The Role of Modified Biochar for the Remediation of Coal Mining-Impacted Contaminated Soil: A Review
Abstract
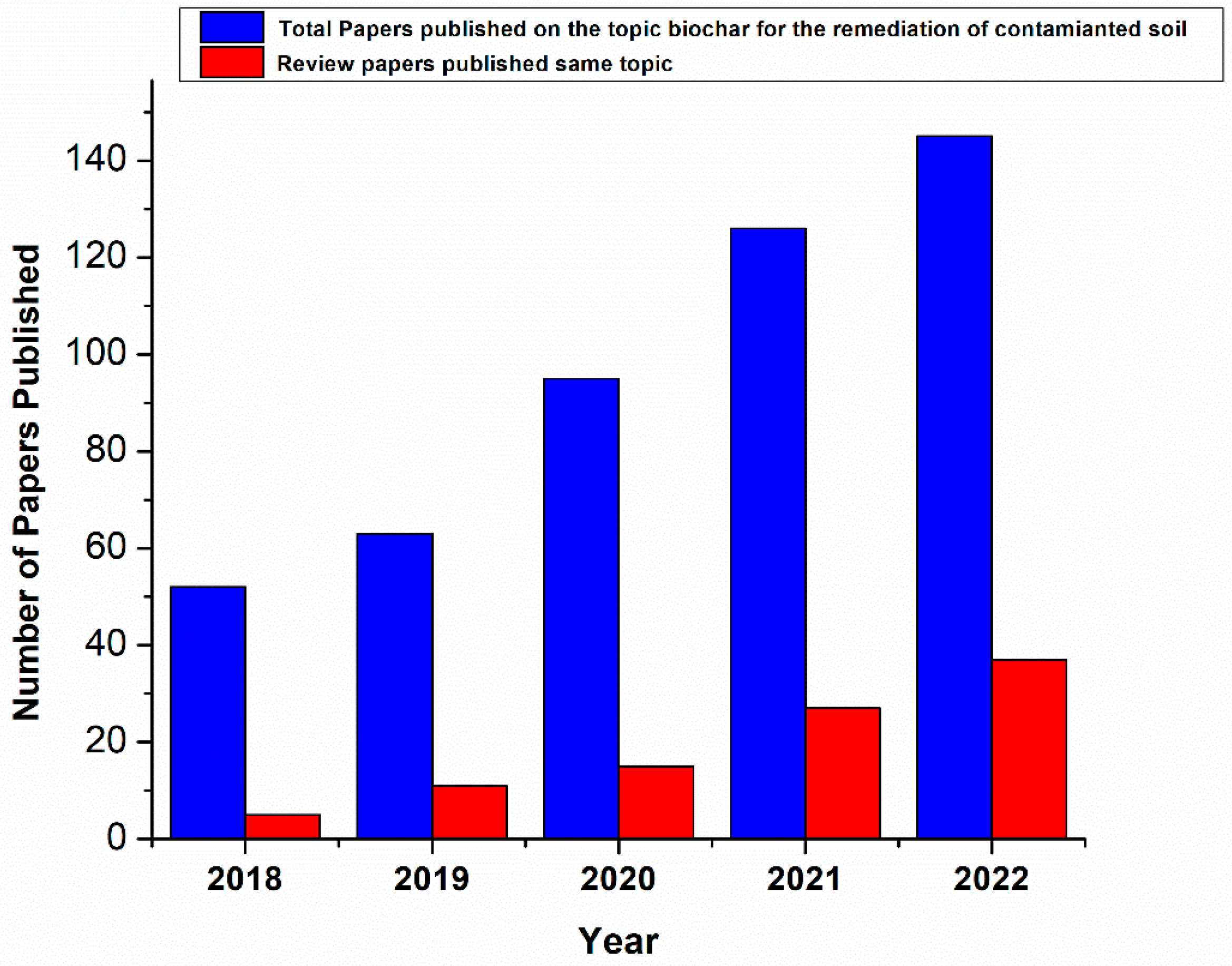
:1. Introduction
2. Degraded and Contaminated Soils in Mining Areas
2.1. Physicochemical Properties
2.2. Impact on Soil Ecosystem
2.3. Heavy Metals Pollution and Health Impacts
3. Remediation of Heavy Metal-Contaminated Soil
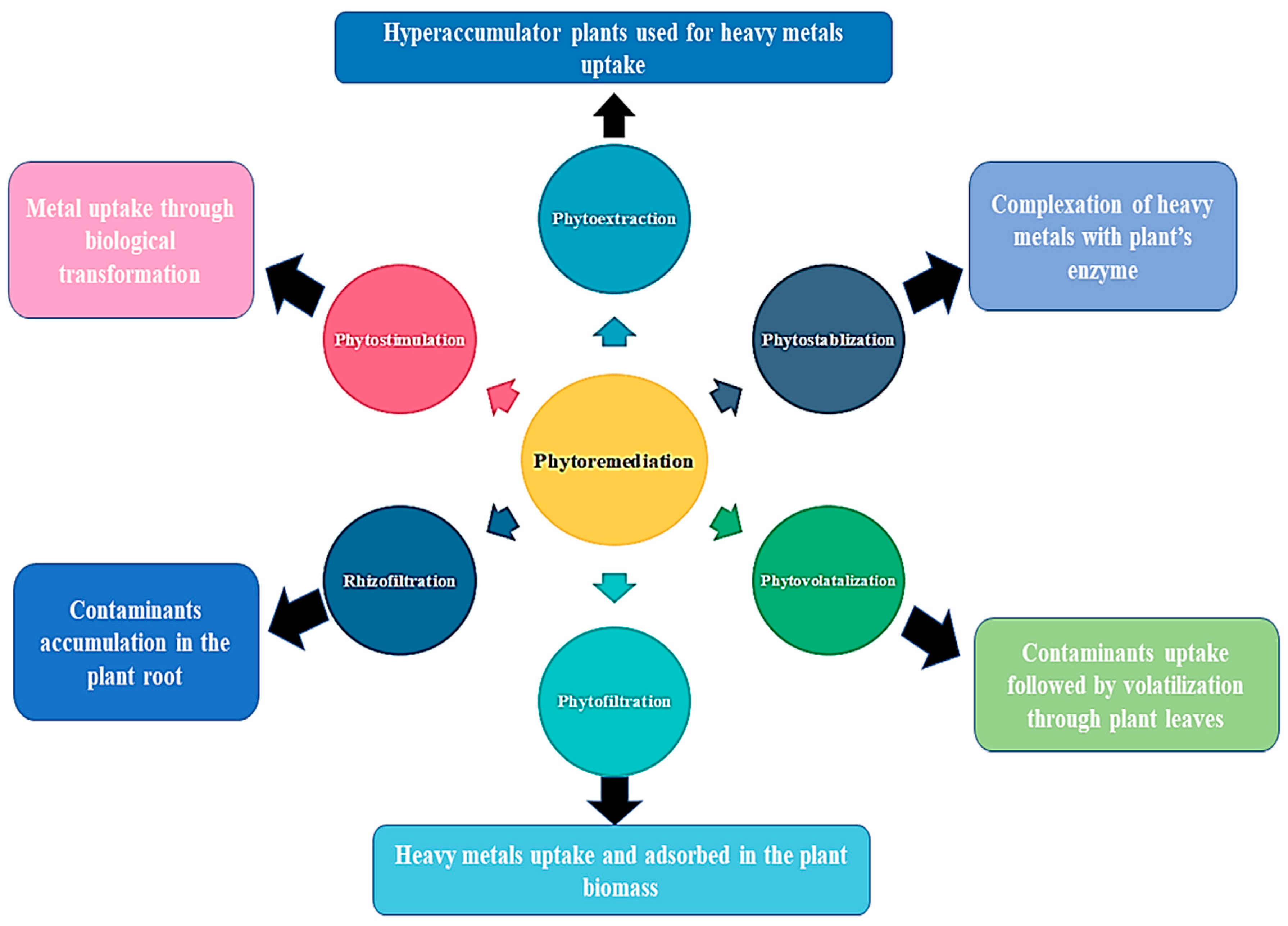
3.1. Phytoremediation
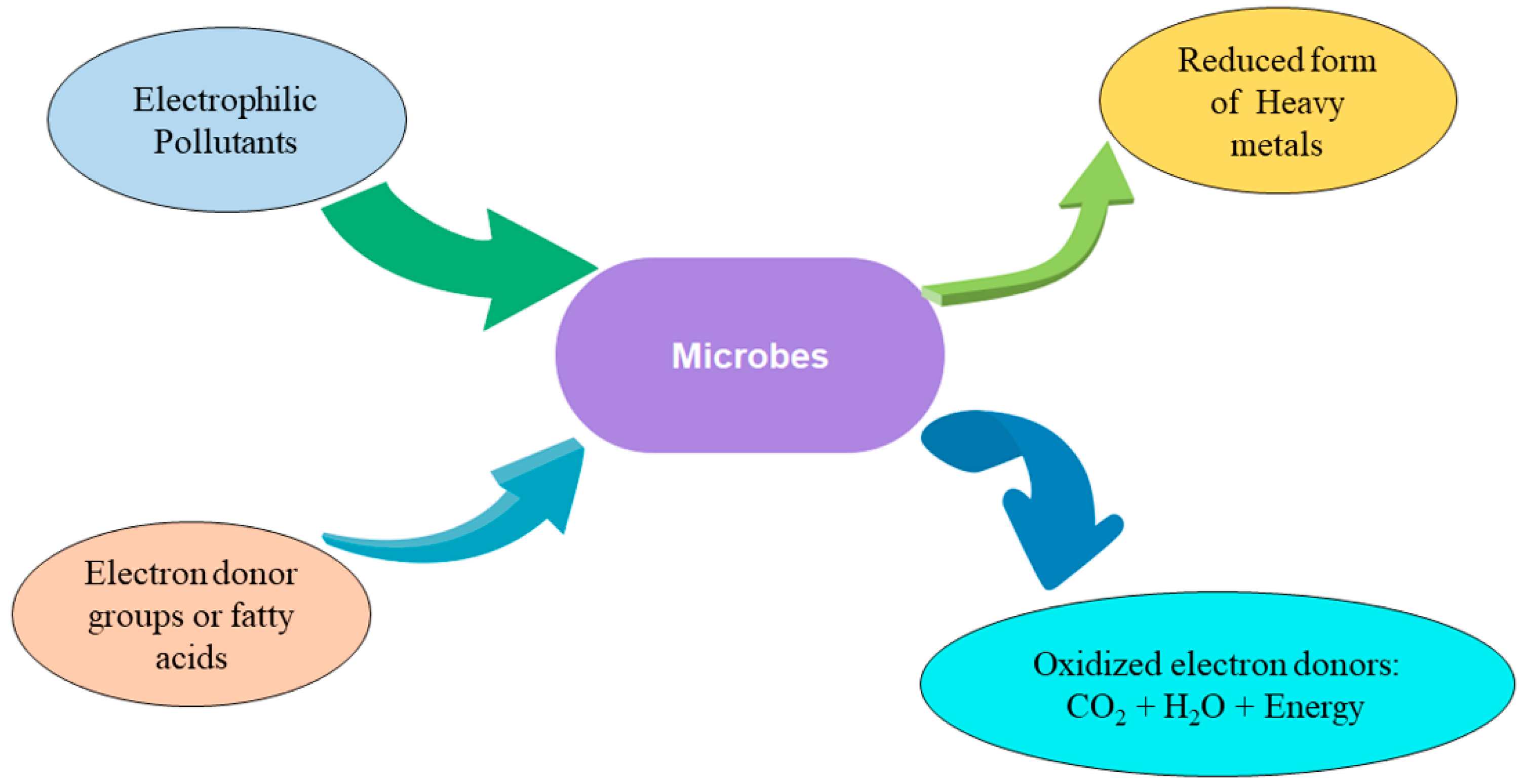
3.2. Bioremediation
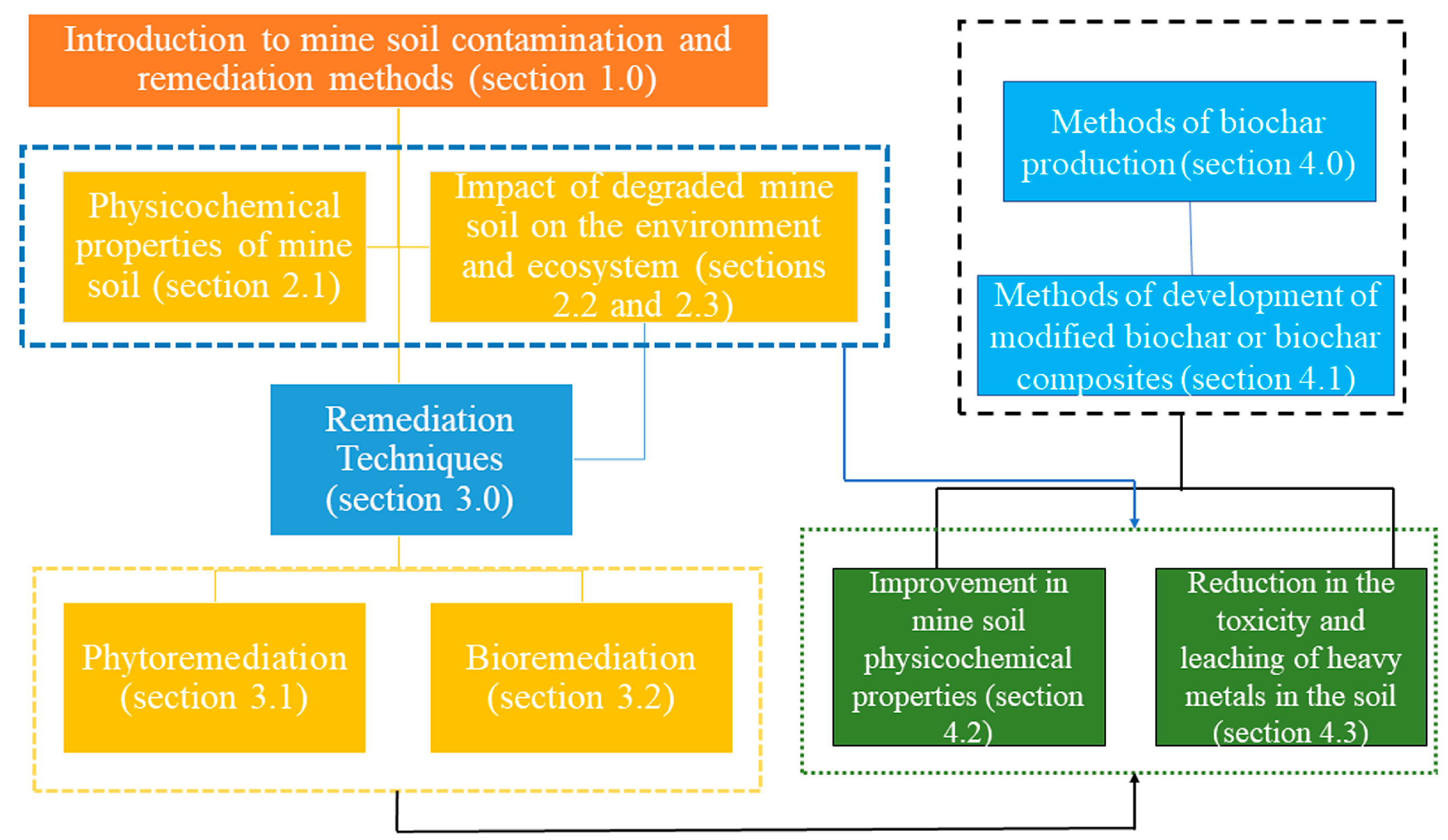
4. Biochar and Its Composites for Soil Remediation
4.1. Various Synthesis Methods of Biochar Composites
4.1.1. Modifications in the Treatment Methods
- (a)
- Pre-treatment methods
- (b)
- Post-treatment methods
4.1.2. Co-Doped Biochar Composites
4.2. Effect of Biochar and Its Composites on Soil Physicochemical Properties
4.3. Effect on Heavy Metals
5. Conclusions and Future Prospects
- (i)
- The coal mining activities destroy the landscape, soil quality, and ecosystem in the impacted area to such an extent that restoring such degraded lands to their original state becomes difficult.
- (ii)
- The degraded landscape triggers the release of heavy metals such as Cr, Cd, As, Mn, Co, Ni, and Zn from the contaminated mine soil into various surface and groundwater resources, which may have significant health impacts on humans and animals.
- (iii)
- Phytoremediation is the most common and low-cost method to restore such degraded and contaminated mine soil by promoting the growth of metal-tolerant species in such soil. However, the poor physiochemical properties of the mine soil further limit the growth of hyperaccumulators in the mine soil.
- (iv)
- The surface properties of pristine biochar can be modified by pre- and post-treatment methods. The acid- or alkali-treated biochar shows the change in the abundance and characteristics of functional groups and surface porosity. The biochar can also be combined with other minerals through thermal and chemical treatment methods to produce biochar composites having enhanced surface properties compared with the pristine biochar.
