1. Introduction
The temperate region vegetation of the Khyber Pakhtunkhwa province, distributed at elevation ranges from 1200–2000 m above agricultural fields and below the sub-alpine woodlands, is dominated by oak forests. These communities are either pure or admixed with coniferous forests forming a distinct vegetation structure and composition [
1]. According to Alamgir [
2], the overall area of oak forests is 16,700 hectares (41,500 acres) and is mostly used for fuel, small timber, fencing around agricultural fields, agricultural equipment, charcoal wood, tannin extraction, and thatching roofs [
3]. Plant communities are typically complex ecosystems that constantly change in response to external factors and internal dynamics [
4].
Communities and ecosystems in transition zones are often the first to change because they may be affected by climate change, especially in areas with unfavorable conditions for plant growth [
3]. This phenomenon is prevalent in Pakistan’s Swat Mountains, which form ecotones between the countries’ wet and dry temperate zones [
1]. The Himalayas are part of the Sino-Japanese phytogeographical region, including Afghanistan and Gilgit-Karakorum Baltistan’s Range as the continuation of the Hindu Raj, making up the Great Hindukush escarpment [
5]. Climate, topography, and soil factors are the main determinants of vegetation patterns [
6,
7]. Because of the variety of habitats, the edaphic conditions, and its transitional position between wet and dry regions, the Swat Hindukush range of mountains is a suitable study site to ascertain if these factors affect the structure of the flora. Steep temperature gradients and high-elevation habitats may be essential buffers against climatic changes [
8]. It is critical to document the current plant groups and comprehend how they interact with their surroundings because deforestation, excessive grazing, and land clearing for terrace farming all contribute to the degradation of these forests [
9].
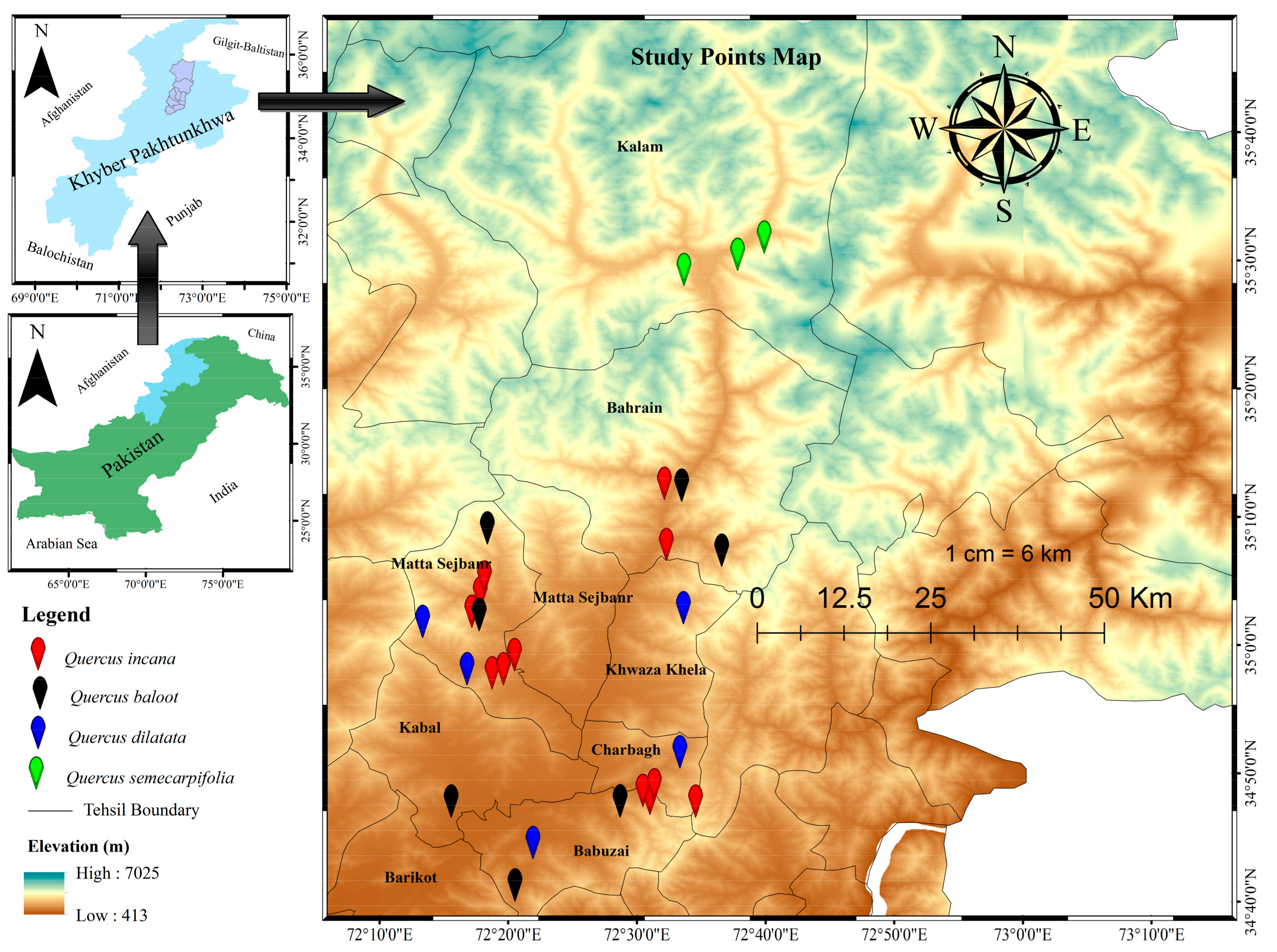
The District Swat in the Hindukush Range Mountains is a region sustaining these natural forests [
5]. It has attracted the attention of various organizations and tourists due to the high quality of the wood, accompanying wildlife, and plenty of non-timber forest products [
10]. It may create pure and mixed stands by establishing broad-scale ecological gradients [
11]. These stands have less focus, and more attention needs to be paid to how they relate to environmental factors [
