In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Material and Its Viability
2.2. In Vitro Seed Germination
2.3. In Vitro Culture Establishment Stage
2.4. Shoot Multiplication Stage
2.5. In Vitro Rooting
2.6. Ex Vitro Acclimatization and Plant Growth
2.7. In Vitro Culture Conditions
2.8. Statistical Analysis
3. Results
3.1. Seed Material and Its Viability
3.2. In Vitro Seed Germination
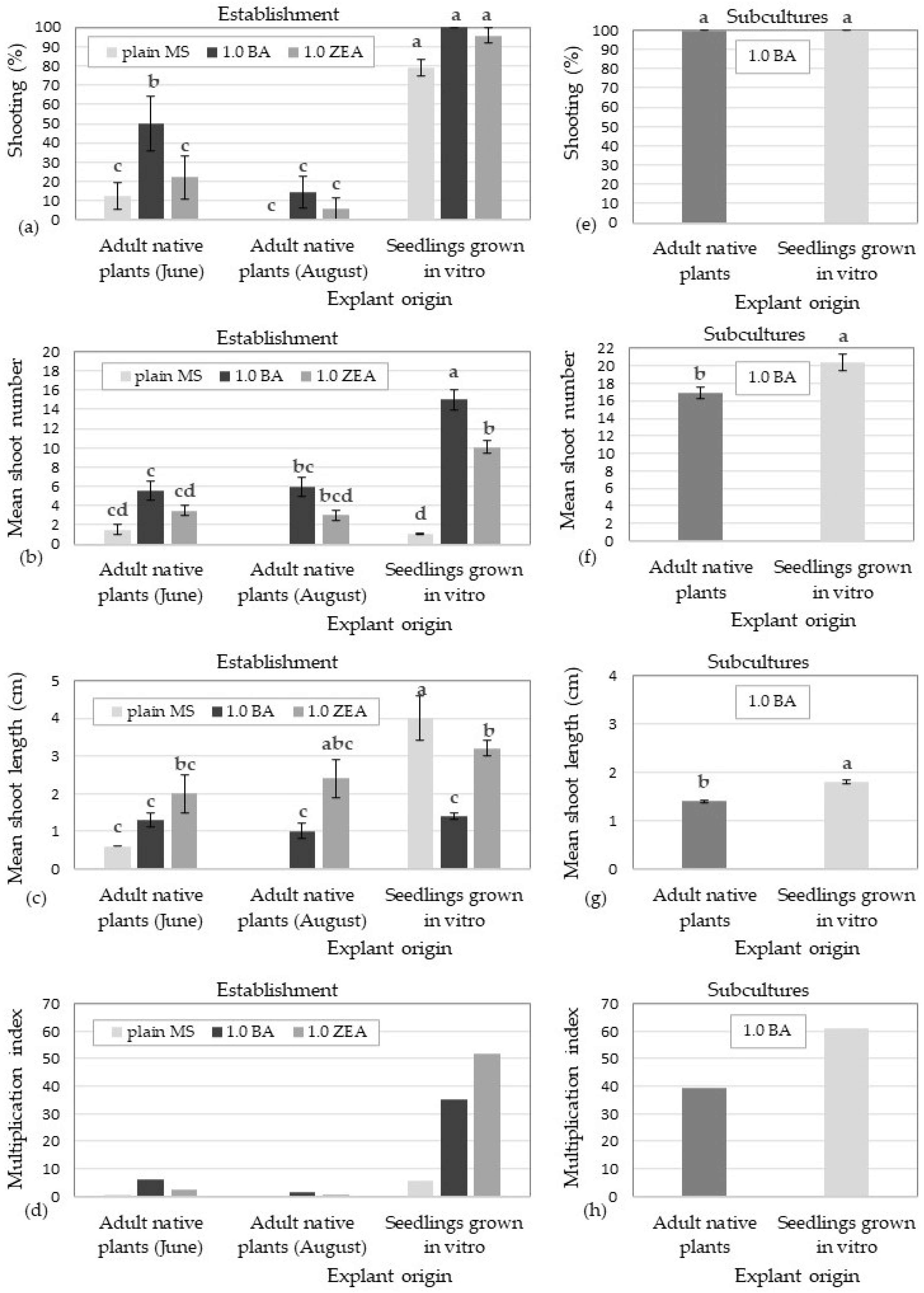
3.3. In Vitro Culture Establishment Stage
3.4. Shoot Multiplication Stage
3.5. In Vitro Rooting
3.6. Ex Vitro Acclimatization and Plant Growth
4. Discussion
4.1. Collection of Seed and Tissue Culture Material
4.2. In Vitro Seed Germination
4.3. In Vitro Clonal Propagation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kabbadas, D.S. Illustrated Botanical Botanical Dictionary; Pelekanos Publications: Athens, Greece, 1956; Volume I, pp. 479–480. [Google Scholar]
- Polunin, O. Flowers of Greece and the Balkans. A Field Guide; Oxford University Press: London, UK, 1980; p. 308. [Google Scholar]
- Arabatzis, T.I. Shrubs and Trees in Greece; Ecological Movement of Drama; Technological Educational Institute of Kavala: Drama, Greece, 2001; Volume II, p. 83. [Google Scholar]
- Anthyllis hermanniae L. Plants of the World Online. Board of Trustees of the Royal Botanic Gardens, Kew. 2017. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:474699-1 (accessed on 7 July 2020).
