Spatio-Temporal Variability of Soil Properties and Nutrient Uptake for Sustainable Intensification of Rainfed Pigeon Pea (Cajanus cajana) in Semi-Arid Tropics of India
Abstract
1. Introduction
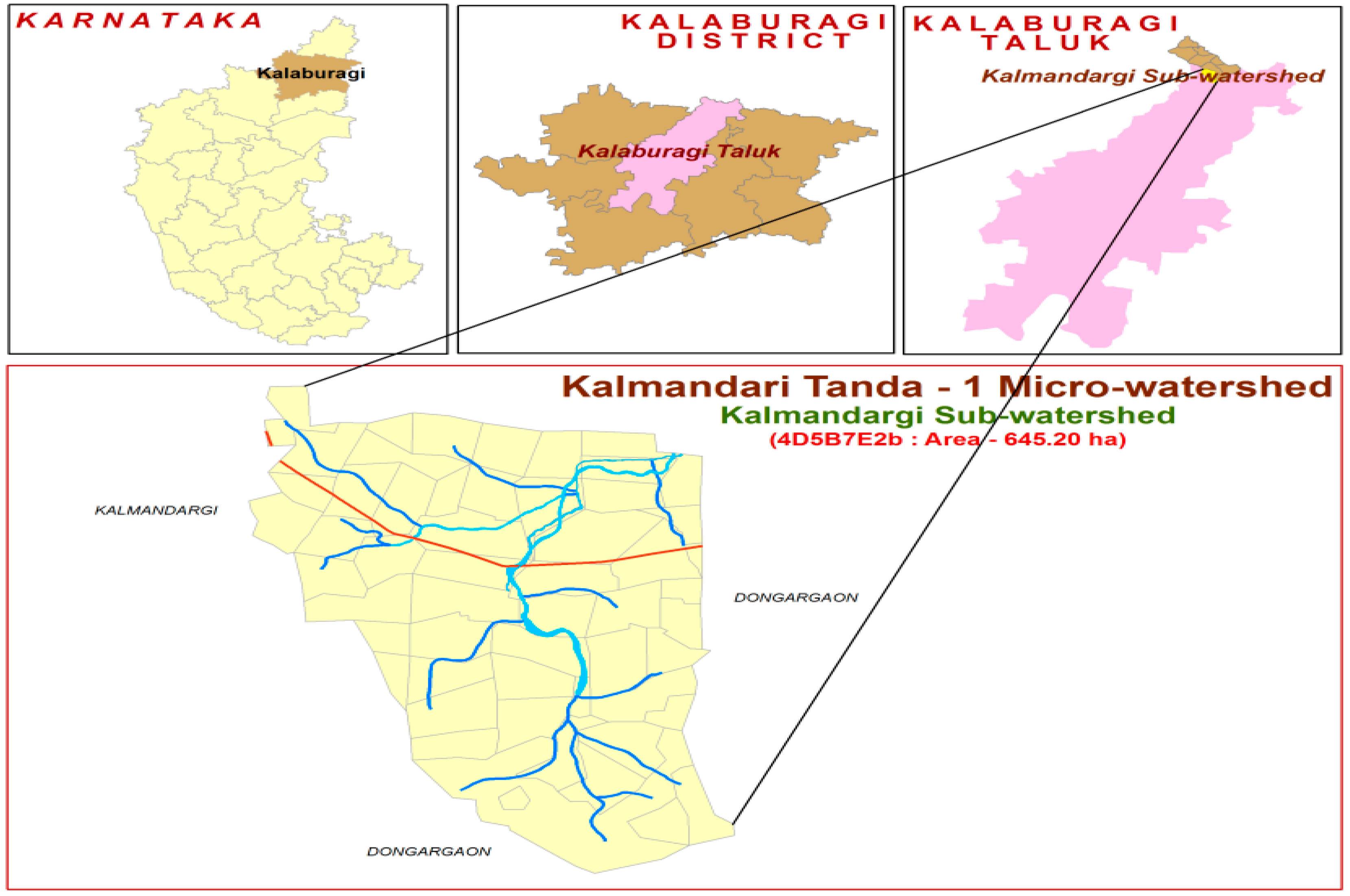
2. Material and Methods
3. Soil Sampling
4. Classification of Soil Series and Phases
5. Results and Discussion
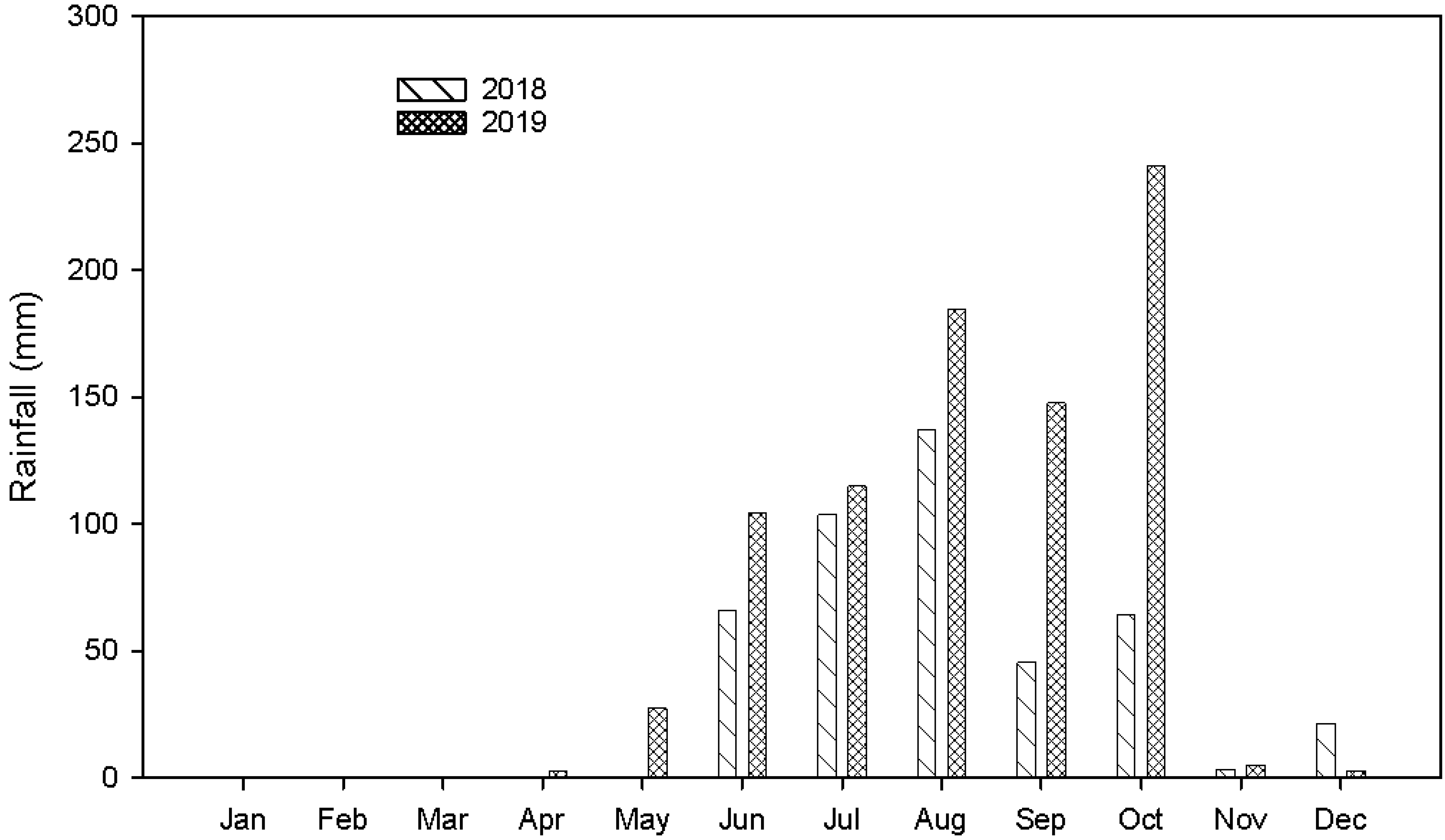
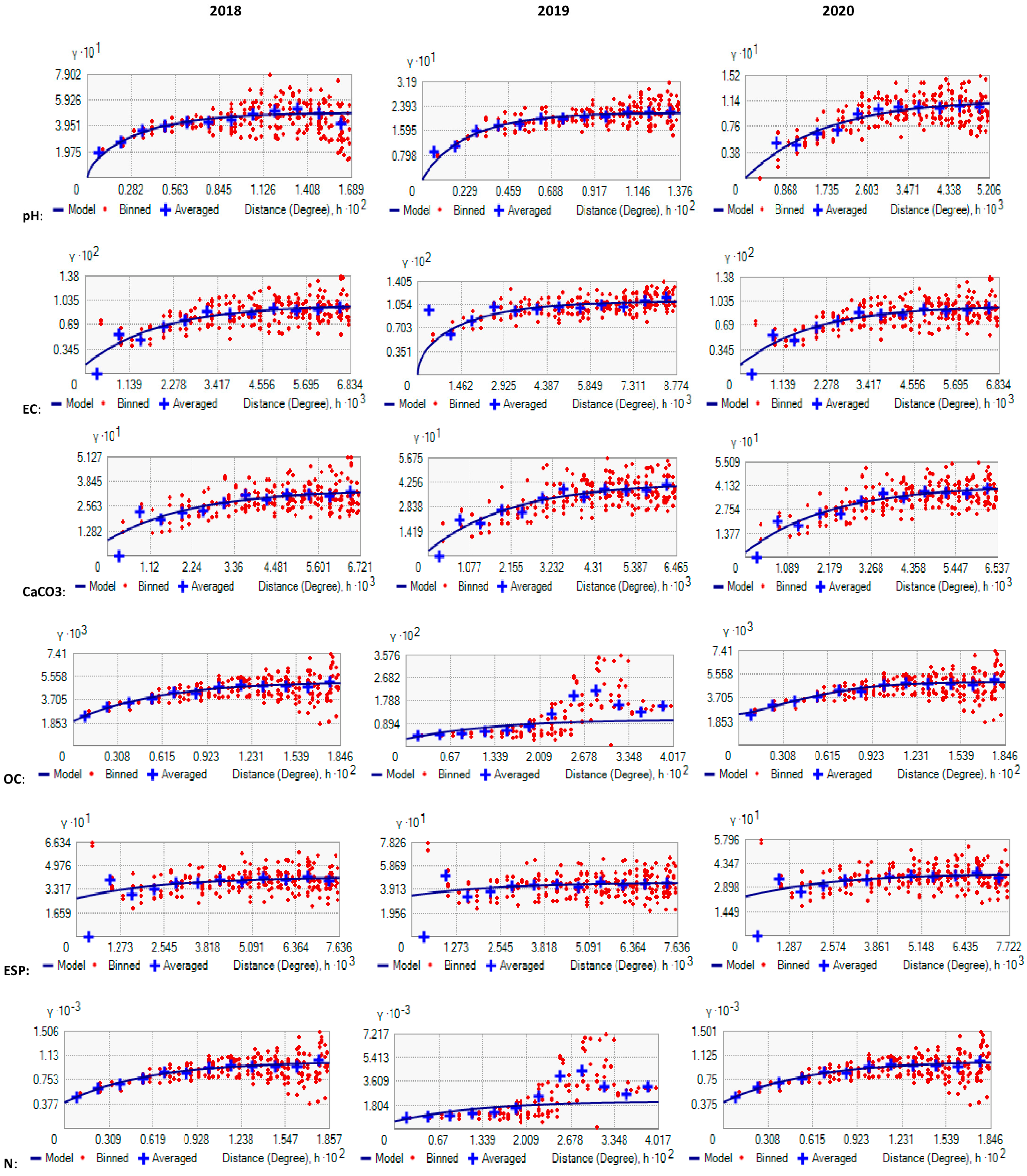
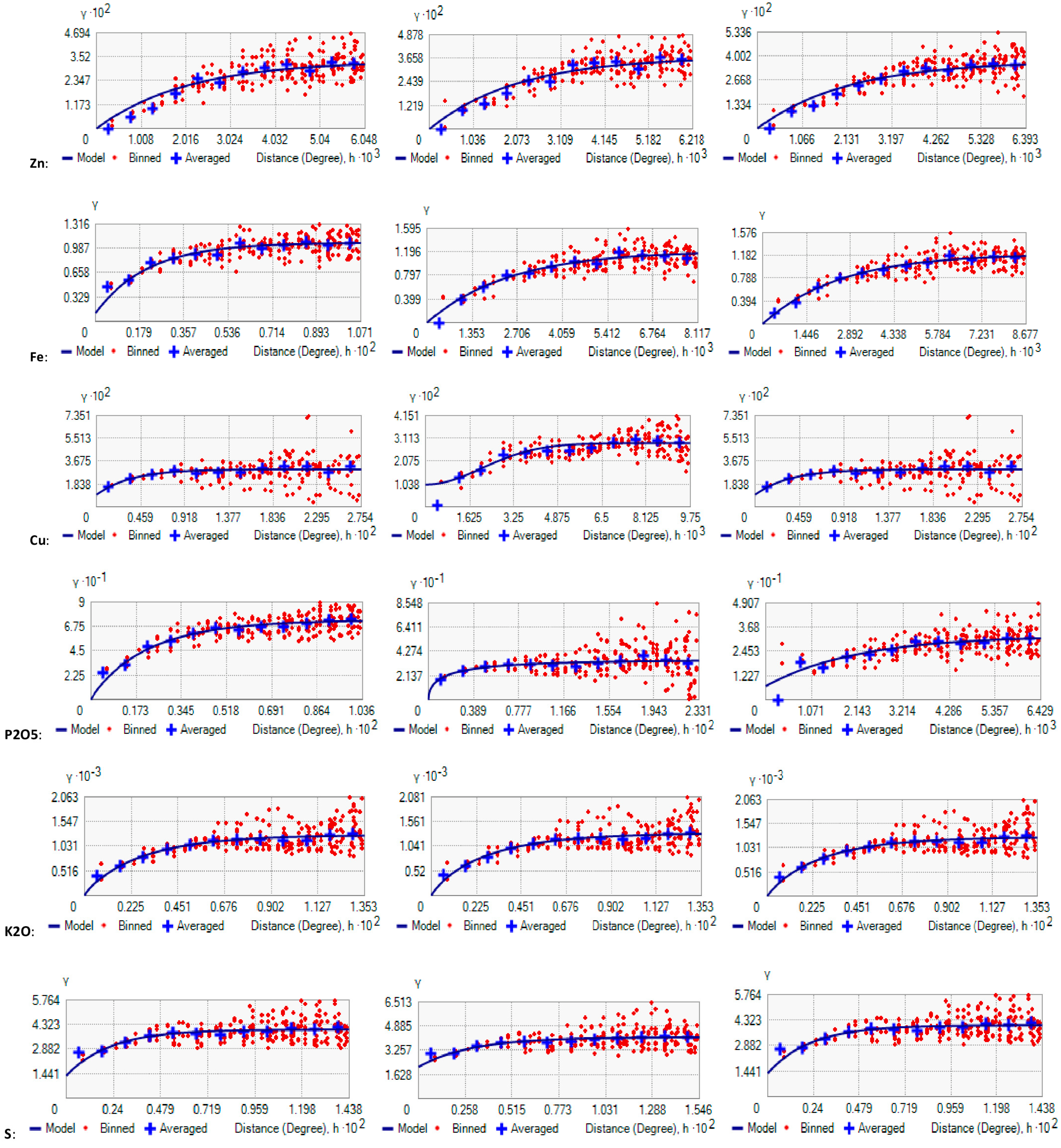
6. Spatial Dependency of Soil Fertility Parameters of Kalmandari Tanda-1 MWS
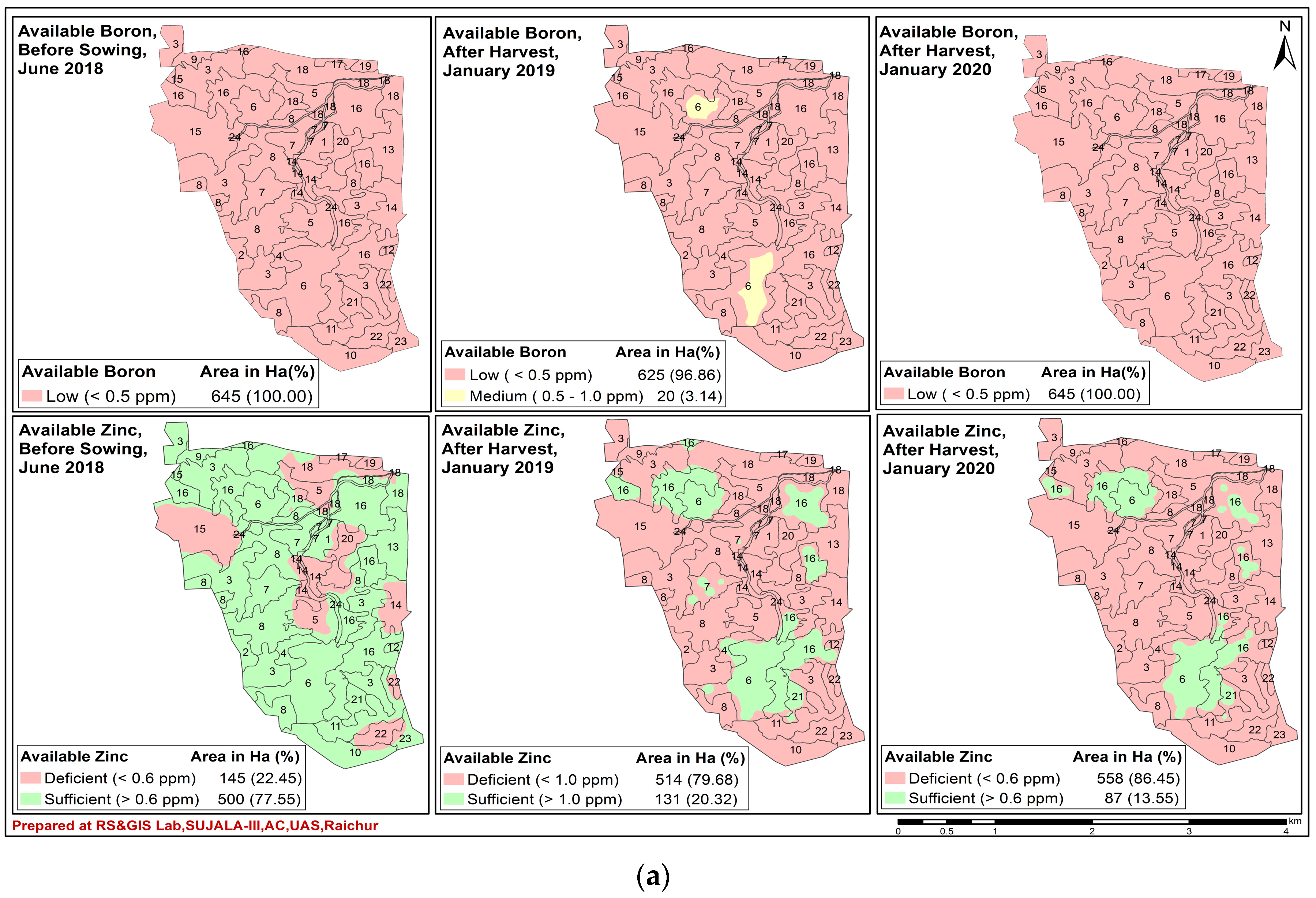
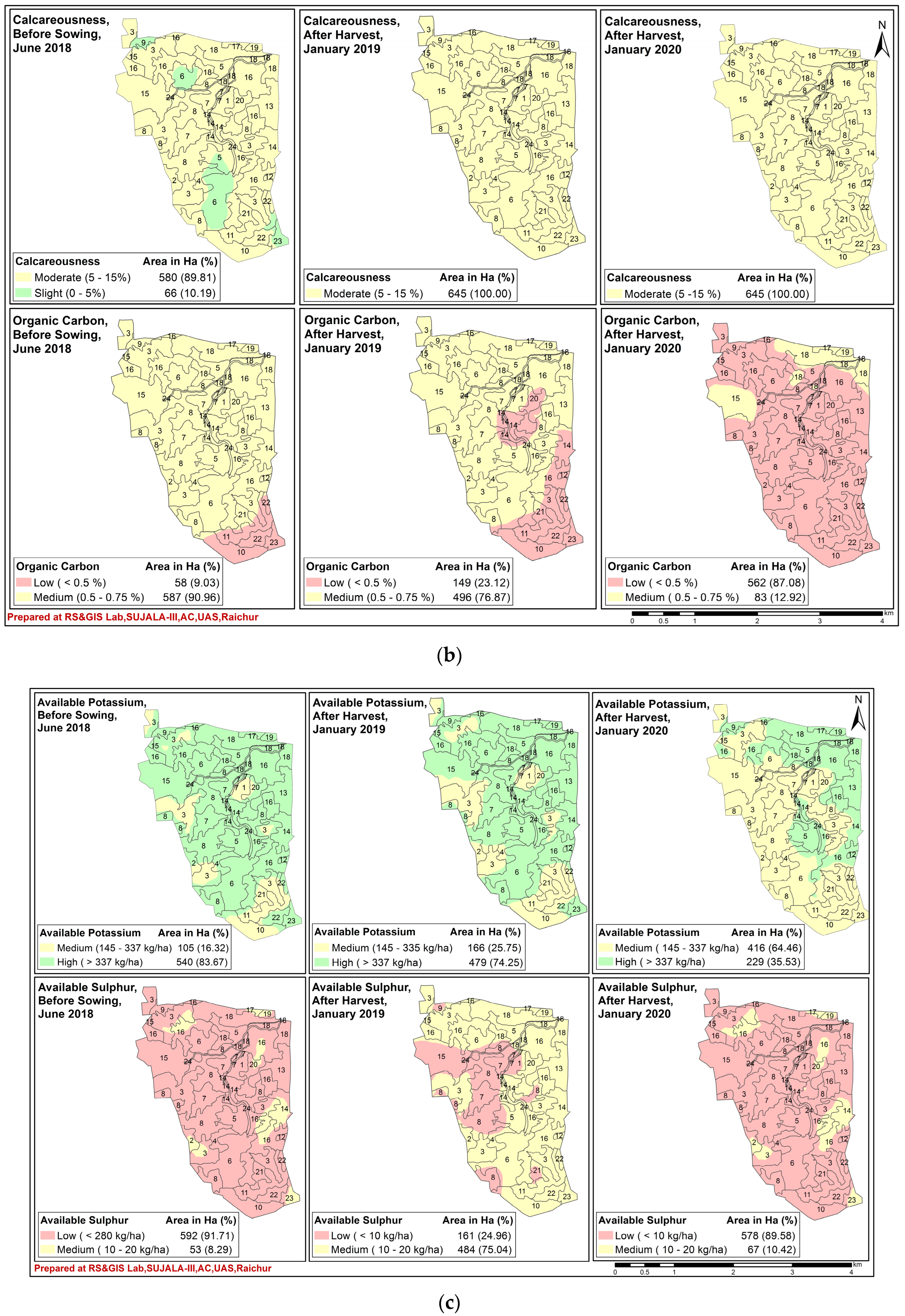
7. Spatio-Temporal Variability of Soil Fertility of Kalmandari Tanda-1 MWS
8. Nutrient Uptake in Pigeon Pea
9. Seed Yield
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Görres, J.H.; Amador, J.A. Spatial Patterns. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 562–570. [Google Scholar] [CrossRef]
- Lark, R.M.; Cullis, B.R.; Welham, S.J. On spatial prediction of soil properties in the presence of a spatial trend: The empirical best linear unbiased predictor (E-BLUP) with REML. Eur. J. Soil Sci. 2006, 57, 787–799. [Google Scholar] [CrossRef]
- Rakesh, S.; Kunal, S. Characterization of spatial variability of soil parameters in apple orchards of Himalayan region using geostatistical analysis. Commun. Soil Sci. Plant Anal. 2020, 51, 1065–1077. [Google Scholar]
- Berkes, F.; Colding, J.; Folke, C. Rediscovery of traditional ecological knowledge as adaptive management. Ecol. Appl. 2000, 10, 1251–1262. [Google Scholar] [CrossRef]
- Natarajan, A.; Reddy, R.S.; Niranjana, K.V.; Rajendra, H.; Srinivasan, R.; Dharumarajan, S.; Srinivas, S.; Dhanokar, B.A.; Vasundhara, R. Field Guide for Land Resources Inventory, Sujala III Project, Karnataka; NBSS Publ., ICAR-NBSS & LUP: Bangalore, India, 2016; p. 154. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 8th ed.; SCS, USDA: Washington, DC, USA, 2008. [Google Scholar]
- Evans, R. Assessment and monitoring of accelerated water erosion of cultivated land–when will reality be acknowledged? Soil Use Manag. 2013, 29, 105–118. [Google Scholar] [CrossRef]
- Walkley, A.; Black, C.A. Organic carbon. Methods of soil analysis. Am. Soc. Agron. 1965, 37, 1372–1375. [Google Scholar]
- Subbaiah, B.Y.; Asija, G.L. A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Academic Press: New York, NY, USA, 1966; p. 367. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India, Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Kenay, D.R. Methods of Soil Analysis. Part-II Chemical and Microbiological Properties; American Society of Agronomy Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Oliver, M.A. (Ed.) Geostatistical Applications for Precision Agriculture; Springer Science & Business Media: Berlin, Germany, 2010; p. 146. [Google Scholar]
- Goovaerts, P. Geostatistics in Soil Science: State-of-the-Art and Perspectives. Geoderma 1999, 89, 1–45. [Google Scholar] [CrossRef]