6]. Clarifying these relationships is necessary to comprehend the make-up and structure of plant species in a particular habitat, terrain, and location [
12,
13]. Plant communities are dynamic and continually change in response to internal and external environments [
4,
14,
15].
Although a preliminary inventory of plant species for the study area was compiled [
16,
17], while [
17,
18] assessed vegetation characteristics, these authors ignored or just superficially examined the woody vegetation, focusing instead on the understory vegetation or classification. Other studies on related subjects, such as species niche modeling and ethnobotany, have also been conducted [
19,
20,
21,
22]. However, there have yet to be many studies on the stand structure of woody vegetation and its relationships with environmental conditions, even though the area includes thickets of dry and wet forests with various plant species. The objective of this research was to determine the structure of plant communities in the Swat Hindukush Mountains to understand better the main ecological and environmental gradients affecting the distribution of the oak population. This research will assist in understanding how to forecast the expansion of oak plant communities and their likely causes since they have significant ecological significance as a benchmark across the northern parts of Pakistan and adjacent countries.
The primary objectives of this study were to describe the stand structures of oak-dominated oak forests in the Swat Hindukush Mountains and to assess the relationship between stand structure and environmental variables. In addition, we intend to investigate any apparent changes in structure over time by examining tree age and size distributions. There were five questions listed specifically (i) How different is the tree and stand architectures of oak-dominated woodlands distributed over the terrain of the study region? (ii) Are there causal relationships between the current stand structures and the environmental factors? (iii) If true, what connections exist between ages and stand structures? (iv) How well-developed is oak regeneration in the sampled stands and does it predictably respond to the environment? (v) Do the size and age distributions suggest that the military operations and the 2010 flood altered the disturbance regimes in oak woodlands? We provide new details on the status, environmental linkages, and history of oak populations in a poorly-studied region. The findings of this research will fill important knowledge gaps concerning oak woodlands and might contribute to developing location-specific management strategies.