- GBIF Backbone Taxonomy. “Anthyllis hermanniae L.” gbif.org. GBIF Secretariat. Available online: https://www.gbif.org/species/5352341 (accessed on 7 July 2020).
- Papafotiou, M.; Bertsouklis, K.; Martini, A.N.; Vlachou, G.; Akoumianaki-Ioannidou, A.; Kanellou, E.; Kartsonas, E. Evaluation of the establishment of native Mediterranean plant species suggested for landscape enhancement in archaeological sites of Greece. Acta Hortic. 2017, 1189, 177–180. [Google Scholar] [CrossRef]
- Egerer, M.; Cecala, J.; Cohen, H. Wild Bee Conservation within Urban Gardens and Nurseries: Effects of Local and Landscape Management. Sustainability 2019, 12, 293. [Google Scholar] [CrossRef]
- Yang, Y.; Battesti, M.-J.; Paolini, J.; Costa, J. Pollen Diversity and Volatile Variability of Honey from Corsican Anthyllis hermanniae L. Habitat. Chem. Biodivers. 2014, 11, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Spera, K.; Flamini, G.; Mele, S.; Morelli, I. Isoflavonoids and chalcones from Anthyllis hermanniae. Phytochemistry 1996, 42, 1455–1458. [Google Scholar] [CrossRef]
- Halabalaki, M.; Paschali, A.; Mitakou, S.; Skaltsounis, A.L. New flavonoid triglycosides from Anthyllis hermanniae. Planta Medica 2007, 73, 345. [Google Scholar] [CrossRef]
- Paschali, A.; Halabalaki, M.; Tsiripillou, P.; Skaltsounis, A.L. Total synthesis of Hermannioside A, a novel flavonoid triglycoside from Anthyllis hermanniae. Planta Med. 2008, 74, PG92. [Google Scholar] [CrossRef]
- Halabalaki, M.; Urbain, A.; Paschali, A.; Mitakou, S.; Tillequin, F.; Skaltsounis, A.-L. Quercetin and Kaempferol 3-O-[α-l-Rhamnopyranosyl-(1→2)-α-l-arabinopyranoside]-7-O-α-l-rhamnopyranosides from Anthyllis hermanniae: Structure Determination and Conformational Studies. J. Nat. Prod. 2011, 74, 1939–1945. [Google Scholar] [CrossRef]
- Paschali, A.; Termentzi, A.; Halabalaki, M.; Skaltsounis, A. Phytochemical analysis of Anthyllis hermanniae—Leguminosae, and development of a sensitive UHPLC-HRMS/MS method for the rapid analysis of the phenolic content. Planta Med. 2011, 77, PA26. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Physiology of dormancy and germination in relation to seed bank ecology. In Ecology of Soil Seed Banks; Leck, M.A., Parker, V.T., Simpson, R.L., Eds.; Academic Press: San Diego, CA, USA, 1989; pp. 53–66. [Google Scholar]
- Bevilacqua, L.R.; Roti-Michelozzi, G.; Modenesi, P. The watertight dormancy of Melilotus alba seeds: Further observations on the palisade cell wall. Can. J. Bot. 1989, 67, 3453–3456. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Wangwang, Y.; Hanson, J.; Mariam, Y. Effect of sulfuric acid pretreatment on breaking hard seed dormancy in diverse accessions of five wild Vigna species. Seed Sci. Technol. 2007, 35, 550–559. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Breaking Seed Dormancy during Dry Storage: A Useful Tool or Major Problem for Successful Restoration via Direct Seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds-Physiology of Development and Germination, 2nd ed.; Plenum Press: New York, NY, USA, 1994; p. 445. [Google Scholar]
- Doussi, M.A.; Thanos, C.A. Post-fire regeneration of hardseeded plants: Ecophysiology of seed germination. In Proceedings of the 2nd International Conference on Forest Fire Research, Coimbra, Portugal, 21–24 November 1994; Volume 2, pp. 1035–1044. [Google Scholar]
- Papafotiou, M.; Martini, A.N. In Vitro Seed and Clonal Propagation of the Mediterranean Aromatic and Medicinal Plant Teucrium capitatum. Hortscience 2016, 51, 403–411. [Google Scholar] [CrossRef]
- Máthé, A.; Hassan, F.; Abdul Kader, A. In vitro micropropagation of medicinal and aromatic plants. In Medicinal and Aromatic Plants of the World; Máthé, A., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 1, pp. 305–336. [Google Scholar]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitr. Cell. Dev. Biol. Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Lemma, D.T.; Banjaw, D.T.; Megersa, H.G. Micropropagation of medicinal plants. Int. J. Plant Breed Crop. Sci. 2020, 7, 796–802. [Google Scholar]