- (v)
- The application of biochar to the contaminated and degraded soil improves the overall soil physicochemical properties and promotes the immobilization of heavy metals within the soil matrix, thus, promoting the phytoremediation of the soil. Precipitation, surface complexation, electrostatic attraction, and ion exchange are the dominant methods through which biochar adsorbs and immobilizes heavy metals in the soil matrix.
- (vi)
- Remediating mine soils contaminated with heavy metals such as As, Cr, and Cd using pristine biochar is challenging. The application of modified biochar or biochar composites enhances the remediation process. The modified biochar’s surface properties can be changed through the modification process to remove a particular class of heavy metals with anionic characteristics in the aqueous soil solution.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benoit, P. Energy and Development in a Changing World: A Framework for the 21st Century. Available online: https://www.energypolicy.columbia.edu/ (accessed on 15 July 2022).
- WEC. World Energy Resources 2016; WEC: Milwaukee, WI, USA, 2016. [Google Scholar]
- Philalay, M.; Drahos, N.; Thurtell, D.; Pratley, L.; Brooks, L.; Nguyen, T.; Moloney, J.; Wakefield, E.; Bray, M.; Samuels, R. Coal in India; IEA: Paris, France, 2020. [Google Scholar]
- Ahirwal, J.; Kumar, A.; Pietryzekowski, M.; Maiti, S.K. Reclamation of coal mine spoil and its effect on Technosol quality and carbon sequestration: A case study from India. Environ. Sci. Pollut. Res. 2018, 25, 27992–28003. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Cooke, S.J.; Lesser, P.; Macura, B.; Nilsson, A.E.; Taylor, J.J.; Raito, K. Evidence of the Impacts of Metal Mining and the Effectiveness of Mining Mitigation Measures on Social-Ecological Systems in Arctic and Boreal Regions: A Systematic Map Protocol. Environ. Evid. 2019, 8, 1. [Google Scholar] [CrossRef]
- Coal, M. Production and Supplies. Available online: https://coal.gov.in/ (accessed on 16 July 2022).
