced Settleability. ACS Omega 2020, 5, 27269–27277. [Google Scholar] [CrossRef]
- Kumar, K.; Banerjee, D.; Das, D. Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana. Bioresour. Technol. 2014, 152, 225–233. [Google Scholar] [CrossRef]
- Hosseini, N.S.; Shang, H.; Ross, G.M.; Scott, J.A. Microalgae cultivation in a novel top-lit gas-lift open bioreactor. Bioresour. Technol. 2015, 192, 432–440. [Google Scholar] [CrossRef]
- Da Rosa, G.M.; Moraes, L.; Cardias, B.B.; Souza, M.D.R.A.Z.D.; Costa, J.A.V. Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour. Technol. 2015, 192, 321–327. [Google Scholar] [CrossRef]
- Jerney, J.; Spilling, K. Large Scale Cultivation of Microalgae: Open and Closed Systems. In Biofuels from Algae. Methods in Molecular Biology; Spilling, K., Ed.; Humana: New York, NY, USA, 2018; Volume 1980, pp. 1–8. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, M.; Moreno, J.; Cañavate, J.P.; Anguis, V.; Prieto, A.; Manzano, C.; Florencio, F.J.; Guerrero, M. Conditions for open-air outdoor culture of Dunaliella salina in southern Spain. J. Appl. Phycol. 2003, 15, 177–184. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, X.; Wang, J.; Han, D.; Li, Y.; Xi, Y.; Tang, Y.; Chi, Z. Large-scale cultivation of Spirulina in a floating horizontal photobioreactor without aeration or an agitation device. Appl. Microbiol. Biotechnol. 2018, 102, 8979–8987. [Google Scholar] [CrossRef]
- Acién, F.; Molina, E.; Reis, A.; Torzillo, G.; Zittelli, G.; Sepúlveda, C.; Masojídek, J. Photobioreactors for the production of microalgae-based biofuels and bioproducts. In Microalgae-Based Biofuels and Bioproducts—From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing Series in Energy: Cambridge, UK, 2017; pp. 1–44. [Google Scholar]
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef]
- Paul, S.; Bera, S.; Dasgupta, R.; Mondal, S.; Roy, S. Review on the recent structural advances in open and closed systems for carbon capture through algae. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, A.K.; Ranganathan, P.; Singh, S.; Udayan, A.; Awasthi, M.K.; Hoang, A.T.; Chilakamarry, C.R.; Kim, S.H.; Sim, S.J. Design and applications of photobioreactors—A review. Bioresour. Technol. 2022, 349, 126858. [Google Scholar] [CrossRef]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Rippka, R. [1] Isolation and purification of cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar] [CrossRef]
- Shirai, M.; Matumaru, K.; Ohotake, A.; Takamura, Y.; Aida, T.; Nakano, M. Development of a Solid Medium for Growth and Isolation of Axenic Microcystis Strains (Cyanobacteria). Appl. Environ. Microbiol. 1989, 55, 2569–2571. [Google Scholar] [CrossRef]
- Castenholz, R.W. [3] Culturing methods for cyanobacteria. Methods Enzymol. 1988, 167, 68–93. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process.-Process. Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chen, Y.; Vaidyanathan, S. Simultaneous assay of pigments, carbohydrates, proteins and lipids in microalgae. Anal. Chim. Acta 2013, 776, 31–40. [Google Scholar] [CrossRef]
- Belal, E.B. Use of Spirulina (Arthrospira fusiformis) for promoting growth of Nile Tilapia fingerlings. Afr. J. Microbiol. Res. 2012, 6, 6423–6431. [Google Scholar] [CrossRef]
- Thangavel, K.; Krishnan, P.R.; Nagaiah, S.; Kuppusamy, S.; Chinnasamy, S.; Rajadorai, J.S.; Olaganathan, G.N.; Dananjeyan, B. Growth and metabolic characteristics of oleaginous microalgal isolates from Nilgiri biosphere Reserve of India. BMC Microbiol. 2018, 18, 1. [Google Scholar] [CrossRef]
- Bouyam, S.; Choorit, W.; Sirisansaneeyakul, S.; Chisti, Y. Heterotrophic production of Chlorella sp. TISTR 8990-biomass growth and composition under various production conditions. Biotechnol. Prog. 2017, 33, 1589–1600. [Google Scholar] [CrossRef]
- Vaičiulytė, S.; Padovani, G.; Kostkevičienė, J.; Carlozzi, P. Batch Growth of Chlorella vulgaris CCALA 896 versus Semi-Continuous Regimen for Enhancing Oil-Rich Biomass Productivity. Energies 2014, 7, 3840–3857. [Google Scholar] [CrossRef]
- Teoh, M.-L.; Chu, W.-L.; Marchant, H.; Phang, S.-M. Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J. Appl. Phycol. 2004, 16, 421–430. [Google Scholar] [CrossRef]