- White, J.G.; Welch, R.M.; Norvell, W.A. Soil zinc map of the USA using geostatistics and geographic information systems. Soil Sci. Soc. Am. J. 1997, 61, 185–194. [Google Scholar] [CrossRef]
- Mishra, B.B. Indian system of soil classification: A way forward. Agric. Res. Technol. 2016, 3, 555–606. [Google Scholar] [CrossRef]
- Subehia, S.K.; Dhanika; Rana, S.S. Effect of continuous cropping and fertilization on availability of nutrients in acidic soil. Agropedology 2011, 21, 18–22. [Google Scholar]
- Swarup, A. Long-term effect of green manuring (Sesbania aculeata) on soil properties and sustainability of rice and wheat yield on sodic soil. J. Indian Soc. Soil Sci. 1991, 39, 777–780. [Google Scholar]
- Ramesh, P.; Singh, M.; Panwar, N.R.; Singh, A.B.; Ramana, S. Response of pigeonpea varieties to organic manures and their influence on fertility and enzyme activity of soil. Indian J. Agric. Sci. 2006, 76, 252–254. [Google Scholar]
- Gunjal, B.S.; Chitodkar, S.S. Effect of integrated nutrient management on soil properties and soil fertility under in sweet corn-potato cropping sequence in Vertisols of Deccan plateau of India. Int. J. Chem. Stud. 2017, 5, 1343–1351. [Google Scholar]
- Dasog, G.S.; Patil, P.L. Genesis and Classification of Black, Red and Lateritic Soils of North Karnataka. In Proceedings of the Special Publ. on Soil Science Research in north Karnataka, 76th Annual Convention of ISSS, Dharwad Chapter of ISSS, Dharwad, India, 15–18 November 2011; pp. 1–10. [Google Scholar]
- Sainju, U.M.; Allen, B.L.; Caesar-TonThat, T.; Lenssen, A.W. Dryland soil chemical properties and crop yields affected by long-term tillage and cropping sequence. Springer Plus 2015, 4, 320. [Google Scholar] [CrossRef]
- Landey, R.I.; Hirekerur, L.R.; Krishnamurthy, P. Morphology, genesis and classification of black soils. Review of Soil Research in India Part–II. In Proceedings of the 12th International Congress of Soil Science, New Delhi, India, 8–17 February 1982; pp. 484–498. [Google Scholar]
- Das, D.K. Role of soil information systems in sustainable use of land resources. J. Indian Soc. Soil Sci. 1999, 47, 584–610. [Google Scholar]
- Srinath, K. A study of physico-chemical characteristics of some salt affected soils of Tungabhadra river valley project left bank canal area. Master´s Thesis, University of Agricultural Sciences, Bangalore, India, 1979. [Google Scholar]
- Almagro, M.; Ruiz-Navarro, A.; Díaz-Pereira, E.; Albaladejo, J.; Martínez-Mena, M. Plant residue chemical quality modulates the soil microbial response related to decomposition and soil organic carbon and nitrogen stabilization in a rainfed Mediterranean agroecosystem. Soil Biol. Biochem. 2021, 156, 108198. [Google Scholar] [CrossRef]
- Tamhane, R.V.; Motiramani, D.P.; Bali, V.D.; Roy, L.D. Soil: Their Chemistry and Fertility in Tropical Asia; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1964. [Google Scholar]