- Koner, R.; Chakravarty, D. Characterisation of Overburden Dump Materials: A Case Study from the Wardha Valley Coal Field. Bull. Eng. Geol. Environ. 2016, 75, 1311–1323. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Kumar, M.S. Eco-Restoration Approach for Mine Spoil Overburden Dump through Biotechnological Route. Environ. Monit. Assess. 2019, 191, 772. [Google Scholar] [CrossRef]
- Lei, L.Q.; Song, C.A.; Xie, X.L.; Li, Y.H.; Wang, F. Acid Mine Drainage and Heavy Metal Contamination in Groundwater of Metal Sulfide Mine at Arid Territory (BS Mine, Western Australia). Trans. Nonferrous Met. Soc. China 2010, 20, 1488–1493. [Google Scholar] [CrossRef]
- Pająk, M.; Błońska, E.; Szostak, M.; Gąsiorek, M.; Pietrzykowski, M.; Urban, O.; Derbis, P. Restoration of Vegetation in Relation to Soil Properties of Spoil Heap Heavily Contaminated with Heavy Metals. Water. Air. Soil Pollut. 2018, 229, 392. [Google Scholar] [CrossRef] [Green Version]
- Bech, J.; Duran, P.; Roca, N.; Poma, W.; Sánchez, I.; Roca-Pérez, L.; Boluda, R.; Barceló, J.; Poschenrieder, C. Accumulation of Pb and Zn in Bidens Triplinervia and Senecio Sp. Spontaneous Species from Mine Spoils in Peru and Their Potential Use in Phytoremediation. J. Geochem. Explor. 2012, 123, 109–113. [Google Scholar] [CrossRef]
- Maiti, S.K. Ecorestoration of the Coalmine Degraded Lands; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 9788578110796. [Google Scholar]
- Prasad, M.N.V.; de Campos Favas, P.J.; Maiti, S.K. Fly Ash and Lime-Stabilized Biosolid Mixtures in Mine Spoil Reclamation. In Bio-Geotechnologies for Mine Site Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 159–180. [Google Scholar]
- Pandey, V.C.; Singh, N. Impact of Fly Ash Incorporation in Soil Systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Mossa, A.W.; Bailey, E.H.; Usman, A.; Young, S.D.; Crout, N.M.J. The Impact of Long-Term Biosolids Application (>100 Years) on Soil Metal Dynamics. Sci. Total Environ. 2020, 720, 137441. [Google Scholar] [CrossRef]
- Adnan, M.; Shah, Z.; Sharif, M.; Rahman, H. Liming Induces Carbon Dioxide (CO2) Emission in PSB Inoculated Alkaline Soil Supplemented with Different Phosphorus Sources. Environ. Sci. Pollut. Res. 2018, 25, 9501–9509. [Google Scholar] [CrossRef]
- Qu, J.; Tian, X.; Zhang, X.; Yao, J.; Xue, J.; Li, K.; Zhang, B.; Wang, L.; Zhang, Y. Free Radicals-Triggered Reductive and Oxidative Degradation of Highly Chlorinated Compounds via Regulation of Heat-Activated Persulfate by Low-Molecular-Weight Organic Acids. Appl. Catal. B Environ. 2022, 310, 121359. [Google Scholar] [CrossRef]
- Narayanan, M.; Ma, Y. Influences of Biochar on Bioremediation/Phytoremediation Potential of Metal-Contaminated Soils. Front. Microbiol. 2022, 13, 929730. [Google Scholar] [CrossRef]
- Wang, Y.Y.; You, L.C.; Lyu, H.H.; Liu, Y.X.; He, L.L.; Hu, Y.D.; Luo, F.C.; Yang, S.M. Role of Biochar–Mineral Composite Amendment on the Immobilization of Heavy Metals for Brassica Chinensis from Naturally Contaminated Soil. Environ. Technol. Innov. 2022, 28, 102622. [Google Scholar] [CrossRef]
- Shentu, J.; Li, X.; Han, R.; Chen, Q.; Shen, D.; Qi, S. Effect of Site Hydrological Conditions and Soil Aggregate Sizes on the Stabilization of Heavy Metals (Cu, Ni, Pb, Zn) by Biochar. Sci. Total Environ. 2022, 802, 149949. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, B.; Tong, H.; Liu, Y.; Wang, S.; Wei, S.; Wang, L.; Wang, Y.; Zhang, Y. High-Efficiency Decontamination of Pb ( II ) and Tetracycline in Contaminated Water Using Ball-Milled Magnetic Bone Derived Biochar. J. Clean. Prod. 2023, 385, 135683. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar Systems. In Biochar for Environmental Management; Routledge: London, UK, 2009. [Google Scholar]
- Qu, J.; Xu, Y.; Zhang, X.; Sun, M.; Tao, Y.; Zhang, X.; Zhang, G.; Ge, C.; Zhang, Y. Ball Milling-Assisted Preparation of N-Doped Biochar Loaded with Ferrous Sulfide as Persulfate Activator for Phenol Degradation: Multiple Active Sites-Triggered Radical/Non-Radical Mechanism. Appl. Catal. B Environ. 2022, 316, 121639. [Google Scholar] [CrossRef]
- Wu, W.X.; Yang, M.; Feng, Q.B.; McGrouther, K.; Wang, H.L.; Lu, H.H.; Chen, Y.X. Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Kane, S.; Ryan, C. Biochar from Food Waste as a Sustainable Replacement for Carbon Black in Upcycled or Compostable Composites. Compos. Part C Open Access 2022, 8, 100274. [Google Scholar] [CrossRef]
- Papageorgiou, A.; Azzi, E.S.; Enell, A.; Sundberg, C. Biochar Produced from Wood Waste for Soil Remediation in Sweden: Carbon Sequestration and Other Environmental Impacts. Sci. Total Environ. 2021, 776, 145953. [Google Scholar] [CrossRef]
- Ng, L.Y.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Farid, M.A.A.; Hassan, M.A. High-Energy Ball Milling for High Productivity of Nanobiochar from Oil Palm Biomass. Nanomaterials 2022, 12, 3251. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Qiao, J.; Ai, J.; Ning, S.; Sun, H.; Chen, M.; Zhao, L.; Zhang, G.; Lian, F. Systematic Research on the Transport of Ball-Milled Biochar in Saturated Porous Media: Effect of Humic Acid, Ionic Strength, and Cation Types. Nanomaterials 2022, 12, 988. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of Biochar on Chemical Properties of Acidic Soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Ogura, T.; Date, Y.; Masukujane, M.; Coetzee, T.; Akashi, K.; Kikuchi, J. Improvement of Physical, Chemical, and Biological Properties of Aridisol from Botswana by the Incorporation of Torrefied Biomass. Sci. Rep. 2016, 6, 28011. [Google Scholar] [CrossRef] [Green Version]
- Turan, V. Calcite in Combination with Olive Pulp Biochar Reduces Ni Mobility in Soil and Its Distribution in Chili Plant. Int. J. Phytoremed. 2022, 24, 166–176. [Google Scholar] [CrossRef]
- Turan, V. Arbuscular Mycorrhizal Fungi and Pistachio Husk Biochar Combination Reduces Ni Distribution in Mungbean Plant and Improves Plant Antioxidants and Soil Enzymes. Physiol. Pantarum 2021, 173, 418–429. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar Composites: Emerging Trends, Field Successes and Sustainability Implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of Biochar and Biochar Composites and Their Application in a Fenton-like Process for Wastewater Decontamination: A Review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Sun, L.; Gong, P.; Sun, Y.; Qin, Q.; Song, K.; Ye, J.; Zhang, H.; Zhou, B.; Xue, Y. Modified Chicken Manure Biochar Enhanced the Adsorption for Cd2+ in Aqueous and Immobilization of Cd in Contaminated Agricultural Soil. Sci. Total Environ. 2022, 851, 158252. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Bhattacharya, J. Potassium-Iron Rice Straw Biochar Composite for Sorption of Nitrate, Phosphate, and Ammonium Ions in Soil for Timely and Controlled Release. Sci. Total Environ. 2020, 712, 136337. [Google Scholar] [CrossRef]
- He, X.; Nkoh, J.N.; Shi, R.Y.; Xu, R.k. Application of Chitosan- and Alginate-Modified Biochars in Promoting the Resistance to Paddy Soil Acidification and Immobilization of Soil Cadmium. Environ. Pollut. 2022, 313, 120175. [Google Scholar] [CrossRef]
- Li, A.; Xie, H.; Qiu, Y.; Liu, L.; Lu, T.; Wang, W.; Qiu, G. Resource Utilization of Rice Husk Biomass: Preparation of MgO Flake-Modified Biochar for Simultaneous Removal of Heavy Metals from Aqueous Solution and Polluted Soil. Environ. Pollut. 2022, 310, 119869. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, W.; Liu, F.; Wang, G.; Wan, J.; Zhang, W.; Peng, C.; Yang, J. Simultaneous Immobilization of Arsenic, Lead and Cadmium by Magnesium-Aluminum Modified Biochar in Mining Soil. J. Environ. Manag. 2022, 310, 114792. [Google Scholar] [CrossRef]