3. Results
The 30 sampled oak forest stands of different species revealed the dominance of
Q. incana (mean IVI = 84 ± 1.2) with the lowest cumulative variance percentage (
Table 1). The structural characteristics of stand density (232 stems/stand) and basal area (1348/stand) were higher for
Q. incana. Likewise, the numbers of seedlings were higher than saplings and trees, indicating good regeneration potential in the oak forest stands. Among the oak species,
Q. semecarpifolia attained the highest basal area (1172/4000 m
2) and was found to be a fairly regenerating species (Trees density < saplings > seedlings). Conversely,
Q. dilatata and
Q. baloot showed poor regeneration potential (Tree density > saplings > seedlings). Species richness was 8–12 tree/stand had been found in communities with fourteen associated species, of which
P. roxburghii (IV = 10.9 ± 10%) and
O. ferruginea (7.8 ± 4.9%) shared higher importance values after oak specie and emerged as the most common co-dominant species forming the second tree stratum (
Table 1).
Generally, the oak forest in the area has always been threatened by human intervention, especially the cutting down of trees in the forest canopy. The local inhabitants chopped down almost the entire forest patches in some areas, which led to little or no oak tree populations. The communities of
Q. semecarpifolia (30.8 ± 13.0 stem/stand) and
Q. incana (29.4 ± 9.4 stem/stand) were mostly affected with the highest number of cut trees. In contrast, the lowest number of cut trees and other anthropogenic disturbances were recorded in
Q. baloot and
Q. dilatata communities (
Table 2).
Furthermore, the pairwise statistical analysis (
Table 3) showed significant differences in the structural and dendrometric parameters such as Importance values (
p = 0.004), especially between
Q.
semecarpifolia and
Q.
incana (
p < 0.001) and
Q.
dilatata and
Q.
incana (
p = 0.006). The four Oak species’ significant difference was recorded among the basal area m
2/ha (
p = 0.025) and density ha
−1 (
p = 0.008). However, the density of
Q.
semecarpifolia and
Q.
incana (
p = 0.001) and between
Q. dilatata and Q. incana (
p = 0.010) forest stands varied significantly when compared with the basal area of these species. Correspondingly, seedling density among the four oak species exhibited significant variation (
p < 0.001) and identical patterns. For instance, the pairwise differences between
Q. semecarpifolia and
Q. incana (
p < 0.001) and
Q. dilatata and Q. incana (
p < 0.001), and
Q. baloot and Q. incana (
p = 0.001) were obtained (
Table 3).
The height size classes of the Oak species show inverse J-shape distribution (
Figure 2). The number of seedlings was lower than saplings in species indicating low regeneration potential. Similarly, the number of individuals in height size classes progressively decreases with an increase in height. The pattern of diameter at breast height (DBH) was the same for
Q. semecarpifolia and
Q. incana, in which the frequencies decreased with an increase in diameter at breast height. However, the pattern is a little irregular in
Q. baloot and
Q. dilatata e.g., in
Q. dilatata frequencies of 11–20 cm DBH and 51–60 cm DBH were highest while the remaining DBH classes follow progressive decreasing trends in frequencies while in
Q.
baloot the trend is uniformed except for 51–60 cm dbh where the frequencies increases than the proceeding and preceding DBH size class.
The stems stumped of three Oak species collected have age ranges between 13–42 years. The age structure of stands was dominated by
Q. incana 19–25 age group (45%), followed by 25–30 (34%), while the remaining were trees aged between 31–42 years. Similarly, in
Q. dilatata, the age group 13–18 years was highest (65%) while the rest ranged from 19–36 years. In
Q. baloot dominated stands the age group 19–25 years was frequent (32%) followed by the age group 13–18 years and 25–30 years (25% each) while the rest were having age of 31–36 years. Overall, the age structure represents young communities with lower class classes in most of the stands dominated by Oak species (
Figure 3).
There were 18 distinct tree species linked to the four groups of oak-dominated woodland. According to a life table for seedlings, saplings, and tree species, saplings make up most of the population in forests where oak trees predominate (
Table 4). While gigantic trees have a short lifespan, saplings, and little trees have a long one. The highest rate of survival (
Ix) was revealed by saplings, followed by seedlings, and the lowest rate of survival was found for giant trees, most of which were old and fell for various economic reasons. Compared to other tree plants, extremely few small trees have been found to have a modest survival rate. Stage (age) specific mortality (
Qx): Mortality was shown to be lowest for seedlings and very tiny trees due to their limited utility and environmental adaptation and greatest for gigantic trees, followed by medium size trees. Trees of little and medium sizes die out at a moderate pace. The mean expectancy of additional life (
Ex) for communities where oak predominates revealed that seedlings reached the highest value of 0.55. Very tiny trees in the community size class had a maximum mean expectancy of life value of −1.54, while saplings displayed −0.46 values (
Table 4). All stands showed a −0.213 life expectancy for giant trees. In addition, the individuals’ oak species life tables are presented in
Appendix A (
Table A1,
Table A2,
Table A3 and
Table A4).