- Rana, N.S.; Singh, G.V.; Ahlawat, I.P.S. Effect of nitrogen, Rhizobium inoculation and phosphorous on root nodulation, dry matter yield and nutrient uptake in pigeonpea (Cajanus cajan). Indian J. Agron. 1998, 43, 102–105. [Google Scholar]
- Sutaria, G.S.; Akbari, K.N.; Vora, V.D.; Hirpara, D.S.; Padmani, D.R. Response of legume crops to enriched compost and vermicompost on Verticustochrept under rain fed agriculture. Leg. Res. 2010, 33, 128–130. [Google Scholar]
- Rana, R.; Badiyala, D. Effect of integrated nutrient management on seed yield, quality and nutrient uptake of soybean (Glycine max) under mid hill conditions of Himachal Pradesh. Indian J. Agron. 2014, 59, 641–645. [Google Scholar]
- Mere, V.; Singh, A.K.; Singh, M.; Jamir, Z.; Gupta, R.C. Effect of nutritional schedule on productivity and quality of soybean varieties and soil fertility. Leg. Res. 2013, 36, 528–534. [Google Scholar]
- Gupta, S.; Handore, K. Direct and residual effect of zinc and zinc amended organic manures on the zinc nutrition of field crop. Int. J. Agric. Sci. 2009, 1, 26–29. [Google Scholar]
- Tiwari, A.; Dwivedi, A.K.; Dikshit, P.R. Long term influence of organic and inorganic fertilization on soil fertility and productivity of soybean-wheat system in Vertisol. J. Indian Soc. Soil Sci. 2002, 50, 472–475. [Google Scholar]
- Jadhav, A.B.; Kadlag, A.D.; Patil, V.S.; Bachkar, S.R.; Dale, R.M. Response of chickpea to conjoint application of inorganic fertilizers based on STCR approach and vermicompost on Inceptisol. J. Maharashtra Agric. Univ. 2009, 34, 125–127. [Google Scholar]
- NarayanaRao, K.; Rajath, E.; Kumara, K. Yield, quality parameters and economics of sunflower (Helianthus annuus L.) as influenced by micronutrient mixture foliar application. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1999–2005. [Google Scholar]
- Jat, H.S.; Ahlawat, I.P.S. Response of pigeonpea (Cajanus cajan) + groundnut (Arachis hypogea) intercropping system to planting and phosphorus management. Indian J. Agron. 2003, 48, 156–159. [Google Scholar]
- Abdel Rahman, M.A.; Natarajan, A.; Hegde, R. Assessment of land suitability and capability by integrating remote sensing and GIS for agriculture in Chamarajanagar district, Karnataka, India. Egypt. J. Remote Sens. Space Sci. 2016, 19, 125–141. [Google Scholar]
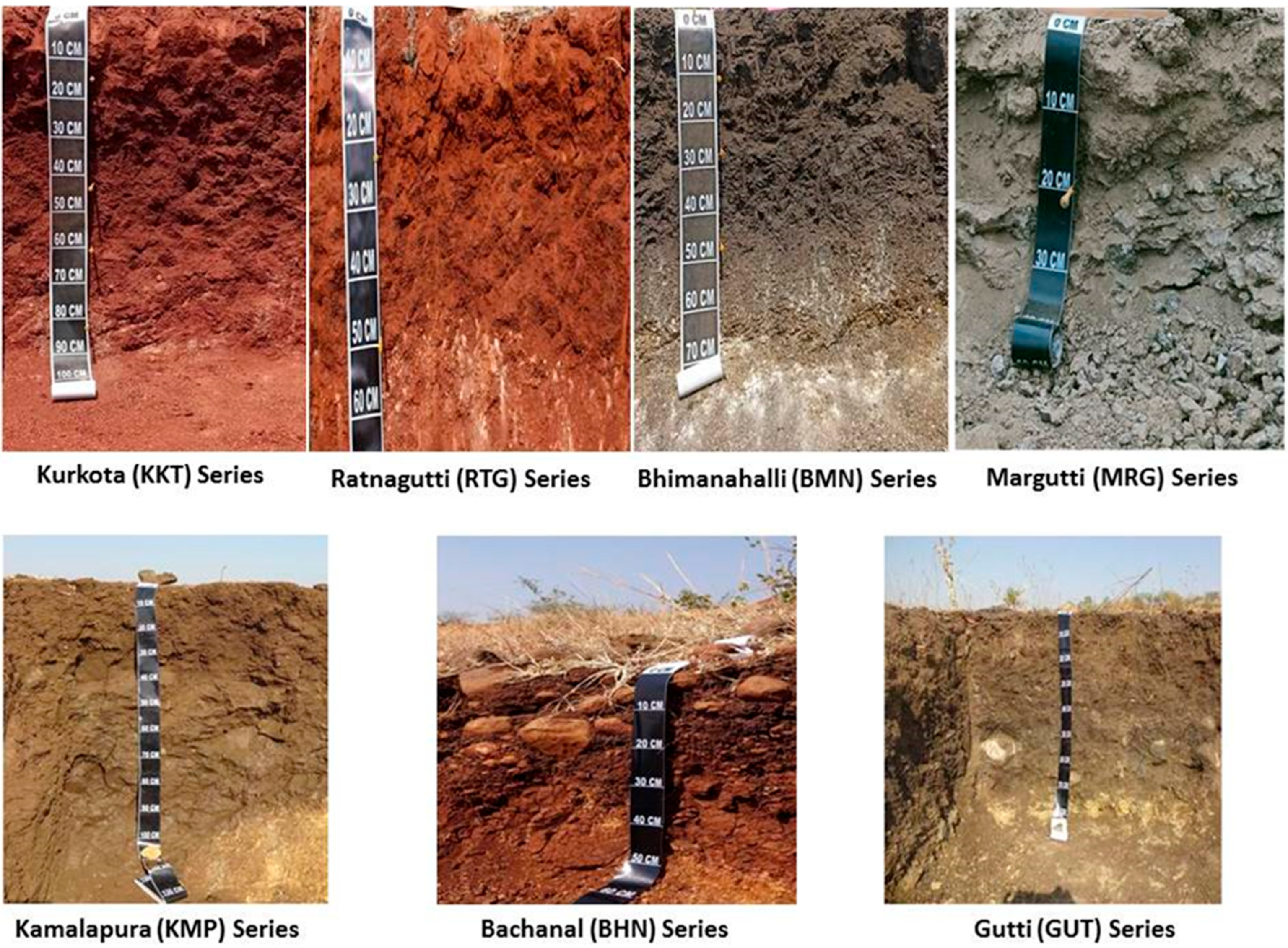
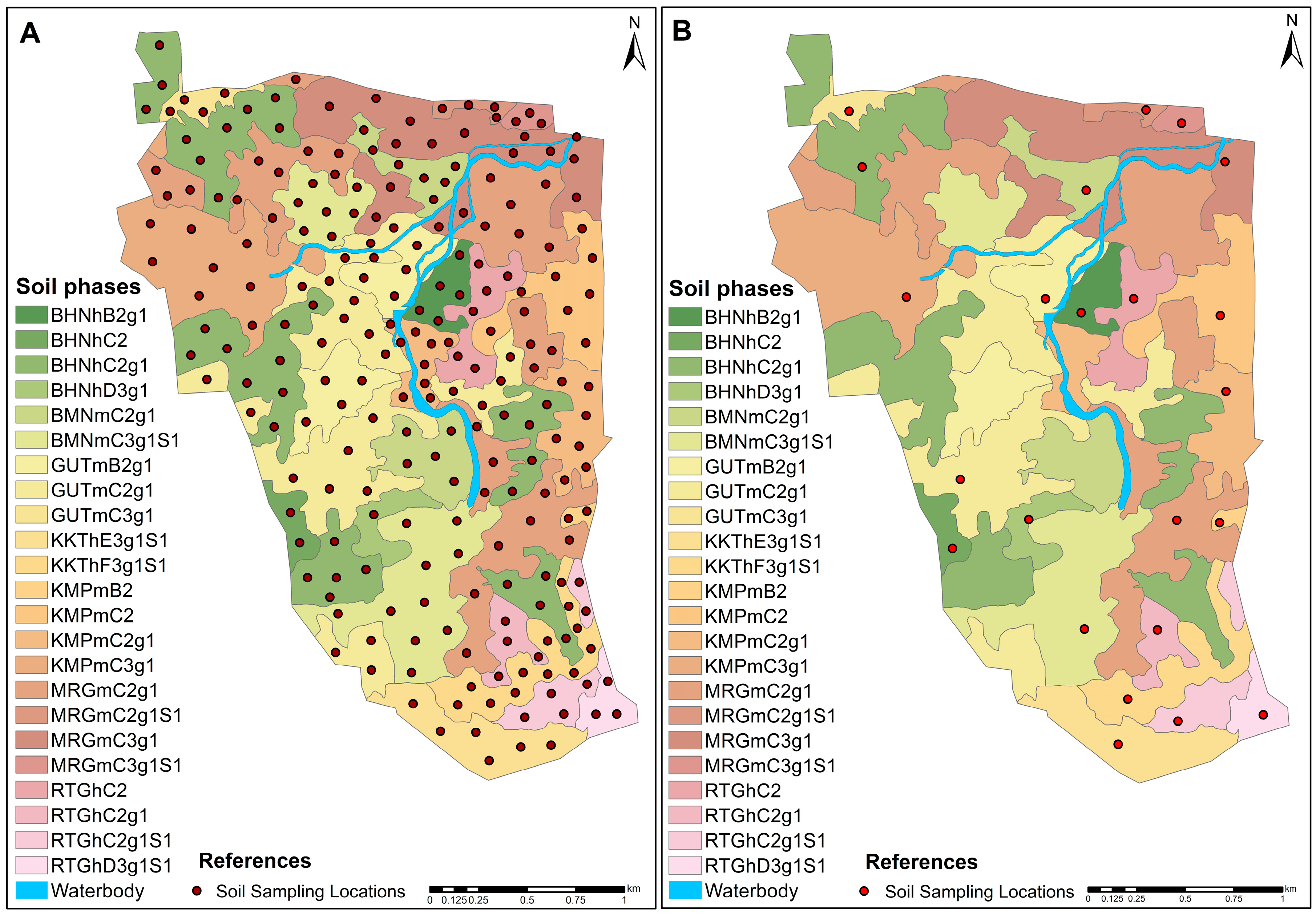
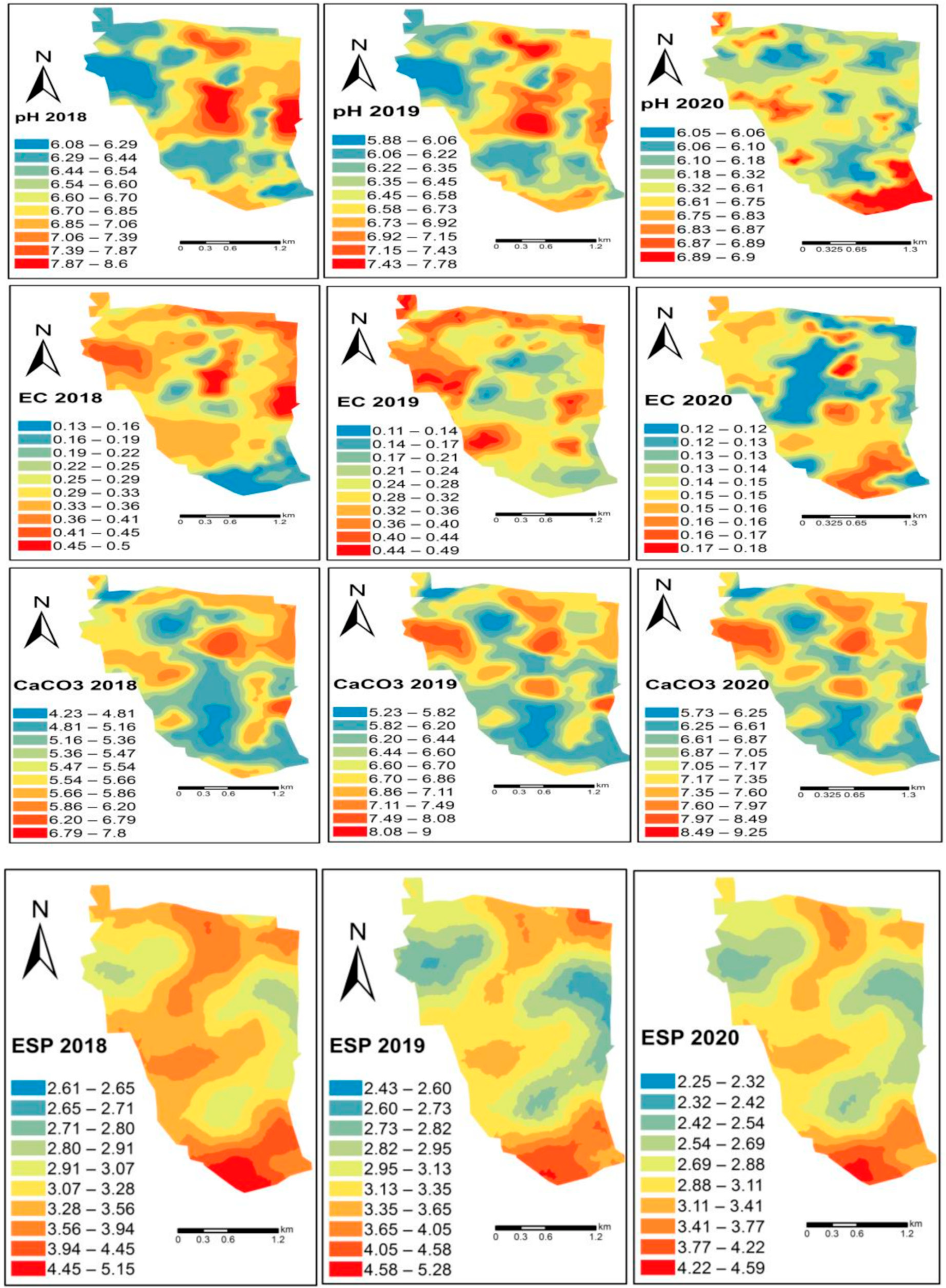
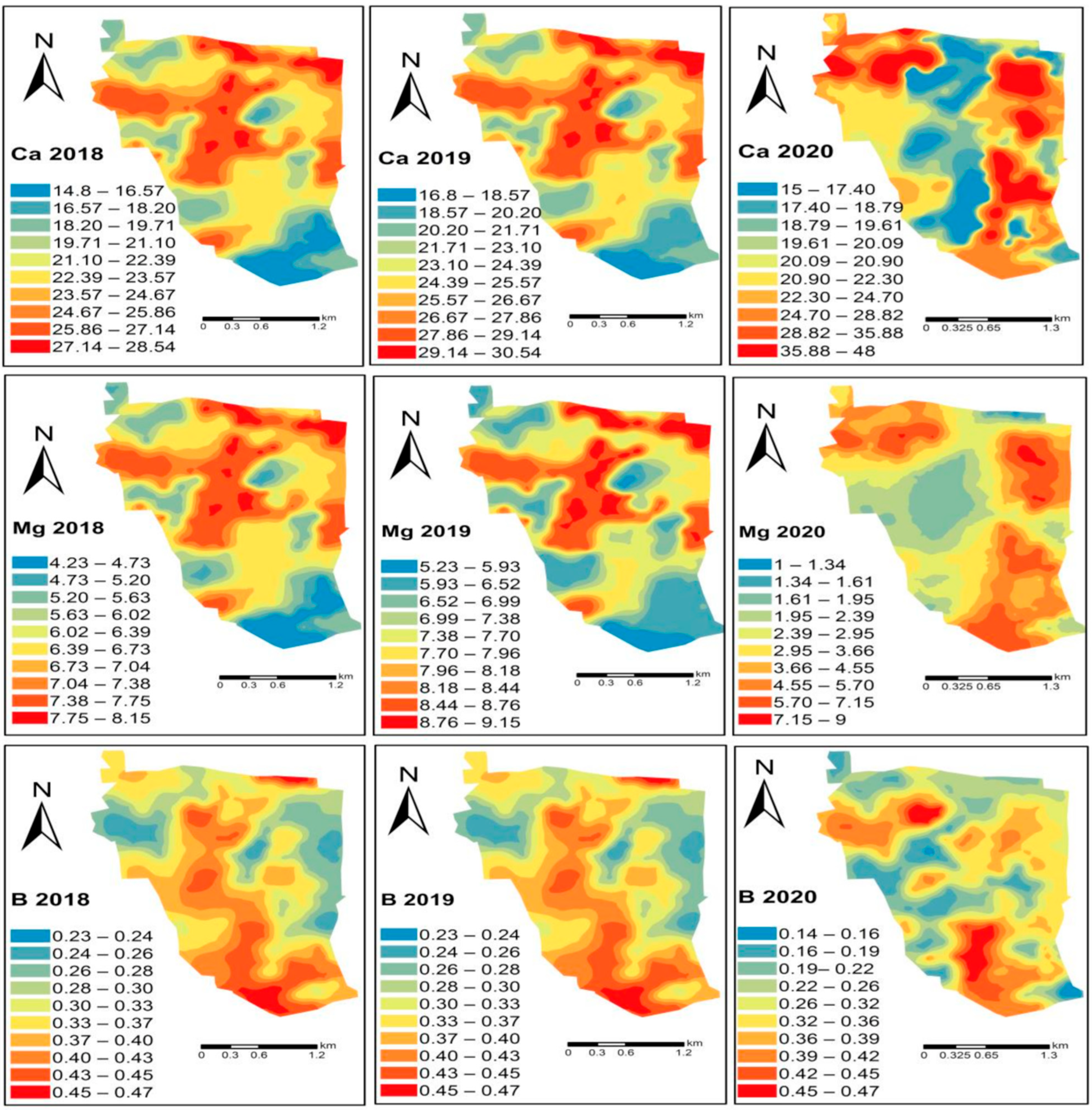
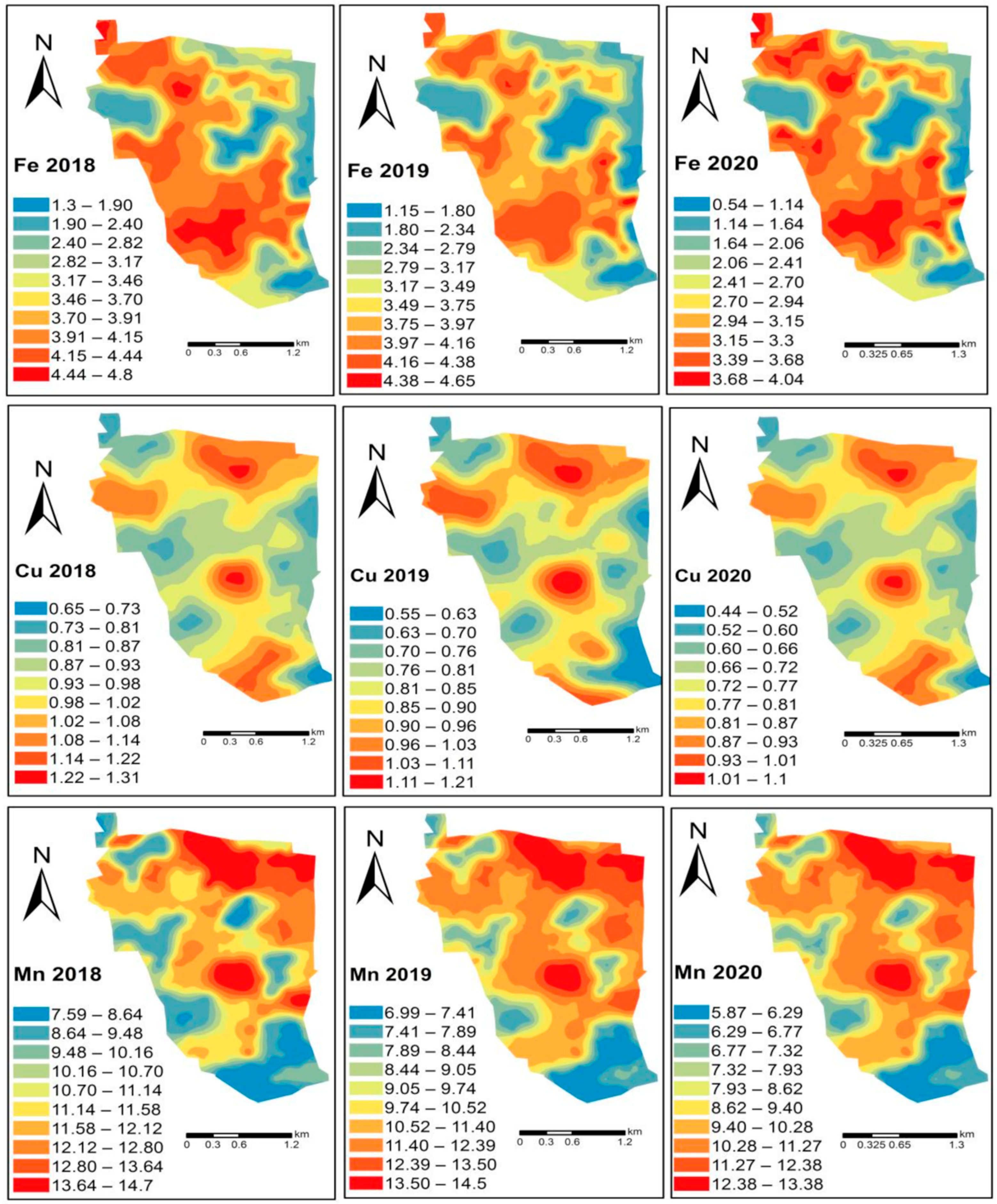



| Sl. No. | Soil Series | Mapping Unit | Depth (cm) | Slope (%) | Color | Texture | Drainage | Physiography | Geology | Consistency | Stoniness | Erosion | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface | Sub Surface | Surface | Sub Surface | |||||||||||
| 1 | Bachnal (BHN) | BHNhB2g1 | 25–50 | 1–3 | 5 YR 3/2 | 5 YR 4/3 | scl | MWD | Pd | Basalt * | sh, fri, ss, sp | h, fri, ss, sp | - | M |
| BHNhC2 & g1 | 25–50 | 3–5 | 5 YR 3/2 | 5 YR 4/3 | scl | MWD | Pd | sh, fri, ss, sp | h, fri, ss, sp | - | M | |||