- Gholami, L.; Rahimi, G. Efficiency of CH4N2S− Modified Biochar Derived from Potato Peel on the Adsorption of Cd, Zn, and Cu in the Contaminated Soil.Pdf. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100468. [Google Scholar] [CrossRef]
- Yang, X.; Pan, H.; Shaheen, S.M.; Wang, H.; Rinklebe, J. Immobilization of Cadmium and Lead Using Phosphorus-Rich Animal-Derived and Iron-Modified Plant-Derived Biochars under Dynamic Redox Conditions in a Paddy Soil. Environ. Int. 2021, 156, 106628. [Google Scholar] [CrossRef]
- Song, P.; Ma, W.; Gao, X.; Ai, S.; Wang, J.; Liu, W. Remediation Mechanism of Cu, Zn, As, Cd, and Pb Contaminated Soil by Biochar-Supported Nanoscale Zero-Valent Iron and Its Impact on Soil Enzyme Activity. J. Clean. Prod. 2022, 378, 134510. [Google Scholar] [CrossRef]
- Wong, M. Ecological Restoration of Mine Degraded Soils, with Emphasis on Metal Contaminated Soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Johnson, C.D.; Skousen, J.G. Minesoil Properties of 15 Abandoned Mine Land Sites in West Virginia. J. Environ. Qual. 1995, 24, 635–643. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of Surface Coal Mining and Land Reclamation on Soil Properties: A Review. Earth-Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, S.K.; Masto, R.E. Use of Reclaimed Mine Soil Index (RMSI) for Screening of Tree Species for Reclamation of Coal Mine Degraded Land. Ecol. Eng. 2013, 57, 133–142. [Google Scholar] [CrossRef]
- Dejun, Y.; Zhengfu, B.; Shaogang, L. Impact on Soil Physical Qualities by the Subsidence of Coal Mining: A Case Study in Western China. Environ. Earth Sci. 2016, 75, 652. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the Influence of Soil Microbial Community on the Long-Term Heavy Metal Pollution of Different Land Use Types and Depth Layers in Mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.; Niu, S.; Li, X.; Wang, Y.; Bai, Z. Reclamation Promotes the Succession of the Soil and Vegetation in Opencast Coal Mine: A Case Study from Robinia Pseudoacacia Reclaimed Forests, Pingshuo Mine, China. Catena 2018, 165, 72–79. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Bierza, W.; Błońska, A.; Sierka, E.; Magurno, F.; Chmura, D.; Besenyei, L.; Radosz, Ł.; Woźniak, G. Vegetation Diversity on Coal Mine Spoil Heaps—How Important Is the Texture of the Soil Substrate? Biologia 2019, 74, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Zhang, Y.; Ruan, M.; Guo, J.; Chai, T. Land Subsidence in a Coal Mining Area Reduced Soil Fertility and Led to Soil Degradation in Arid and Semi-Arid Regions. Int. J. Environ. Res. Public Health 2019, 16, 3929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wang, J.; Li, B. Determining the Influence Factors of Soil Organic Carbon Stock in Opencast Coal-Mine Dumps Based on Complex Network Theory. Catena 2019, 173, 433–444. [Google Scholar] [CrossRef]
- Acosta, J.A.; Abbaspour, A.; Martínez, G.R.; Martínez-martínez, S.; Zornoza, R. Phytoremediation of Mine Tailings with Atriplex Halimus and Organic/Inorganic Amendments: A Fi ve-Year Fi Eld Case Study. Chemosphere 2018, 204, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, I.; Suryaningtyas, D.T.; Baskoro, D.P.T.; Budi, S.W.; Gozali, I.; Saridi, S.; Masyhuri, M.; Dultz, S. The Regulatory Role of Mine Soil Properties in the Growth of Revegetation Plants in the Post-Mine Landscape of East Kalimantan. Ecol. Indic. 2022, 139, 108877. [Google Scholar] [CrossRef]
- Yunanto, T.; Amanah, F.; Wulansari, A.R.; Wisnu, N.P. Effect of Soil Properties on Plant Growth and Diversity at Various Ages of Coal Mine Reclamation in Indonesia. Biodiversitas 2022, 23, 459–468. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Jing, M.; Wang, S.; Huang, Y.; Geng, B.; Cao, Y. Changes in Reconstructed Soil Physicochemical Properties in an Opencast Mine Dump in the Loess Plateau Area of China. Int. J. Environ. Res. Public Health 2022, 19, 706. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Bhattacharya, J.; Vanapalli, K.R.; Samal, B. Effect of the Co-Application of Eucalyptus Wood Biochar and Chemical Fertilizer for the Remediation of Multimetal (Cr, Zn, Ni, and Co) Contaminated Soil. Sustainabiblity 2022, 14, 7266. [Google Scholar] [CrossRef]
- Franklin, J.A.; Buckley, D.S. Effects of Seedling Size and Ground Cover on the First-Year Survival of Planted Pine and Hardwoods over an Extreme Drought. In Proceedings of the American Society of Mining and Reclamation, Bbillings, MT, USA, 30 May–5 June 2009; pp. 474–484. [Google Scholar]
- Helingerová, M.; Frouz, J.; Šantrůčková, H. Microbial Activity in Reclaimed and Unreclaimed Post-Mining Sites near Sokolov (Czech Republic). Ecol. Eng. 2010, 36, 768–776. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Sołek-Podwika, K.; Wieczorek, J. Enzyme Activity as an Indicator of Soil-Rehabilitation Processes at a Zinc and Lead Ore Mining and Processing Area. J. Environ. Manag. 2014, 132, 250–256. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a Potential Strategy for Remediation of Contaminated Mining Soils: Mechanisms, Applications, and Future Perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Guo, R.P.; Yue, Q.L.; Zhou, K.; Wu, Z.F. Environmental Quality Assessment and Spatial Pattern of Potentially Toxic Elements in Soils of Guangdong Province, China. Environ. Earth Sci. 2013, 70, 1903–1910. [Google Scholar] [CrossRef]
- Liu, X.; Bai, Z.; Shi, H.; Zhou, W.; Liu, X. Heavy Metal Pollution of Soils from Coal Mines in China. Nat. Hazards 2019, 99, 1163–1177. [Google Scholar] [CrossRef]
- Singh, M.; Ansari, A.A.; Müller, G.; Singh, I.B. Heavy Metals in Freshly Deposited Sediments of the Gomati River (a Tributary of the Ganga River) Effects of Human Activities. Environ. Geol. 1997, 29, 246–252. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Mahurpawar, M. Effects of Heavy Metals on Human Health. Int. J. Res.-GRANTHAALAYAH 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Bai, Z.; Zhou, W.; Liu, K.; Wang, M.; He, Y. Heavy Metal Concentrations of Soils near the Large Opencast Coal Mine Pits in China. Chemosphere 2020, 244, 125360. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Maiti, S.K. Evaluation of Toxic Metal(Loid)s Concentration in Soils around an Open-cast Coal Mine (Eastern India).Pdf. Environ. Earth Sci. 2019, 78, 645. [Google Scholar] [CrossRef]
- Chakraborty, B.; Bera, B.; Roy, S.; Adhikary, P.P.; Sengupta, D.; Shit, P.K. Assessment of Non-Carcinogenic Health Risk of Heavy Metal Pollution: Evidences from Coal Mining Region of Eastern India. Environ. Sci. Pollut. Res. 2021, 28, 47275–47293. [Google Scholar] [CrossRef]
- Yan, T.; Zhao, W.; Yu, X.; Li, H.; Gao, Z.; Ding, M.; Yue, J. Evaluating Heavy Metal Pollution and Potential Risk of Soil around a Coal Mining Region of Tai’an City, China. Alex. Eng. J. 2022, 61, 2156–2165. [Google Scholar] [CrossRef]
- Siddiqui, A.U.; Jain, M.K.; Masto, R.E. Pollution Evaluation, Spatial Distribution, and Source Apportionment of Trace Metals around Coal Mines Soil: The Case Study of Eastern India. Environ. Sci. Pollut. Res. 2020, 27, 10822–10834. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.A.; Chowdhury, A.I.H.; Mullick, M.R.A.; Hoque, A. Heavy Metal Pollution Status and Health Risk Assessment Vicinity to Barapukuria Coal Mine Area of Bangladesh. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100469. [Google Scholar] [CrossRef]