Two distinct and obvious gradients in the stand structure were found in RDA-ordination, coupled with a third, weaker gradient, across all the stands. Axis 1 and 2 contributed 32.7% of the variance, whereas the ordination accounted for 41.3% of the variability in stand structure data (
Table 5). The strong gradient on axis 1 was elevation (
r = 0.47,
p < 0.01), precipitation (
r = −0.47,
p < 0.01), relative humidity (
r = −0.77,
p < 0.001), and Phosphorusphosphorus (
r = −0.33,
p < 0.05). Similarly, on axis 2, the gradient of environmental factors was strongly explained by Potassium (
r = −0.66,
p < 0.001) and elevation (
r = −0.51,
p < 0.05). In contrast, axis 3 has a strong gradient of precipitation (
r = 0.47,
p < 0.01) and maximum temperature (
r = 0.45,
p < 0.01). Likewise, in biplot scores, the bulk of explainable variables accommodated on axes 1 and 2 (
Table 5 and
Figure 4).
Axis 1 has the maximum biplot score for relative humidity (2.72), followed by precipitation (−1.65) and elevation (1.66). Similarly, on axis 2, Potassium (−1.88) and elevation (−1.46) revealed the highest biplot scores, respectively. In the two-dimensional biplot, these factors are presented as red arrows indicating their vital role in shaping the stand structure of an Oak-dominated forest.
4. Discussion
The present study describes the tree vegetation composition, age structure, and distribution pattern of oak-dominated forests in Pakistan’s Swat Hindu-Kush ranges. Even though four oak species predominated the vegetation, showing structural and floristic heterogeneity and having a complex relationship with the environment [
3]. The three oak communities were found in the research region at a moderate height of 1160 to 2289 m a.s.l. and were dominated by
Q. incana,
Q. baloot, and
Q. dilatata. While the pure population of
Q. semecarpifolia was found at high elevations, the species-rich
Q. incana community thrived at lower altitudes (1160 m). The areas with medium elevations had most of the
Q. baloot and
Q. dilatata groups. The oak communities’ co-dominant species were the broad-leaved
Olea ferruginea and coniferous
Pinus roxburghii. Numerous studies categorizing the northern Pakistani Himalayan, Hindukush, and Karakoram regions have shown a similar communal structure.
Pinus roxburghii co-dominate with
Q. baloot,
Q. dilatata, and
Olea ferruginea [
30]; these reports were subsequently confirmed by [
17,
18,
19] in
Pinus wallachiana populations in the same area. Additionally, they showed how the communities were distributed depending on elevation, which is consistent with the present research results. The current study’s findings on the distribution pattern and occurrences of various species in oak ecosystems are also compatible with [
42].
The regeneration status could have been higher due to the number of trees reported in the communities. In stands of oak forests, older trees often start to regenerate after 30 to 40 years, and saplings frequently survive in significant numbers in harsh environments [
43]. Most of the stated forest ages in our research region were relatively young, which might indicate inadequate levels of regeneration. It is evident from this research that if forests are adequately maintained, oak will regenerate, especially in good seed years as the forest ages. However, other conditions start to matter after this stage and impact the oak saplings’ survival ability. As shown for oak species in the Hindukush regions in the present research and the same genus [
44], this demonstrates that ontogeny significantly influences resource requirements. Competition within the regeneration layer is essential to how well oak regenerates.
Oak seedlings have been observed to be hampered by several trees, shrubs, and plant species [
45,
46,
47]. Since oaks are considered species that need high quantities of light [
48], any disruption of the canopy cover in closed forests improves conditions for oak seedlings and saplings. Competing
Pinus species and
Olea ferruginea regeneration had a role in this situation. Where
Pinus species and
Olea ferruginea regeneration was abundant, oak regeneration will be lagged, and vice versa.