| BHNhD3g1 | 25–50 | 5–10 | 5 YR 3/2 | 5 YR 4/3 | scl | MWD | Pd | sh, fri, ss, sp | sh, fri, ss, sp | - | S | |||
| 2 | Bheemanhalli (BMN) | BMNmC2g1 | 25–50 | 3–5 | 10 YR 3/1 | 10 YR 4/3 | c | WD | Pd | Basalt | sh, fm, s, p | vh, fm, s, p | - | M |
| BMNmC3g1S1 | 25–50 | 3–5 | 10 YR 3/1 | 10 YR 4/3 | c | WD | Pd | sh, fm, s, p | vh, fm, s, p | Strong | S | |||
| 3 | Gutti (GUT) | GUTmB2g1 | 50–75 | 1–3 | 10 YR 3/2 | 10 YR 3/1 | c | SPWD | Lpdp | sh, fm, s, p | vh, fm, s, p | - | M | |
| GUTmC2g1 | 50–75 | 3–5 | 10 YR 3/2 | 10 YR 3/1 | c | SPWD | Lpdp | sh, fm, s, p | vh, fm, s, p | - | M | |||
| GUTmC3g1 | 50–75 | 3–5 | 10 YR 3/2 | 10 YR 3/1 | c | SPWD | Lpdp | sh, fm, s, p | vh, fm, s, p | - | S | |||
| 4 | Kurkota (KKT) | KKThE3g1S1 | 75–100 | 10–15 | 5 YR 3/4 | 5 YR 3/4 | scl | WD | Lpdp | Basalt * | sh, fri, ss, sp | h, fri, ss, sp | Strong | S |
| 5 | Kamalapur (KMP) | KMPmB2 | 0.75–1.0 | 1–3 | 10 YR 3/2 | 10 YR 3/1 | c | SPWD | Lpdp | Basalt | sh, fm, s, p. | vh, fm, s, p | - | M |
| KMPmC2 & g1 | 75–100 | 3–5 | 10 YR 3/2 | 10 YR 3/1 | c | SPWD | Lpdp | Basalt | sh, fm, s, p | vh, fm, s, p | - | M | ||
| KMPmC3g1 | 75–100 | 3–5 | 10 YR 3/2 | 10 YR 3/1 | c | SPWD | Lpdp | sh, fm, s, p | vh, fm, s, p | - | S | |||
| 6 | Margutti (MRG) | MRGmC2g1 | 0–25 | 3–5 | 10 YR 4/3 | 10 YR 4/3 | c | MWD | Pd | sh, fm, s, p | sh, fm, s, p | - | M | |
| MRGmC2g1S1 | 0–25 | 3–5 | 10 YR 4/3 | 10 YR 4/3 | c | MWD | Pd | sh, fm, s, p | sh, fm, s, p | Strong | M | |||
| MRGmC3g1 | 0–25 | 3–5 | 10 YR 4/3 | 10 YR 4/3 | c | MWD | Pd | sh, fm, s, p | sh, fm, s, p | - | S | |||
| MRGmC3g1S1 | 0–25 | 3–5 | 10 YR 4/3 | 10 YR 4/3 | c | MWD | Pd | sh, fm, s, p | sh, fm, s, p | Strong | S | |||
| 7 | Ratnagutti (RTG) | RTGhC2 & g1 | 25–50 | 3–5 | 5 YR 3/3 | 5 YR 3/3 | scl | MWD | Lpdp | Basalt * | sh, fri, ss, sp | h, fri, ss, p | - | M |
| RTGhC2g1S1 | 25–50 | 3–5 | 5 YR 3/3 | 5 YR 3/3 | scl | MWD | Lpdp | sh, fri, ss, sp | h, fri, ss, p | Strong | M | |||
| RTGhD3g1S1 | 25–50 | 5–10 | 5 YR 3/3 | 5 YR 3/3 | scl | MWD | Lpdp | sh, fri, ss, sp | h, fri, ss, p | Strong | S | |||
| Parameter | 2018 | 2019 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Skewness | Kurtosis | Min | Max | Mean | SD | Skewness | Kurtosis | |
| pH | 5.88 | 7.78 | 6.63 | 0.44 | 0.71 | 0.65 | 6.05 | 6.9 | 6.50 | 0.33 | −0.14 | −1.66 |
| EC | 0.11 | 0.49 | 0.27 | 0.09 | 0.64 | 0.23 | 0.12 | 0.18 | 0.15 | 0.02 | −0.06 | −1.08 |
| CaCO3 | 5.23 | 9.00 | 6.67 | 0.84 | 0.84 | 1.52 | 5.73 | 9.25 | 7.16 | 0.81 | 0.64 | 0.85 |
| OC | 0.27 | 0.69 | 0.50 | 0.09 | −0.52 | 1.12 | 0.19 | 0.61 | 0.42 | 0.09 | −0.52 | 1.12 |
| N | 121.5 | 310.5 | 225.6 | 40.7 | −0.51 | 1.14 | 104.0 | 283.5 | 199.4 | 39.7 | −0.37 | 0.75 |
| P2O5 | 20.2 | 44.7 | 30.7 | 6.3 | 0.88 | 0.27 | 20.3 | 41.7 | 28.5 | 6.2 | 0.72 | −0.34 |
| K2O | 302.4 | 449.8 | 361.7 | 45.7 | 0.45 | −0.91 | 273.3 | 417.9 | 333.9 | 41.9 | 0.57 | −0.67 |
| S | 7.00 | 13.60 | 10.69 | 2.08 | −0.27 | −1.10 | 5.05 | 11.65 | 8.74 | 2.08 | −0.27 | −1.10 |