- Bu, Q.; Li, Q.; Zhang, H.; Cao, H.; Gong, W.; Zhang, X.; Ling, K.; Cao, Y. Concentrations, Spatial Distributions, and Sources of Heavy Metals in Surface Soils of the Coal Mining City Wuhai, China. J. Chem. 2020, 2020, 4705954. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An Untapped Plant Resource for the Phytoremediation of Heavy Metal Contaminated Soils. Chemosphere 2020, 246, 125750. [Google Scholar] [CrossRef] [PubMed]
- Eraknumen; Agbontalor, A. Phytoremediation: An Environmentally Sound Technology for Pollution Prevention, Control and Remediation in Developing Countries. Educ. Res. Rev. 2007, 2, 151–156. [Google Scholar]
- Kanwar, V.S.; Sharma, A.; Srivastav, A.L.; Rani, L. Phytoremediation of Toxic Metals Present in Soil and Water Environment: A Critical Review. Environ. Sci. Pollut. Res. 2020, 27, 44835–44860. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A Review on Phytoremediation of Heavy Metals and Utilization of It’s by Products. Asian J. Energy Environ. 2005, 6, 214–231. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; De La Rosa, G.; Parsons, J.G. Phytoremediation of Heavy Metals and Study of the Metal Coordination by X-Ray Absorption Spectroscopy. Coord. Chem. Rev. 2005, 249, 1797–1810. [Google Scholar] [CrossRef]
- Ullah, R.; Hadi, F.; Ahmad, S.; Jan, A.U.; Rongliang, Q. Phytoremediation of Lead and Chromium Contaminated Soil Improves with the Endogenous Phenolics and Proline Production in Parthenium, Cannabis, Euphorbia, and Rumex Species. Water. Air. Soil Pollut. 2019, 230, 40. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Wu, S.; Zhang, X.; Yang, C.; Xiong, X. Comparison of Heavy Metal Phytoremediation in Monoculture and Intercropping Systems of Phyllostachys Praecox and Sedum Plumbizincicola in Polluted Soil. Int. J. Phytoremed. 2018, 20, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Effects of Sophorolipids Augmentation on the Plant Growth and Phytoremediation of Heavy Metal Contaminated Soil. J. Clean. Prod. 2021, 280, 124406. [Google Scholar] [CrossRef]
- Azab, E.; Hegazy, A. Monitoring the Efficiency of Rhazya Stricta L. plants in phytoremediation of heavy metal-contaminated soil. Plants 2020, 9, 1057. [Google Scholar] [CrossRef]
- Shrestha, P.; Bellitürk, K.; Görres, J.H. Phytoremediation of Heavy Metal-Contaminated Soil by Switchgrass: A Comparative Study Utilizing Different Composts and Coir Fiber on Pollution Remediation, Plant Productivity, and Nutrient Leaching. Int. J. Environ. Res. Public Health 2019, 16, 1261. [Google Scholar] [CrossRef] [Green Version]
- Elbehiry, F.; Elbasiouny, H.; Ali, R.; Brevik, E.C. Enhanced Immobilization and Phytoremediation of Heavy Metals in Landfill Contaminated Soils. Water. Air. Soil Pollut. 2020, 231, 204. [Google Scholar] [CrossRef]
- Kang, W.; Bao, J.; Zheng, J.; Xu, F.; Wang, L. Phytoremediation of Heavy Metal Contaminated Soil Potential by Woody Plants on Tonglushan Ancient Copper Spoil Heap in China. Int. J. Phytoremed. 2018, 20, 1–7. [Google Scholar] [CrossRef]
- Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment; Intech: London, UK, 2020; pp. 225–240. [Google Scholar]
- Li, X.; Wang, X.; Chen, Y.; Yang, X.; Cui, Z. Optimization of Combined Phytoremediation for Heavy Metal Contaminated Mine Tailings by a Field-Scale Orthogonal Experiment. Ecotoxicol. Environ. Saf. 2019, 168, 1–8. [Google Scholar] [CrossRef]
- El Fantroussi, S.; Agathos, S.N. Is Bioaugmentation a Feasible Strategy for Pollutant Removal and Site Remediation? Curr. Opin. Microbiol. 2005, 8, 268–275. [Google Scholar] [CrossRef]
- Naik, M.G.; Duraphe, M.D. Review Paper on—Parameters Affecting Bioremediation. Adv. Res. Pharm. Biol. 2012, 2, 77–80. [Google Scholar]
- Omokhagbor Adams, G.; Tawari Fufeyin, P.; Eruke Okoro, S.; Ehinomen, I. Bioremediation, Biostimulation and Bioaugmention: A Review. Int. J. Environ. Bioremediation Biodegrad. 2020, 3, 28–39. [Google Scholar] [CrossRef]
- Ivask, A.; Dubourguier, H.C.; Põllumaa, L.; Kahru, A. Bioavailability of Cd in 110 Polluted Topsoils to Recombinant Bioluminescent Sensor Bacteria: Effect of Soil Particulate Matter. J. Soils Sediments 2011, 11, 231–237. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ossai, I.C.; Hamid, F.S. Bioaugmentation Assisted Mycoremediation of Heavy Metal and/Metalloid Landfill Contaminated Soil Using Consortia of Filamentous Fungi. Biochem. Eng. J. 2020, 157, 66–80. [Google Scholar] [CrossRef]
- Lebrazi, S.; Fikri-Benbrahim, K. Rhizobium-Legume Symbioses: Heavy Metal Effects and Principal Approaches for Bioremediation of Contaminated Soil. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 205–233. [Google Scholar]
- Li, X.; Dong, S.; Yao, Y.; Shi, W.; Wu, M.; Xu, H. Inoculation of Bacteria for the Bioremediation of Heavy Metals Contaminated Soil by Agrocybe Aegerita. RSC Adv. 2016, 6, 65816–65824. [Google Scholar] [CrossRef]
- Alsamhary, K. Vermi-Cyanobacterial Remediation of Cadmium-Contaminated Soil with Rice Husk Biochar: An Eco-Friendly Approach. Chemosphere 2023, 311, 136931. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fan, Y.; Xia, X.; Liu, Z.; Yang, S. Effect of Ginkgo Biloba Leaves on the Removal Efficiency of Cr(VI) in Soil and Its Underlying Mechanism. Environ. Res. 2022, 216, 114431. [Google Scholar] [CrossRef]
- Zha, F.; Chen, S.; Kang, B.; Xu, L.; Shen, Y.; Wang, R. Synergistic Solidification of Lead-Contaminated Soil by Magnesium Oxide and Microorganisms. Chemosphere 2022, 308, 136422. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, P.; Zhang, S.; Zhang, S.; Sun, Y. Contribution of Arbuscular Mycorrhizal Fungi and Soil Amendments to Remediation of a Heavy Metal-Contaminated Soil Using Sweet Sorghum. Pedosphere 2022, 32, 844–855. [Google Scholar] [CrossRef]
- He, N.; Hu, L.; Jiang, C.; Li, M. Remediation of Chromium, Zinc, Arsenic, Lead and Antimony Contaminated Acidic Mine Soil Based on Phanerochaete Chrysosporium Induced Phosphate Precipitation. Sci. Total Environ. 2022, 850, 157995. [Google Scholar] [CrossRef]
- Zuo, W.; Song, B.; Shi, Y.; Zupanic, A.; Guo, S.; Huang, H.; Jiang, L.; Yu, Y. Using Bacillus Thuringiensis HM-311@hydroxyapatite@biochar Beads to Remediate Pb and Cd Contaminated Farmland Soil. Chemosphere 2022, 307, 135797. [Google Scholar] [CrossRef]
- Li, M.; Yao, J.; Sunahara, G.; Hawari, J.; Duran, R.; Liu, J.; Liu, B.; Cao, Y.; Pang, W.; Li, H.; et al. Novel Microbial Consortia Facilitate Metalliferous Immobilization in Non-Ferrous Metal(Loid)s Contaminated Smelter Soil: Efficiency and Mechanisms. Environ. Pollut. 2022, 313, 120042. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2009; Volume 1, pp. 1–12. [Google Scholar]
- Laird, D. a Chapter 16 Pyrolysis and Biochar-Opportunities for Distributed Production and Soil Quality Enhancement. Production 2010, 257–281. [Google Scholar]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Lai, W.Y.; Lai, C.M.; Ke, G.R.; Chung, R.S.; Chen, C.T.; Cheng, C.H.; Pai, C.W.; Chen, S.Y.; Chen, C.C. The Effects of Woodchip Biochar Application on Crop Yield, Carbon Sequestration and Greenhouse Gas Emissions from Soils Planted with Rice or Leaf Beet. J. Taiwan Inst. Chem. Eng. 2013, 44, 1039–1044. [Google Scholar] [CrossRef]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The Effect of Paper Sludge and Biochar Addition on Brown Peat and Coir Based Growing Media Properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Chandra, S.; Bhattacharya, J. Influence of Temperature and Duration of Pyrolysis on the Property Heterogeneity of Rice Straw Biochar and Optimization of Pyrolysis Conditions for Its Application in Soils. J. Clean. Prod. 2019, 215, 1123–1139. [Google Scholar] [CrossRef]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent Trends in Biochar Production Methods and Its Application as a Soil Health Conditioner: A Review. SN Appl. Sci. 2020, 2, 1307. [Google Scholar] [CrossRef]