- Makaroglou, G.; Marakas, H.; Fodelianakis, S.; Axaopoulou, V.A.; Koumi, I.; Kalogerakis, N.; Gikas, P. Optimization of biomass production from Stichococcous sp. biofilms coupled to wastewater treatment. Biochem. Eng. J. 2021, 169, 107964. [Google Scholar] [CrossRef]
- Psachoulia, P.; Chatzidoukas, C. Illumination Policies for Stichococcus sp. Cultures in an Optimally Operating Lab-Scale PBR toward the Directed Photosynthetic Production of Desired Products. Sustainability 2021, 13, 2489. [Google Scholar] [CrossRef]
| # | Microalgal Strain | Reference Code | Cultivation Medium |
|---|---|---|---|
| 1 | Arthrospira cf. fusiformis | TAU-MAC 0113 | Zarrouk |
| 2 | Arthrospira cf. maxima | TAU-MAC 0213 | Zarrouk |
| 3 | Chlamydomonadales | TAU-MAC 3510 | 3N-BBM + V_Mod |
| 4 | Chlorella sp. | ACA17 | BG11 |
| 5 | Chlorella sp. | ASP14 | BG11 |
| 6 | Chlorella vulgaris | TAU-MAC 1110 | BG11 |
| 7 | Chlorella vulgaris | TAU-MAC 3210 | BG11 |
| 8 | Chlorophyta | TAU-MAC 3917 | BG11 |
| 9 | Komarekiella sp. | TAU-MAC 0117 | BG11 |
| 10 | Stichococcus sp. | Wild-TUC | 3N-BBM + V_Mod |
| 11 | Stichococcus sp. | TAU-MAC 0119 | 3N-BBM + V_Mod |
| 12 | Stichococcus sp. | EMS1-TUC | 3N-BBM + V_Mod |
| 13 | Stichococcus sp. | EMS3-TUC | 3N-BBM + V_Mod |
| Component | Concentration (g∙L−1) | ||
|---|---|---|---|
| BG11 | 3N-BBM + V_Mod | Zarrouk | |
| NaNO3 | 1.5 | 0.75 | 2.5 |
| K2HPO4∙3H2O | 0.04 | 0.075 | 0.5 |
| K2SO4 | - | - | 1.0 |
| KH2PO4 | - | 0.175 | - |
| NaCl | - | 0.25 | 1.0 |
| NaHCO3 | - | - | 16.8 |
| MgSO4∙7H2O | 0.075 | 0.075 | 0.2 |
| CaCl2∙2H2O | 0.036 | 0.025 | 0.04 |
| Na2CO3 | 0.02 | - | 0.2 |
| DiNa-EDTA | 0.001 | 0.0045 | 0.08 |
| Citric acid | 0.006 | - | - |
| Ammonium ferric citrate | 0.006 | - | - |
| TES + V * | 1 | 1 | 1 |
| Microalgal Strain | Carbohydrates (g·L−1) | Proteins (g·L−1) | Lipids (g·L−1) | Total Biochemicals Products (g·L−1) | Total Product Contents (% w/w) |
|---|---|---|---|---|---|
| Arthrospira cf. fusiformis TAU-MAC 0113 | 0.17 | 0.48 | 0.42 | 1.07 | 76.98 |
| Arthrospira cf. maxima TAU-MAC 0213 | 0.02 | 0.01 | 0.03 | 0.06 | 60.00 |
| Chlamydomonadales TAU-MAC 3510 | 0.03 | 0.03 | 0.03 | 0.09 | 9.47 |
| Chlorella sp. ACA17 | 0.16 | 0.33 | 0.35 | 0.84 | 60.00 |
| Chlorella sp. ASP14 | 0.27 | 0.63 | 0.33 | 1.23 | 83.67 |
| Chlorella vulgaris TAU-MAC 1110 | 0.21 | 0.27 | 0.76 | 1.24 | 84.93 |
| Chlorella vulgaris TAU-MAC 3210 | 0.17 | 0.21 | 0.56 | 0.94 | 88.68 |
| Chlorophyta TAU-MAC 3917 | 0.03 | 0.02 | 0.02 | 0.07 | 63.64 |
| Komarekiella sp. TAU-MAC 0117 | 0.04 | 0.02 | 0.02 | 0.08 | 72.73 |
| Stichococcus sp. Wild-TUC | 0.32 | 0.69 | 0.39 | 1.40 | 83.33 |
| Stichococcus sp. TAU-MAC 0119 | 0.23 | 0.26 | 0.35 | 0.84 | 77.06 |
| Stichococcus sp. EMS1-TUC | 0.09 | 0.14 | 0.08 | 0.31 | 73.81 |
| Stichococcus sp. EMS3-TUC | 0.07 | 0.12 | 0.08 | 0.27 | 65.85 |
| Photobioreactor (PBR) Configuration | Carbohydrates Concentration (g·L−1) | Proteins Concentration (g·L−1) | Lipids Concentration (g·L−1) | Total Biochemical Products (g·L−1) |
|---|---|---|---|---|
| Stirred Tank: ST-PBR | 0.39 | 0.56 | 0.48 | 1.43 |
| Horizontal Tubular: HT-PBR | 1.36 | 1.00 | 0.97 | 3.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlou, A.; Penloglou, G.; Kiparissides, C. Evaluation of Tolerant to CO2 Excess Microalgae for the Production of Multiple Biochemicals in a 3G Biorefinery. Sustainability 2023, 15, 3889. https://doi.org/10.3390/su15053889
Pavlou A, Penloglou G, Kiparissides C. Evaluation of Tolerant to CO2 Excess Microalgae for the Production of Multiple Biochemicals in a 3G Biorefinery. Sustainability. 2023; 15(5):3889. https://doi.org/10.3390/su15053889
Chicago/Turabian StylePavlou, Alexandros, Giannis Penloglou, and Costas Kiparissides. 2023. "Evaluation of Tolerant to CO2 Excess Microalgae for the Production of Multiple Biochemicals in a 3G Biorefinery" Sustainability 15, no. 5: 3889. https://doi.org/10.3390/su15053889
APA StylePavlou, A., Penloglou, G., & Kiparissides, C. (2023). Evaluation of Tolerant to CO2 Excess Microalgae for the Production of Multiple Biochemicals in a 3G Biorefinery. Sustainability, 15(5), 3889. https://doi.org/10.3390/su15053889