| Zn | 0.22 | 0.75 | 0.44 | 0.18 | 0.69 | −0.91 | 0.19 | 0.69 | 0.38 | 0.17 | 0.81 | −0.85 |
| Fe | 1.15 | 4.65 | 2.97 | 1.12 | −0.18 | −1.57 | 0.54 | 4.04 | 2.36 | 0.2 | −0.04 | −0.81 |
| Cu | 0.55 | 1.21 | 0.85 | 0.19 | −0.06 | −0.79 | 0.44 | 1.10 | 0.74 | 2.53 | 0.1 | −1.4 |
| Mn | 6.99 | 14.50 | 10.44 | 2.55 | 0.08 | −1.44 | 5.87 | 13.38 | 9.32 | 4.08 | 0.02 | −1.15 |
| Ca | 16.8 | 30.54 | 23.88 | 4.07 | 0.01 | −1.17 | 15.00 | 48.00 | 21.89 | 1.13 | 0.04 | −1.19 |
| Mg | 5.23 | 9.15 | 7.25 | 1.15 | 0.03 | −1.18 | 1.00 | 9.00 | 3.28 | 0.15 | −0.34 | −1.39 |
| B | 0.19 | 0.52 | 0.37 | 0.10 | −0.35 | −1.38 | 0.14 | 0.47 | 0.32 | 0.29 | 1.35 | 1.25 |
| Na | 0.74 | 1.85 | 1.09 | 0.28 | 1.33 | 1.24 | 0.71 | 1.80 | 1.04 | 0.85 | 1.36 | 0.85 |
| ESP | 2.43 | 5.28 | 3.28 | 0.84 | 1.34 | 0.89 | 2.25 | 5.04 | 3.06 | 2.36 | −0.45 | −1.44 |
| CEC | 28.54 | 35.70 | 33.16 | 2.35 | −0.48 | −1.50 | 29.29 | 36.45 | 33.91 | 9.44 | −0.04 | −0.53 |
| Parameters | EC | OC | N | P2O5 | K2O | Zn | Mn | Ca | Mg | Na | ESP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | 1 | ||||||||||
| OC | 0.484 * | 1 | |||||||||
| N | 0.483 * | 1.000 ** | 1 | ||||||||
| P2O5 | 0.041 | 0.157 | 0.160 | 1 | |||||||
| K2O | 0.013 | 0.343 | 0.345 | −0.209 | 1 | ||||||
| Zn | 0.084 | 0.128 | 0.127 | −0.010 | −0.109 | 1 | |||||
| Fe | 0.118 | 0.009 | 0.010 | −0.030 | 0.065 | 0.532 ** | |||||
| Cu | 0.133 | 0.456 * | 0.459 * | −0.168 | −0.031 | 0.018 | |||||
| Mn | 0.002 | 0.479 * | 0.483 * | −0.293 | 0.778 ** | 0.044 | 1 | ||||
| Ca | 0.091 | 0.590 ** | 0.591 ** | −0.269 | 0.616 ** | −0.141 | 0.786 ** | 1 | |||
| Mg | 0.059 | 0.491 * | 0.493 * | −0.349 | 0.599 ** | −0.178 | 0.775 ** | 0.980 ** | 1 | ||
| Na | 0.106 | −0.183 | −0.180 | −0.486 * | 0.068 | −0.213 | 0.102 | 0.080 | 0.154 | 1 | |
| ESP | 0.090 | −0.335 | −0.332 | −0.403 | −0.131 | −0.226 | −0.143 | −0.160 | −0.085 | 0.963 ** | 1 |
| CEC | 0.074 | 0.481 * | 0.483 * | −0.347 | 0.700 ** | 0.079 | 0.879 ** | 0.833 ** | 0.834 ** | 0.160 | −0.105 |
| Parameters | pH | OC | N | P2O5 | K2O | Zn | Fe | Cu | Mn | Ca | Mg | B | Na | ESP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OC | −0.461 * | |||||||||||||
| N | −0.465 * | 0.999 ** | ||||||||||||
| P2O5 | −0.139 | 0.170 | 0.160 | |||||||||||
| K2O | −0.017 | 0.327 | 0.323 | 0.016 | ||||||||||
| Zn | −0.213 | 0.163 | 0.158 | −0.040 | −0.210 | |||||||||
| Fe | 0.071 | 0.009 | 0.013 | 0.025 | 0.026 | 0.506 * | ||||||||
| Cu | −0.452 * | 0.456 * | 0.451 * | −0.030 | −0.050 | 0.063 | 0.030 | |||||||
| Mn | −0.176 | 0.479 * | 0.478 * | −0.230 | 0.721 ** | 0.024 | 0.258 | 0.190 | ||||||
| Ca | −0.277 | −0.049 | −0.060 | 0.221 | 0.161 | 0.210 | 0.183 | 0.113 | 0.130 | |||||
| Mg | −0.264 | −0.088 | −0.097 | 0.067 | 0.131 | 0.315 | 0.217 | 0.239 | 0.080 | 0.845 ** | ||||
| B | −0.320 | −0.083 | −0.073 | −0.290 | −0.459 * | 0.440 * | 0.082 | 0.205 | −0.200 | 0.100 | 0.346 | |||