- Dai, L.; Fan, L.; Liu, Y.; Ruan, R.; Wang, Y.; Zhou, Y.; Zhao, Y.; Yu, Z. Production of Bio-Oil and Biochar from Soapstock via Microwave-Assisted Co-Catalytic Fast Pyrolysis. Bioresour. Technol. 2017, 225, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Microwave Pyrolysis of Lignocellulosic Biomass: Heating Performance and Reaction Kinetics. Energy 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of Functionalized Magnetic Biochar in Environmental Remediation: A Review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Liu, S.B.; Wang, X.; Liu, S.M.; Li, J. Biochar-Based Nano-Composites for the Decontamination of Wastewater: A Review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and Characterization of a Novel Magnetic Biochar for Arsenic Removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Tang, J.; Cui, M.; Gao, B.; Shen, B. Biochar/Iron (BC/Fe) Composites for Soil and Groundwater Remediation: Synthesis, Applications, and Mechanisms. Chemosphere 2020, 246, 125609. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Fan, J.; Xu, X.; Cao, X. Application of Iron-Biochar Composite in Topsoil for Simultaneous Remediation of Chromium-Contaminated Soil and Groundwater: Immobilization Mechanism and Long-Term Stability. J. Hazard. Mater. 2021, 405, 124226. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, L.; Huang, Y.; Song, Z.; Qiu, W. Effects of a Manganese Oxide-Modified Biochar Composite on Adsorption of Arsenic in Red Soil. J. Environ. Manag. 2015, 163, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Pap, S.; Bezanovic, V.; Radonic, J.; Babic, A.; Saric, S.; Adamovic, D.; Turk Sekulic, M. Synthesis of Highly-Efficient Functionalized Biochars from Fruit Industry Waste Biomass for the Removal of Chromium and Lead. J. Mol. Liq. 2018, 268, 315–325. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, N.; Xu, Z.; Hu, X.; Ding, Z. Integrated Comparisons of Thorium (IV) Adsorption onto Alkali-Treated Duckweed Biomass and Duckweed-Derived Hydrothermal and Pyrolytic Biochar. Environ. Sci. Pollut. Res. 2019, 26, 2523–2530. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Hung, C.M. Synthesis of Magnetic Biochar from Bamboo Biomass to Activate Persulfate for the Removal of Polycyclic Aromatic Hydrocarbons in Marine Sediments. Bioresour. Technol. 2017, 245, 188–195. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of Hexavalent Chromium from Aqueous Solutions by a Novel Biochar Supported Nanoscale Iron Sulfide Composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Imran-Shaukat, M.; Wahi, R.; Rosli, N.R.; Aziz, S.M.A.; Ngaini, Z. Chemically Modified Palm Kernel Shell Biochar for the Removal of Heavy Metals from Aqueous Solution. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012019. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(III) and As(V) by Fe Coated Biochars and Biochars Produced from Empty Fruit Bunch and Rice Husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent Advances in Engineered Biochar Productions and Applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Y.; Ge, W.; Zhang, Y.; Liu, L.; Qiu, G. Removal of Heavy Metals from Wastewaters with Biochar Pyrolyzed from MgAl-Layered Double Hydroxide-Coated Rice Husk: Mechanism and Application. Bioresour. Technol. 2022, 347, 126425. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Zhang, Z. A Versatile EDTA and Chitosan Bi-Functionalized Magnetic Bamboo Biochar for Simultaneous Removal of Methyl Orange and Heavy Metals from Complex Wastewater. Environ. Pollut. 2022, 293, 118517. [Google Scholar] [CrossRef]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Xu, H. Bioremediation of Cadmium Polluted Soil Using a Novel Cadmium Immobilizing Plant Growth Promotion Strain Bacillus Sp. TZ5 Loaded on Biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and Bacteria Inoculated Biochar Enhanced Cd and Cu Immobilization and Enzymatic Activity in a Polluted Soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Peng, B.; Tan, C.; Ma, L.Q. Effect of Biochar and Fe-Biochar on Cd and As Mobility and Transfer in Soil-Rice System. Chemosphere 2017, 186, 928–937. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, Y.; Zhang, X.; Wang, L.; Tao, Y.; Jiang, Z.; Yu, H.; Dong, M.; Zhang, Y. Stabilization of Lead and Cadmium in Soil by Sulfur-Iron Functionalized Biochar: Performance, Mechanisms and Microbial Community Evolution. J. Hazard. Mater. 2022, 425, 127876. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.R.d.S.; de Moraes Silva, W.; de Medeiros, E.V.; Duda, G.P.; Corrêa, M.M.; Martins Filho, A.P.; Clermont-Dauphin, C.; Antonino, A.C.D.; Hammecker, C. Effect of Biochar on Physicochemical Properties of a Sandy Soil and Maize Growth in a Greenhouse Experiment. Geoderma 2018, 319, 14–23. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental Application of Biochar: Current Status and Perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent Advances in Utilization of Biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Yuan, Y.; Bolan, N.; Prévoteau, A.; Vithanage, M.; Biswas, J.K.; Ok, Y.S.; Wang, H. Applications of Biochar in Redox-Mediated Reactions. Bioresour. Technol. 2017, 246, 271–281. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.H.; Bai, Y.; Hou, D. Progress and Future Prospects in Biochar Composites: Application and Reflection in the Soil Environment. Crit. Rev. Environ. Sci. Technol. 2021, 51, 219–271. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; Jiang, C.; Jiang, X.; Guan, Y. Simultaneous and Efficient Capture of Inorganic Nitrogen and Heavy Metals by Polyporous Layered Double Hydroxide and Biochar Composite for Agricultural Nonpoint Pollution Control. ACS Appl. Mater. Interfaces 2018, 49, 43013–43030. [Google Scholar] [CrossRef]
- Chen, L.; Long, X.; Hui, C.; Min, H.; Fu, S. Environmental-Friendly Montmorillonite-Biochar Composites: Facile Production and Tunable Adsorption-Release of Ammonium and Phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Yuan, R.; Wang, P. Biomass Pyrolysis with Alkaline-Earth-Metal Additive for Co-Production of Bio-Oil and Biochar-Based Soil Amendment. Sci. Total Environ. 2020, 743, 140760. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The Effects of Biochar Addition on Soil Physicochemical Properties: A Review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.A.; Zheng, X.; Chen, C. Leaching Behavior of Heavy Metals and Transformation of Their Speciation in Polluted Soil Receiving Simulated Acid Rain. PLoS One 2012, 7, e49664. [Google Scholar] [CrossRef] [Green Version]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation Techniques for Removal of Heavy Metals from the Soil Contaminated through Different Sources: A Review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Land, M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Farraji, H.; Zaman, N.Q.; Tajuddin, R.M.; Faraji, H. Advantages and Disadvantages of Phytoremediation: A Concise Review. Int. J. Env. Tech. Sci. 2016, 2, 69–75. [Google Scholar]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Tan, X.; Hu, X. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of Metal Sorption by Biochars: Biochar Characteristics and Modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Medha, I.; Chandra, S.; Raja, V.K.; Samal, B.; Bhattacharya, J.; Das, B.K. (3-Aminopropyl)Triethoxysilane and Iron Rice Straw Biochar Composites for the Sorption of Cr (VI) and Zn (II) Using the Extract of Heavy Metals Contaminated Soil. Sci. Total Environ. 2021, 771, 144764. [Google Scholar] [CrossRef]