Pinus seedlings naturally outcompete oak saplings because of their exotic nature [
49]. When combined with the older age’s saplings, our findings of low oak seedling-to-sapling ratios and greater sapling-to-tree ratios in many stands may indicate that current seedling recruitment rates are likewise low in these ecosystems. If present regeneration rates are abnormally low, it is unclear what is causing the slowing or stopping of regeneration in this case. However, one of the main co-dominant species in the oak woodlands was identified as
Pinus roxburghii [
50,
51,
52]. Apart from land conversion [
53], conifer encroachment is thought to be the most significant threat to the survival of oak forests; nevertheless, in the oak-dominated stands, this is likely to be the main factor restricting seedling and sapling recruitment [
17,
30]. As a result, it is known that replacing oak with other species following many forms of disturbance is of recurrent concern [
54].
To determine the static life table of the oak-dominated forest population in the various study areas, we calculated the survival number of standardization (
Ix), the number of deaths (
Dx), longevity (
Tx), expected longevity (
Ex), and periodic longevity (
Lx), for each age class, as shown in
Table 3. According to research,
Q. baloot saplings have the highest life expectancy of the tree, while
Quercus dilatata seedlings are the most prevalent in woods. Due to its economic and commercial purposes, it was discovered that trees in all oak-dominated forests had a poor survival rate. Due to their strong tolerance to cold and drought, saplings were found to have the best survival rates across all communities, whereas large trees had the lowest rates. Conferring to [
55], various ecological parameters are crucial for supporting these tree species’ development and survival. Many researchers studying oak forests, such as [
56,
57,
58], observed high death rates for large trees.
Stand structure gradients revealed weakly correlated associations with certain environmental conditions, i.e., lime and nitrogen percentages. The parameters strongly related to the stand structure were climate, elevation, and soil nutrients, which showed the highest correlation across the sampling sites. Negative impacts of precipitation, temperature, relative humidity, altitude, Potassium, and phosphorus content on stand structure are evident. Numerous studies suggested higher temperatures could shorten germination times and decrease seedling richness [
59,
60]. For instance, [
61] reported that increasing temperatures reduced the germination and survival of
A. pseudoplatanus and
A. platanoides. This trend may be explained by the likelihood that warming would lessen the competitive advantage from which this species previously benefited under the earlier colder environment [
62]. Furthermore, since warming increases surface evapotranspiration and decreases soil moisture, creating a water deficit throughout the growing season might hinder recruitment [
61,
63,
64]. For instance, Linares [
65] exposed that yew (
Taxus baccata) regrowth at its southern boundary was limited by water availability [
66,
67,
68]. In addition, tree mortality brought on by physiological stress and temperature, the depletion of starch pools, and interactions with other climate-mediated processes like insect outbreaks and wildfires may increase due to global warming [
69,
70,
71,
72,
73].
5. Conclusions
This research is unique because it assessed oak species’ stand structure, regeneration status, population dynamics, and its relation with the environmental variables. The communities had a fair regeneration status showing strong relation with environmental variables, particularly the elevation gradient. This advances our understanding of the community structure of oak-dominated forests across the elevation gradient, and the species in other regions may follow the same distribution pattern. Apart from local factors, i.e., soil and environmental, regional and global climatic factors may also limit the distribution and dynamics of these forests in the future. In addition, the link between site moisture state, stand architecture, and oak recruitment in the Hindu Kush mountain range requires further study. This study better understood the mechanisms driving vegetation distribution in similar or other vegetation types at adjoining high mountain ranges in Pakistan, India, and Afghanistan. The forests were mostly human-conserved. However, reports of chopped and dead trees suggest human interference. As climate change becomes more significant and land managers are forced to account for these changes in their ecosystem management plans, it may be especially vital to understand the linkages between oak regeneration and site environment state. We suggest a more extensive study at the local and regional levels to reveal the interactions between these forests’ vegetation and environment by including several additional biotic and abiotic components into account.