| Na | 0.310 | −0.186 | −0.171 | −0.439 * | 0.111 | −0.300 | 0.098 | 0.079 | 0.100 | −0.200 | 0.008 | −0.150 | ||
| ESP | 0.391 | −0.332 | −0.317 | −0.380 | −0.060 | −0.310 | 0.035 | 0.024 | −0.100 | −0.200 | 0.016 | −0.110 | 0.967 ** | |
| CEC | −0.295 | 0.481 * | 0.486 * | −0.320 | 0.627 ** | 0.076 | 0.354 | 0.214 | 0.879 ** | 0.120 | 0.053 | −0.060 | 0.160 | −0.090 |
| Parameter | Model | Nugget (Co) | Partial Sill | Range in m | Sill (Co + C) | Ratio Co/ (Co + C) | SD | RMS |
|---|---|---|---|---|---|---|---|---|
| pH | K Bessel | 0.000 | 0.501 | 1181.468 | 0.501 | 0.000 | Strong | 0.829 |
| EC | Exponential | 0.001 | 0.008 | 635.428 | 0.010 | 0.127 | Strong | 0.894 |
| CaCO3 | Exponential | 0.081 | 0.261 | 746.017 | 0.342 | 0.237 | Strong | 0.898 |
| OC | Exponential | 0.002 | 0.003 | 19,060.798 | 0.005 | 0.385 | Moderate | 0.958 |
| N | Exponential | 399.960 | 638.652 | 1899.030 | 1038.612 | 0.385 | Moderate | 0.958 |
| P | Stable | 0.000 | 73.775 | 775.113 | 73.775 | 0.000 | Strong | 0.967 |
| K | K Bessel | 0.000 | 1265.411 | 960.064 | 1265.411 | 0.000 | Strong | 0.956 |
| S | Exponential | 1.326 | 2.716 | 745.684 | 4.042 | 0.328 | Moderate | 0.965 |
| Zn | Exponential | 0.000 | 0.033 | 671.323 | 0.033 | 0.000 | Strong | 0.878 |
| Fe | Exponential | 0.112 | 0.952 | 702.049 | 1.064 | 0.105 | Strong | 0.880 |
| Cu | Exponential | 0.010 | 0.021 | 1186.936 | 0.030 | 0.324 | Moderate | 0.946 |
| Mn | Exponential | 0.000 | 3.818 | 829.023 | 3.818 | 0.000 | Strong | 0.963 |
| Ca | K Bessel | 0.000 | 14.455 | 1024.414 | 14.455 | 0.000 | Strong | 0.973 |
| Mg | K Bessel | 0.000 | 1.247 | 1119.738 | 1.247 | 0.000 | Strong | 0.975 |
| B | Exponential | 0.001 | 0.005 | 749.152 | 0.006 | 0.175 | Strong | 0.914 |
| ESP | Exponential | 0.027 | 0.152 | 847.641 | 0.179 | 0.150 | Strong | 0.921 |
| Parameter | Model | Nugget (Co) | Partial Sill | Range in m | Sill (Co + C) | Ratio Co/(Co + C) | SD Dependence | RMS Statd. |
|---|---|---|---|---|---|---|---|---|
| pH | K Bessel | 0.000 | 0.209 | 863.715 | 0.209 | 0.000 | Strong | 0.870 |
| EC | K Bessel | 0.000 | 0.011 | 586.302 | 0.011 | 0.000 | Strong | 0.971 |
| CaCO3 | Exponential | 0.003 | 0.394 | 717.577 | 0.397 | 0.007 | Strong | 0.926 |
| OC | Exponential | 0.001 | 0.008 | 4458.931 | 0.009 | 0.110 | Strong | 0.976 |
| N | Exponential | 586.331 | 1570.186 | 4457.730 | 2156.517 | 0.272 | Moderate | 0.977 |
| P | Stable | 0.000 | 35.596 | 1794.973 | 35.596 | 0.000 | Strong | 0.901 |
| K | Stable | 0.000 | 1324.721 | 1054.880 | 1324.721 | 0.000 | Strong | 0.952 |
| S | Exponential | 2.147 | 2.006 | 983.912 | 4.153 | 0.517 | Moderate | 0.954 |
| Zn | Exponential | 0.000 | 0.037 | 690.222 | 0.037 | 0.000 | Strong | 0.902 |
| Fe | Exponential | 0.000 | 1.214 | 808.215 | 1.214 | 0.000 | Strong | 0.901 |
| Cu | Exponential | 0.010 | 0.019 | 562.016 | 0.029 | 0.353 | Moderate | 0.973 |
| Mn | K Bessel | 0.000 | 5.796 | 1393.508 | 5.796 | 0.000 | Strong | 0.947 |
| Ca | K Bessel | 0.000 | 14.020 | 1012.739 | 14.020 | 0.000 | Strong | 0.987 |