- Qiao, J.T.; Liu, T.X.; Wang, X.Q.; Li, F.B.; Lv, Y.H.; Cui, J.H.; Zeng, X.D.; Yuan, Y.Z.; Liu, C.P. Simultaneous Alleviation of Cadmium and Arsenic Accumulation in Rice by Applying Zero-Valent Iron and Biochar to Contaminated Paddy Soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, X.; Xiong, T.; Jiang, L.; Wang, H.; Wu, Z. Biochar Facilitated Hydroxyapatite/Calcium Silicate Hydrate for Remediation of Heavy Metals Contaminated Soils. Water. Air. Soil Pollut. 2020, 231, 66. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Xu, H.; Zhang, Y.; Hu, T.; Chen, W.; Hu, H.; Wu, J.; Li, Y.; Jiang, G. A Magnetic Macro-Porous Biochar Sphere as Vehicle for the Activation and Removal of Heavy Metals from Contaminated Agricultural Soil. Chem. Eng. J. 2020, 390, 124638. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Shao, J.; Chen, Y.; Zhang, S.; Chen, H. Multifunctional Carboxymethyl Cellulose Sodium Encapsulated Phosphorus-Enriched Biochar Composites: Multistage Adsorption of Heavy Metals and Controllable Release of Soil Fertilization. Chem. Eng. J. 2023, 453, 139809. [Google Scholar] [CrossRef]
- Sajjadi, B.; Shrestha, R.M.; Chen, W.Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Double-Layer Magnetized/Functionalized Biochar Composite: Role of Microporous Structure for Heavy Metal Removals. J. Water Process Eng. 2021, 39, 101677. [Google Scholar] [CrossRef]
- Qu, J.; Wei, S.; Liu, Y.; Zhang, X.; Jiang, Z.; Tao, Y.; Zhang, G.; Zhang, B.; Wang, L.; Zhang, Y. Effective Lead Passivation in Soil by Bone Char/CMC-Stabilized FeS Composite Loading with Phosphate-Solubilizing Bacteria. J. Hazard. Mater. 2022, 423, 127043. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of Heavy Metals from Soil with Biochar Composite: A Critical Review of the Mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb, and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Chen, S.; Wang, R.; Xu, P.; Cheng, M.; Zhang, C.; Xue, W. Biochar Facilitated the Phytoremediation of Cadmium Contaminated Sediments: Metal Behavior, Plant Toxicity, and Microbial Activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef]
- Rathika, R.; Srinivasan, P.; Alkahtani, J.; Al-Humaid, L.A.; Alwahibi, M.S.; Mythili, R.; Selvankumar, T. Influence of Biochar and EDTA on Enhanced Phytoremediation of Lead Contaminated Soil by Brassica Juncea. Chemosphere 2021, 271, 129513. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of Phytoremediation and Biochar to Remediate Heavy Metal Polluted Soils: A Review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Toxicity of Metals and Arsenic in a Naturally Contaminated Mine Soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef]
- Zhan, F.; Zeng, W.; Yuan, X.; Li, B.; Li, T.; Zu, Y.; Jiang, M.; Li, Y. Field Experiment on the Effects of Sepiolite and Biochar on the Remediation of Cd- and Pb-Polluted Farmlands around a Pb–Zn Mine in Yunnan Province, China. Environ. Sci. Pollut. Res. 2019, 26, 7743–7751. [Google Scholar] [CrossRef]
- Silva Gonzaga, M.I.; de Almeida Silva Matias, M.I.; Andrade, K.R.; de Jesus, A.N.; Cunha, G.D.C.; da Costa Cunha, G.; de Andrade, R.S.; de Jesus Santos, J.S. Aged Biochar Changed Copper Availability and Distribution among Soil Fractions and Influenced Corn Seed Germination in a Copper-Contaminated Soil. Chemosphere 2020, 240, 124828. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Khan, S.; Khan, M.A.; Alam, M.; Ayaz, T. Biochar Reduced the Uptake of Toxic Heavy Metals and Their Associated Health Risk via Rice (Oryza Sativa L.) Grown in Cr-Mn Mine Contaminated Soils. Environ. Technol. Innov. 2020, 17, 100590. [Google Scholar] [CrossRef]
- Meier, S.; Curaqueo, G.; Khan, N.; Bolan, N.; Rilling, J.; Vidal, C.; Fernández, N.; Acuña, J.; González, M.E.; Cornejo, P.; et al. Effects of Biochar on Copper Immobilization and Soil Microbial Communities in a Metal-Contaminated Soil. J. Soils Sediments 2017, 17, 1237–1250. [Google Scholar] [CrossRef]
- Abdin, Y.; Usman, A.; Ok, Y.S.; Tsang, Y.F.; Al-Wabel, M. Competitive Sorption and Availability of Coexisting Heavy Metals in Mining-Contaminated Soil: Contrasting Effects of Mesquite and Fishbone Biochars. Environ. Res. 2020, 181, 108846. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Wu, X.; Sun, Y.; Huang, Z.; Li, H.; Wu, Z.; Zhang, X.; Qin, X.; Zhang, Y.; et al. Effect of Physicochemical Properties of Biochar from Different Feedstock on Remediation of Heavy Metal Contaminated Soil in Mining Area. Surf. Interfaces 2022, 32, 102058. [Google Scholar] [CrossRef]



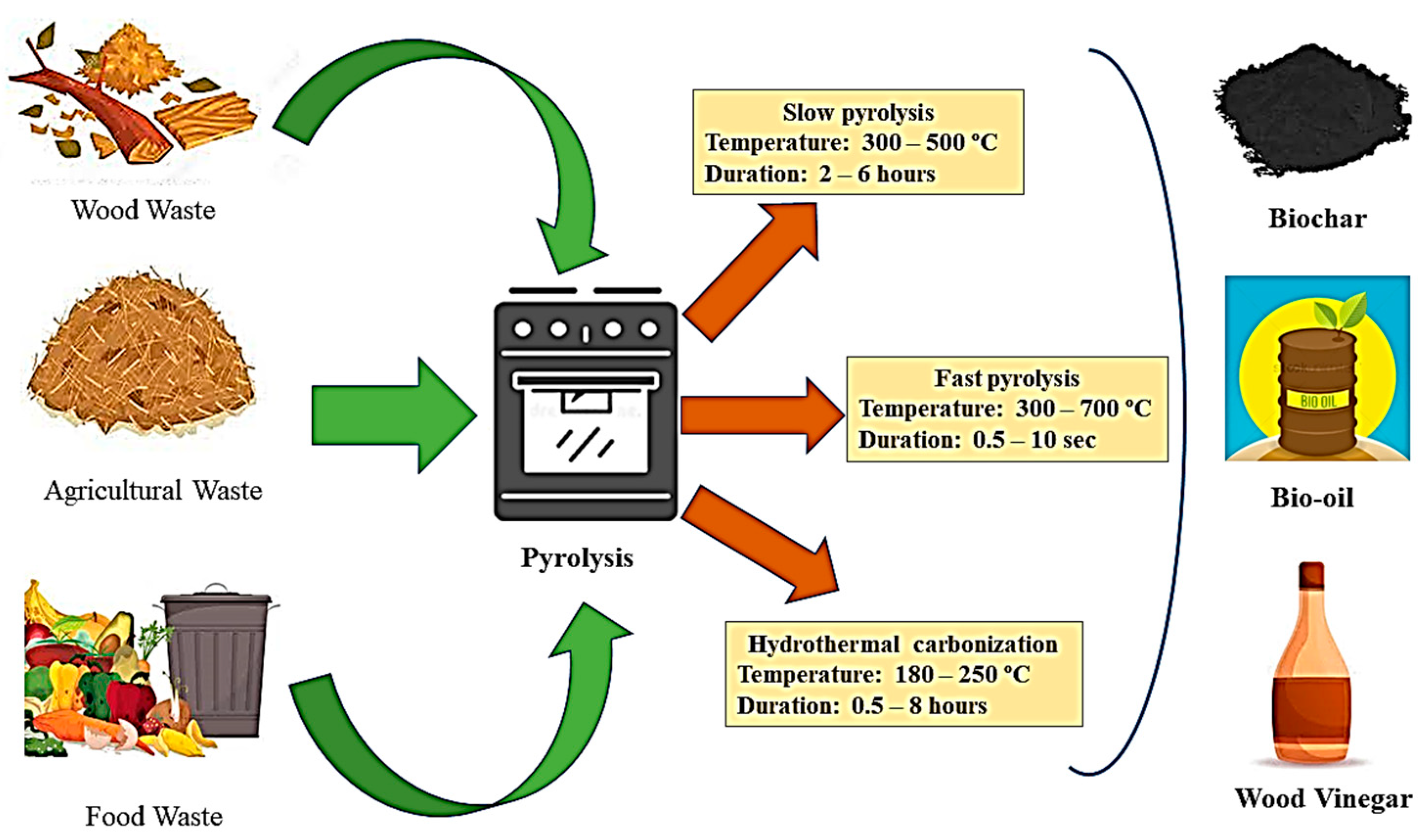


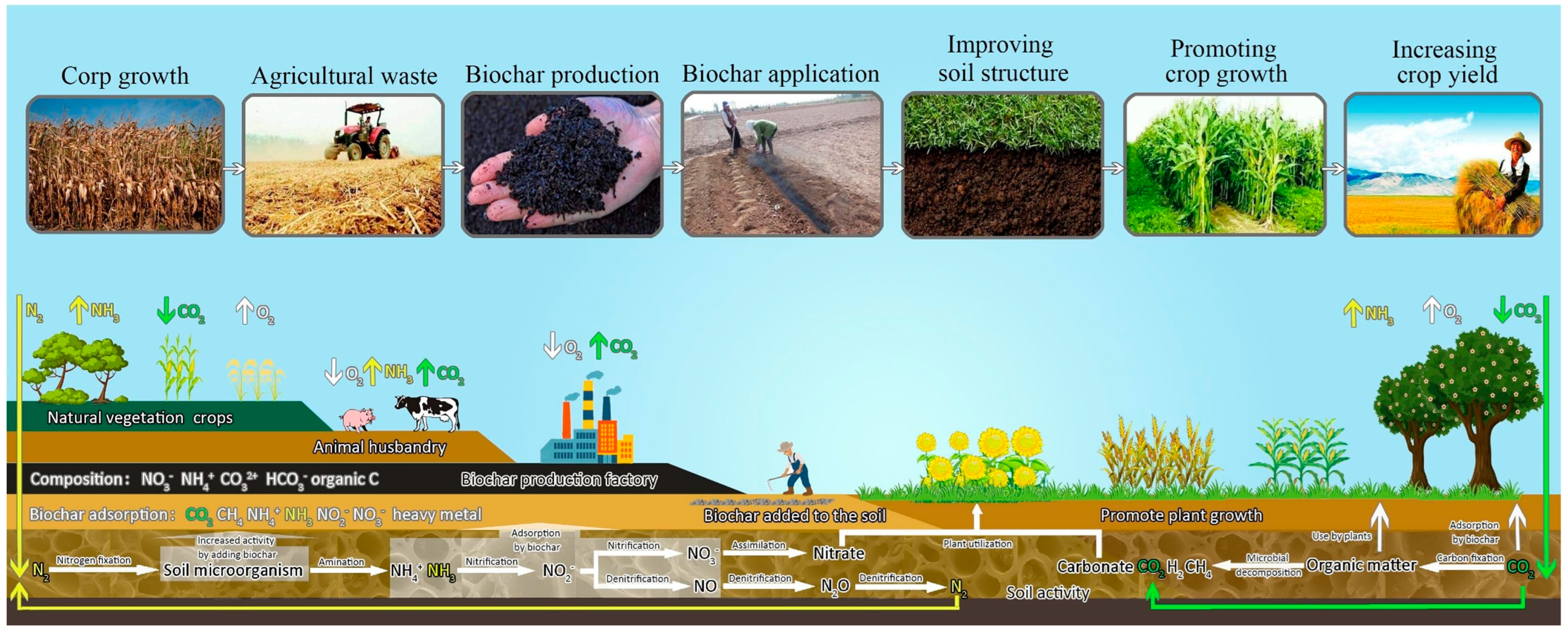
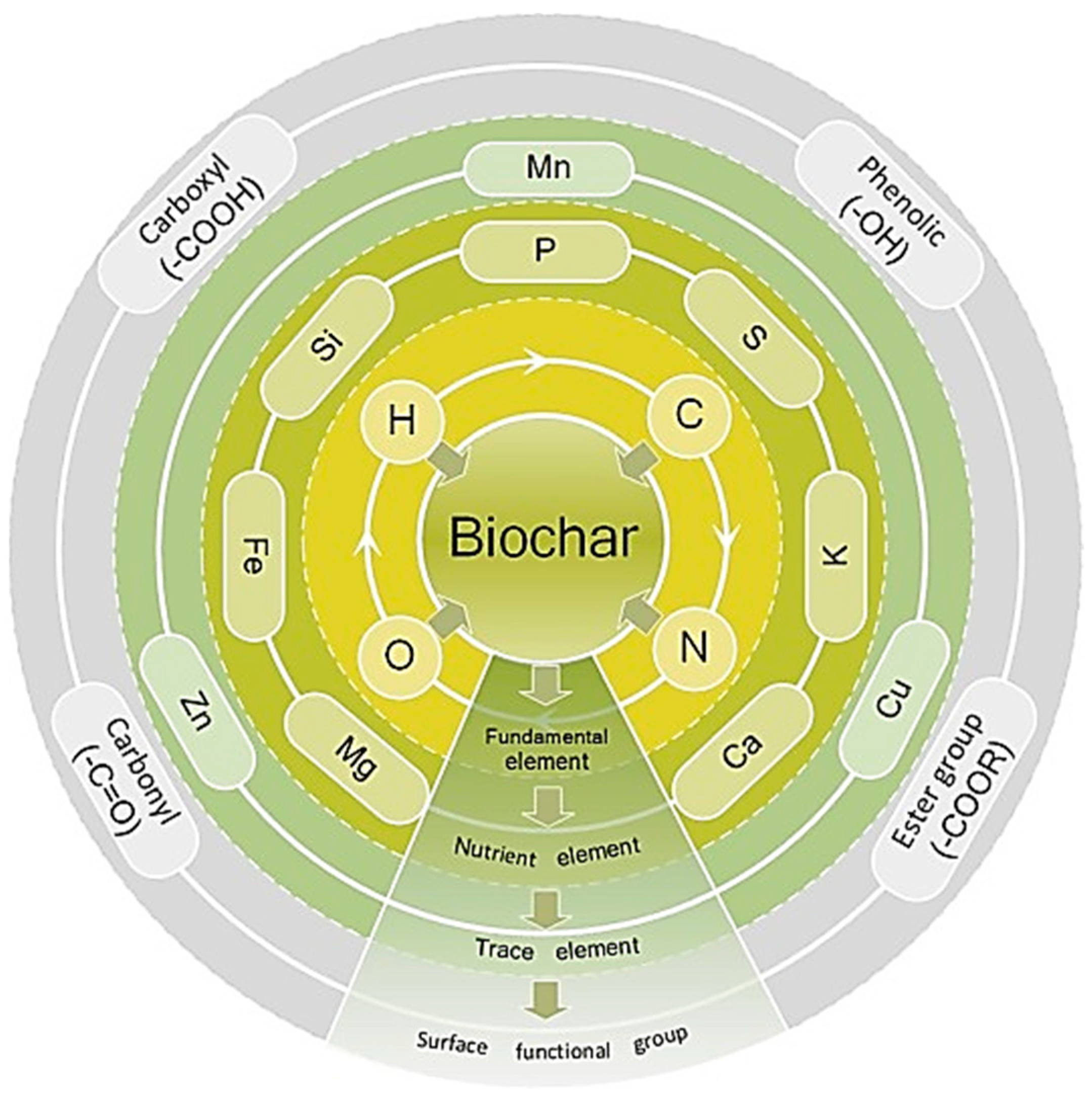


| S. No. | Study Area | Texture Class | Bulk Density (Mg/m3) | Moisture Content % | pH | Organic Matter (%) | Available N (mg kg−1) | Available P (mg kg−1) | Exchangeble K (mg kg−1) | CEC (cmol/100 g Soil) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Viswakarma opencast mines Jharia coal field | Sandy loam | 1.16 | 6.56 | 6.53 | 1.67 | 82.07 | 8.76 | 26.98 | 14.88 | [46] |
| 2 | Daliuta Mining area, Shanxi Province, Northwest China | Sandy loam | 1.60 | 9.07 | 5.67 | 3.06 | - | - | - | - | [47] |
| 3 | Mining city, Anhui Province, China | - | 1.37 | - | 7.92 | 2.15 | 0.72 | 18.96 | 53.82 | - | [48] |
| 4 | Pingshuo mine, China | - | 1.62 | - | 8.70 | 1.78 | 20 | 6.25 | 52 | - | [49] |
| 5 | Silesian Upland in Southern Poland | - | - | 5.70 | - | - | 14.90 | 212.10 | - | [50] | |
| 6 | East-central Ningxia Province, Northwest China | - | - | 7.58 | 8.04 | 3.20 | 3.24 | 5.80 | 158.70 | - | [51] |
| 7 | Antaibao opencast coal mine, Pingshuo, Shanxi Province | Sandy loam | 1.35 | 6.45 | 8.00 | - | - | 4.91 | 151.30 | - | [52] |
| 8 | Cartagena-La Union mining district, SE Spain | - | - | - | 7.40 | 1.64 | - | - | - | - | [53] |
| 9 | Latiand Sambarata open coal mine sites, Berau Regency, East KalimantanProvince | - | 1.65 | - | 4.50 | 0.87 | - | 2.40 | 103 | - | [54] |
| 10 | Mahakam Sumber Jaya open-pit mines, East Kalimantan Province, Indonesia | Silty loam | 1.83 | 19.09 | 3.27 | 1.42 | - | 5.02 | 296.40 | 14.69 | [55] |
| 11 | Pingshuo Mine, China | Sandy loam | 1.42 | - | 8.12 | 1.04 | - | 4.73 | 82.63 | - | [56] |
| 12 | Bastacolla, Jharia Coalfield, Dhanbad, Jharkhand, India | Sandy loam | - | 13.18 | 5.86 | 1.03 | 194.05 | 0.70 | 95.88 | 6.16 | [57] |
| Class | Igeo Values | Pollution Level in the Soil |
|---|---|---|
| 0 | <0 | Practically uncontaminated |
| 1 | 0–1 | Uncontaminated to moderately contaminated |
| 2 | 1–2 | Moderately contaminated |
| 3 | 2–3 | Moderately to heavily contaminated |
| 4 | 3–4 | Heavily contaminated |
| 5 | 4–5 | Heavily to extremely contaminated |
| 6 | >5 | Extremely contaminated |
| S. No. | Heavy Metal | Target Organs | Health Impacts |
|---|---|---|---|
| 1 | Arsenic (As) | Pulmonary, Nervous System, Skin | Perforation of Nasal Septum, Respiratory Cancer, Peripheral Neuropathy, Dermatomes, Skin Cancer |
| 2 | Cadmium (Cd) | Renal, Skeletal, Pulmonary | Proteinuria, Glucosuria, Osteomalacia, Aminoaciduria, Emphysema |
| 3 | Chromium (Cr) | Pulmonary | Ulcer, Perforation of Nasal Septum, Respiratory Cancer |
| 4 | Manganese (Mn) | Nervous System | Central And Peripheral Neuropathies |
| 5 | Lead (Pb) | Nervous System, Hematopoietic System, Renal | Encephalopathy, Peripheral Neuropathy, Central Nervous Disorders, Anemia |
| 6 | Nickel (Ni) | Pulmonary, Skin | Cancer, Dermatitis |
| 7 | Tin (Sn) | Nervous System, Pulmonary | Central Nervous System Disorders, Visual Defects, EEG Changes, Pneumoconiosis |