| Mg | Exponential | 0.030 | 1.045 | 747.200 | 1.075 | 0.028 | Strong | 1.016 |
| B | Exponential | 0.012 | 0.012 | 720.809 | 0.024 | 0.500 | Moderate | 1.007 |
| ESP | Exponential | 0.342 | 0.108 | 847.641 | 0.449 | 0.760 | Weak | 0.915 |
| Parameter | Model | Nugget (Co) | Partial Sill | Range in m | Sill (Co + C) | Ratio Co/ (Co + C) | SD | RMS |
|---|---|---|---|---|---|---|---|---|
| pH | Exponential | 0.000 | 0.116 | 554.547 | 0.116 | 0.000 | Strong | 0.826 |
| EC | Exponential | 0.000 | 0.000 | 446.894 | 0.000 | 0.000 | Strong | 0.894 |
| CaCO3 | Exponential | 0.029 | 0.385 | 725.591 | 0.414 | 0.070 | Strong | 0.974 |
| OC | Exponential | 0.002 | 0.003 | 1906.080 | 0.005 | 0.385 | Moderate | 0.976 |
| N | Exponential | 402.215 | 642.439 | 1905.066 | 1044.654 | 0.385 | Moderate | 0.898 |
| P | Exponential | 6.995 | 25.561 | 713.604 | 32.556 | 0.215 | Strong | 0.958 |
| K | Stable | 0.000 | 1271.490 | 993.881 | 1271.490 | 0.000 | Strong | 0.958 |
| S | Exponential | 1.326 | 2.716 | 745.684 | 4.042 | 0.328 | Moderate | 0.976 |
| Zn | Exponential | 0.000 | 0.037 | 709.653 | 0.037 | 0.000 | Strong | 0.880 |
| Fe | Exponential | 0.000 | 1.214 | 836.776 | 1.214 | 0.000 | Strong | 0.878 |
| Cu | Exponential | 0.010 | 0.021 | 1186.936 | 0.030 | 0.324 | Moderate | 0.957 |
| Mn | Stable | 0.000 | 5.946 | 1617.641 | 5.946 | 0.000 | Strong | 0.965 |
| Ca | Exponential | 0.000 | 112.696 | 624.561 | 112.696 | 0.000 | Strong | 0.946 |
| Mg | Stable | 0.000 | 7.887 | 3320.276 | 7.887 | 0.000 | Strong | 0.963 |
| B | Exponential | 0.001 | 0.012 | 720.045 | 0.013 | 0.073 | Strong | 0.914 |
| ESP | Exponential | 0.236 | 0.140 | 857.106 | 0.377 | 0.628 | Moderate | 0.921 |
| Soil Phase | 2019 | 2020 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | S | Fe | Zn | N | P | K | S | Fe | Zn | |
| BHNhB2g1 | 71.04 | 10.77 | 47.47 | 7.19 | 453.19 | 114.63 | 75.89 | 14.09 | 51.43 | 8.25 | 463.66 | 118.63 |
| BHNhC2 | 64.90 | 9.61 | 37.51 | 6.55 | 425.96 | 102.16 | 72.31 | 13.25 | 42.12 | 7.75 | 442.82 | 107.94 |
| BHNhC2g1 | 66.12 | 9.74 | 35.08 | 5.56 | 421.17 | 104.11 | 71.96 | 13.20 | 39.59 | 6.72 | 442.03 | 110.42 |
| BHNhD3g1 | 61.09 | 9.32 | 36.65 | 8.99 | 401.37 | 96.76 | 63.96 | 12.14 | 39.10 | 9.76 | 398.20 | 97.32 |
| BMNmC2g1 | 69.90 | 10.91 | 46.37 | 5.21 | 439.67 | 111.21 | 76.70 | 14.57 | 50.72 | 6.28 | 448.90 | 115.44 |
| BMNmC3g1S1 | 70.30 | 11.41 | 45.80 | 6.77 | 440.15 | 107.94 | 76.40 | 14.97 | 50.16 | 7.88 | 452.20 | 112.47 |
| GUTmB2g1 | 73.36 | 12.34 | 48.63 | 7.93 | 483.95 | 111.22 | 79.59 | 15.97 | 52.91 | 9.05 | 494.17 | 115.39 |
| GUTmC2g1 | 67.91 | 10.71 | 47.79 | 9.31 | 432.97 | 101.93 | 76.58 | 14.77 | 53.69 | 7.61 | 456.78 | 109.30 |
| GUTmC3g1 | 69.20 | 10.71 | 46.26 | 9.83 | 433.30 | 97.11 | 76.05 | 14.39 | 50.91 | 9.16 | 447.39 | 101.92 |
| KKThE3g1S1 | 62.84 | 9.13 | 41.25 | 7.01 | 395.60 | 108.03 | 66.96 | 12.23 | 44.83 | 8.01 | 404.24 | 111.51 |