| S. No. | Study Area | Heavy Metals (mg/kg) | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Zn | Cr | Ni | Cd | |||
| 1 | Shizishan Mining Area | 79 | 67 | 139 | N.A. | 33 | 0.71 | [48] |
| 2 | All Opencast Coal Mining Pits, China | 28.60 | 28.26 | 77.94 | 63.86 | 27.91 | 0.24 | [66] |
| 3 | Lakhra Coalfield, Province of Sindh, Pakistan | n. a. | 2.38 | n. a. | n. a. | n. a. | 3.46 | [66] |
| 4 | Rohini Opencast Mining Area, North Karnpura, Jharkhand, India | n. a. | 6.57 | n. a. | 15.29 | n. a. | 1.40 | [67] |
| 5 | Raniganj Coal Mining, West Bengal, India | 677 | 256.6 | 893.7 | 851.7 | 811.4 | n. a. | [68] |
| 6 | Coal Mining Region, Tai’an City, Shandong Province, China | 26.55 | 27.62 | 66.68 | 20.66 | 29.61 | 0.20 | [69] |
| 7 | Jharia Coalfield, Jharkhand, India | 11.36 | 11.43 | 19.90 | 23.40 | 11.38 | 0.80 | [70] |
| 8 | Barapukaria Mining Area, Bangladesh | 31.66 | n. a. | 101.96 | 82.37 | 56.54 | n. a. | [71] |
| 9 | Wuhai Coal Mining Area, China | 19.60 | 28 | 55.20 | 61.70 | 24.70 | 0.16 | [72] |
| S. No. | Type of Contamination | Plant Species Used | Initial Concentration (mg kg−1) | BCFshoot | Type of Study | Reference |
|---|---|---|---|---|---|---|
| 1 | Pb and Cr contaminated soil | Parthenium hysterophorus Cannabis sativa | Pb = 14.54 Cr = 4.48 | Parthenium (Pb) = 1.01 (Cr) = 0.58 Cannabis (Pb) = 1.03 (Cr) = 0.54 | Field study | [79] |
| 2 | Cu, Zn, and Cd contaminated soil | Phyllostachys praecox | Cu = 195 Zn = 2980 Cd = 14.5 | Cu = 0.18 Zn = 0.33 Cd = 0.26 | Field study | [80] |
| 3 | Cd contaminated soil | Medicago sativa Bidens Pilosa | Cd = 0.031 | 2.90 | Pot-culture experiment (40 days) | [81] |
| 4 | Multi metals contaminated soil | Rhazya stricta | Cd = 50 Pb = 10 Cu = 10 Zn = 10 | Cd = 1.48 Pb = 0.36 Cu = 0.52 Zn = 1.46 | Pot-culture experiment (3 months) | [82] |
| 5 | Multi metals contaminated soil | Switchgrass | Zn = 68.1 Cr = 35.9 Pb = 16.9 Ni = 27 | - | Pot-culture experiment | [83] |
| 6 | Multi metals contaminated soil | Wheat Crop | Cr = 70.2 Cu = 339 Zn = 202 Pb = 156 Ni = 113 | Cr = 8.2 Cu = 2.2 Zn = 2.6 Pb = 2.8 Ni = 2 | Pot-culture experiment (10 weeks) | [84] |
| 7 | Multi metals contaminated soil | Robina pseudoacacia | Cu = 3166.7 Cd = 3.66 Pb = 137.06 | Cu = 0.044 Pb = 0.170 Cd = 1.358 | Field study | [85] |
| 8 | Anshan Mining, Lioning Province, China | Soybean | Zn = 149.5 Pb = 42.1 Cd = 0.8 | - | Field study (105 days) | [86] |
| S. No. | Type of Microbes Used | Site Description | Heavy Metals Remediated | Reference |
|---|---|---|---|---|
| 1 | M. Circinelloides T. asperellum Mortierella sp. | Metal-contaminated mine tailings, Anshan Mining Group, Lioning Province, China | Zn, Cu, Pb, and Cd | [86] |
| 2 | Bacillus Idriensis strains B. subtilis BR151 | Neighborhood of Lead and Zinc smelters, Northern France | Cd | [91] |
| 3 | Perenniporia subtephropora Cerrena aurantiopora Aspergillus niger MH541017 Aspergillus fumigatus | MSW Landfill, Jinjang Utara, Kuala Lumpur | Ni, Pb, and Zn | [92] |
| 4 | Rhizobium -legume | Heavy metal-contaminated soil | Cd, Cu, and Pb | [93] |
| 5 | Agrocybe Aegerita | Artificially contaminated soil | Ni and Cd | [94] |
| 6 | Eisenia fetida Cylindrospermum stagnale | Artificially contaminated soil | Cd | [95] |
| 7 | Agrococcus Streptomyces Microbacterium | Chromium-contaminated site in Henan Province | Cr (VI) | [96] |
| 8 | Sporosarcina pasteurii | Artificially contaminated soil | Pb | [97] |
| 9 | Acaulospora mellea | Vicinity of a Pb and Zn smelter located in Dongtang Town, Shaoguan City, Guangdong Province, China | Pb and Zn | [98] |
| 10 | Phanerochaete chrysosporium | Abandoned mining area in Hechi City, Guangxi Province, China | As, Cd, Cr, V, Sn, and Zn | [99] |
| 11 | Bacillus thuringiensis HM—311 | Contaminated farmland soil in Jiangning District, Nanjing, Jiangsu Province | Pb and Cd | [100] |
| 12 | SRB 1 (Clostridium, Desulfosporosinus, and Desulfovibrio genera) | Huilong nonferrous metal smelter, Guangxi Province, China | As | [101] |
| S. No. | Feedstock Used | Pyrolysis Conditions | Mine Soil Type | Heavy Metals Present | Plant Species Used | Inferences | References |
|---|---|---|---|---|---|---|---|
| 1 | Orchard pruning residues | Slow pyrolysis at 500 °C | La Mina Monaca Mine site, Spain | Cd, Cu, Pb, As, and Zn | Zea Mays | Biochar amendment significantly reduced the mobility of heavy metals in the aqueous soil medium | [163] |
| 2 | Rice straw | n. a. | Lanping Pb–Zn mines, Yunnan province, China | Cd and Pb | Maize | Biochar application decreased the availability of both total and plant-available contents of Pb and Cd in the soil | [164] |
| 3 | Sewage sludge | Slow pyrolysis at 500 °C | Contaminated soil, Sergipe experimental station, Northeast Brazil | Cu | Corn seeds | Biochar was applied at the rates of 30 t ha−1 and 60 t ha−1 reduced the exchangeable fraction of Cu by 96.2 and 57.5%, respectively | [165] |
| 4 | Hardwood waste | Slow pyrolysis at 500 °C for one hour | Degraded mined-out land | Cr, Cu, Zn, Pb, and Mn | Oryza sativa | Biochar applied at a rate of 3% (w/w) reduced the available contents of Cr, Cu, Zn, Pb, and Mn by 99.1, 71.7, 61.7, 36.4, and 47.9%, respectively | [166] |
| 5 | Chicken manure and Oat hull | Slow pyrolysis at 500 °C and 300 °C, respectively | Ventanas Cu smelter, National Copper Corporation of Chile (CODELCO), Puchuncaví, Valley of Central Chile | Cu | Oenothera picensis | Applications of biochars at the rates of 1 and 5% reduced the exchangeable and carbonate-bounded fractions of Cu in the contaminated soil | [167] |
| 6 | Eucalyptus wood | Slow pyrolysis at 400 and 600 °C for 150 min | Coal mine, Bastacolla area, Dhanbad, Jharkhand, India | Cr, Zn, Ni, and Co | Accacia Auriculiformis | Application of different temperatures biochar applied at the rates of 0.5, 1, 2, 3, and 5% (w/w) significantly reduced the concentrations of total and exchangeable heavy metals in the soil | [57] |
| 7 | Fishbone | Slow pyrolysis at 600 °C | Polluted soil located in an area near a gold mine in the governorate of Mahd ad- Dahab, Saudi Arabia | Pb, Cu, Zn, and Cd | Yecora Rojo | Fishbine biochar application at a rate of 30 g kg−1 reduced the mobility of Pb, Cu, Zn, and Cd by 43.0, 66.2, 55.6, and 33.8%, respectively | [168] |
| 8 | Rice straw Coconut shell Sludge | Slow pyrolysis at 500 °C | Mount Manao abandoned lead-zinc mine areas in Chenzhou city, Hunan Province, China | Cd, Cu, Pb, and Zn | - | Application of rice straw, coconut shell, and sludge-derived biochar at the rates of 0.5, 2.5, and 5% improved the soil’s physicochemical properties and reduced the mobility of heavy metals | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, S.; Medha, I.; Tiwari, A.K. The Role of Modified Biochar for the Remediation of Coal Mining-Impacted Contaminated Soil: A Review. Sustainability 2023, 15, 3973. https://doi.org/10.3390/su15053973
Chandra S, Medha I, Tiwari AK. The Role of Modified Biochar for the Remediation of Coal Mining-Impacted Contaminated Soil: A Review. Sustainability. 2023; 15(5):3973. https://doi.org/10.3390/su15053973
Chicago/Turabian StyleChandra, Subhash, Isha Medha, and Ashwani Kumar Tiwari. 2023. "The Role of Modified Biochar for the Remediation of Coal Mining-Impacted Contaminated Soil: A Review" Sustainability 15, no. 5: 3973. https://doi.org/10.3390/su15053973
APA StyleChandra, S., Medha, I., & Tiwari, A. K. (2023). The Role of Modified Biochar for the Remediation of Coal Mining-Impacted Contaminated Soil: A Review. Sustainability, 15(5), 3973. https://doi.org/10.3390/su15053973