| KKThF3g1S1 | 60.75 | 8.38 | 39.31 | 4.95 | 389.87 | 104.47 | 65.33 | 11.55 | 43.36 | 5.98 | 404.07 | 109.28 |
| KMPmB2 | 71.52 | 11.83 | 49.93 | 6.80 | 462.21 | 107.17 | 81.65 | 16.29 | 57.75 | 8.42 | 506.55 | 118.95 |
| KMPmC2 | 68.17 | 10.88 | 46.09 | 5.73 | 421.36 | 98.18 | 74.83 | 14.60 | 50.78 | 6.87 | 435.73 | 103.15 |
| KMPmC2g1 | 67.24 | 10.27 | 46.91 | 9.33 | 446.45 | 101.86 | 74.54 | 14.10 | 52.65 | 8.38 | 472.90 | 109.31 |
| KMPmC3g1 | 66.52 | 10.81 | 46.29 | 8.28 | 432.00 | 102.16 | 73.93 | 14.66 | 51.53 | 8.81 | 451.61 | 108.44 |
| MRGmC2g1 | 67.38 | 10.36 | 47.42 | 8.81 | 440.71 | 102.60 | 77.41 | 14.73 | 54.86 | 7.27 | 485.99 | 114.51 |
| MRGmC2g1S1 | 64.11 | 10.56 | 47.80 | 10.35 | 448.98 | 103.79 | 75.05 | 15.08 | 55.12 | 11.22 | 484.74 | 114.11 |
| MRGmC3g1 | 63.39 | 10.34 | 46.60 | 5.60 | 435.16 | 106.82 | 72.88 | 14.55 | 51.56 | 6.87 | 455.77 | 114.10 |
| MRGmC3g1S1 | 64.60 | 10.27 | 45.74 | 6.06 | 442.11 | 105.34 | 74.94 | 14.57 | 51.91 | 7.42 | 467.25 | 113.57 |
| RTGhC2 | 61.91 | 9.34 | 36.36 | 5.51 | 440.70 | 107.31 | 67.93 | 12.71 | 39.80 | 6.49 | 444.07 | 110.10 |
| RTGhC2g1 | 62.87 | 9.44 | 37.07 | 8.69 | 426.33 | 108.71 | 69.36 | 12.93 | 41.28 | 10.06 | 439.65 | 113.79 |
| RTGhC2g1S1 | 61.91 | 9.30 | 39.21 | 8.74 | 428.48 | 101.81 | 68.96 | 12.89 | 43.98 | 10.95 | 447.49 | 107.93 |
| RTGhD3g1S1 | 61.09 | 9.03 | 36.48 | 7.94 | 415.85 | 100.18 | 70.64 | 12.94 | 41.45 | 8.92 | 433.22 | 106.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajesh, N.L.; Narayana Rao, K.; Desai, B.R.K.; Sathishkumar, U.; Basavaraj, K.; Rudramurty, H.V.; Wali, V.B.; Umesh, M.R. Spatio-Temporal Variability of Soil Properties and Nutrient Uptake for Sustainable Intensification of Rainfed Pigeon Pea (Cajanus cajana) in Semi-Arid Tropics of India. Sustainability 2023, 15, 3998. https://doi.org/10.3390/su15053998
Rajesh NL, Narayana Rao K, Desai BRK, Sathishkumar U, Basavaraj K, Rudramurty HV, Wali VB, Umesh MR. Spatio-Temporal Variability of Soil Properties and Nutrient Uptake for Sustainable Intensification of Rainfed Pigeon Pea (Cajanus cajana) in Semi-Arid Tropics of India. Sustainability. 2023; 15(5):3998. https://doi.org/10.3390/su15053998
Chicago/Turabian StyleRajesh, Neralikere Lakkappa, Koluru Narayana Rao, Bheemsain Rao K. Desai, Umapathi Sathishkumar, Kasareddy Basavaraj, Halagappala Verappa Rudramurty, Vijay B. Wali, and Mathada Rangappa Umesh. 2023. "Spatio-Temporal Variability of Soil Properties and Nutrient Uptake for Sustainable Intensification of Rainfed Pigeon Pea (Cajanus cajana) in Semi-Arid Tropics of India" Sustainability 15, no. 5: 3998. https://doi.org/10.3390/su15053998
APA StyleRajesh, N. L., Narayana Rao, K., Desai, B. R. K., Sathishkumar, U., Basavaraj, K., Rudramurty, H. V., Wali, V. B., & Umesh, M. R. (2023). Spatio-Temporal Variability of Soil Properties and Nutrient Uptake for Sustainable Intensification of Rainfed Pigeon Pea (Cajanus cajana) in Semi-Arid Tropics of India. Sustainability, 15(5), 3998. https://doi.org/10.3390/su15053998