- Gavidia, I.; Zaragoza, C.; Segura, J.; Pérez-Bermudez, P. Plant regeneration from juvenile and adult Anthyllis cytisoides, a multipurpose leguminous shrub. J. Plant Physiol. 1997, 150, 714–718. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K. Studies on in vitro propagation of Anthyllis barbajovis L. Acta Hortic. 2017, 1155, 317–320. [Google Scholar] [CrossRef]
- Trigka, M.; Papafotiou, M. In vitro propagation of Anthyllis barba-jovis from seedling tissues. Acta Hortic. 2017, 1189, 473–748. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Murthy, H.N.; Ammar, M.H.; Alghamdi, S.S.; Al-Suhaibani, N.A.; Alsadon, A.A.; Paek, K.Y. In vitro rooting of leguminous plants: Difficulties, alternatives, and strategies for improvement. Hortic. Environ. Biotechnol. 2016, 57, 311–322. [Google Scholar] [CrossRef]
- Bonga, J.M. Vegetative propagation in relation to juvenility, maturity and rejuvenation. In Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijdhoff/Dr W Junk: The Hague, The Netherlands, 1982; pp. 387–412. [Google Scholar]
- Martini, A.N.; Papafotiou, M. In Vitro Propagation and NaCl Tolerance of the Multipurpose Medicinal Halophyte Limoniastrum monopetalum. Hortscience 2020, 55, 436–443. [Google Scholar] [CrossRef]
- Papafotiou, M.; Vlachou, G.; Martini, A.N. Investigation of the Effects of the Explant Type and Different Plant Growth Regulators on Micropropagation of Five Mediterranean Salvia spp. Native to Greece. Horticulturae 2023, 9, 96. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Goyne, K.W.; Udawatta, R.P. Environmental Impacts of Enhanced-Efficiency Nitrogen Fertilizers. Crop. Manag. 2008, 7, 1–15. [Google Scholar] [CrossRef]
- Folina, A.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Katsenios, N.; Efthimiadou, A.; Travlos, I.; Roussis, I.; Darawsheh, M.; Papastylianou, P.; et al. Evaluation of Various Nitrogen Indices in N-Fertilizers with Inhibitors in Field Crops: A Review. Agronomy 2021, 11, 418. [Google Scholar] [CrossRef]
- Akoumianaki-Ioannidou, A.; Martini, A.N.; Papafotiou, M. Rooting and establishment of Limoniastrum monopetalum (L.) Boiss stem-tip cuttings. Afr. J. Plant Sci. 2016, 10, 23–31. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Akoumianaki-Ioannidou, A. Vegetative propagation by stem cuttings and establishment of the Mediterranean aromatic and medicinal plant Teucrium capitatum. Acta Hortic. 2017, 455–460. [Google Scholar] [CrossRef]
- Moore, R.P.E. Handbook on Tetrazolium Testing, 1st ed.; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 1985; p. 99. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Molau, U.; Shaver, G. Controls on seed production and seed germinability inEriophorum vaginatum. Glob. Chang. Biol. 1997, 3, 80–88. [Google Scholar] [CrossRef]
- Morgan, J.W. Effects of Population Size on Seed Production and Germinability in an Endangered, Fragmented Grassland Plant. Conserv. Biol. 1999, 13, 266–273. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Vlachou, G.; Trigka, M.; Papafotiou, M. In Vitro Studies on Seed Germination of the Mediterranean Species Anthyllis barbajovis to Facilitate Its Introduction into the Floriculture Industry. Horticulturae 2022, 8, 889. [Google Scholar] [CrossRef]
- Thanos, C.A.; Kadis, C.C.; Skarou, F. Ecophysiology of germination in the aromatic plants thyme, savory and oregano (Labiatae). Seed Sci. Res. 1995, 5, 161–170. [Google Scholar] [CrossRef]
- Thanos, C.A.; Doussi, M.A. Ecophysiology of seed germination in endemic labiates of crete. Isr. J. Plant Sci. 1995, 43, 227–237. [Google Scholar] [CrossRef]
- Morbidoni, M.; Estrelles, E.; Soriano, P.; Martínez-Solís, I.; Biondi, E. Effects of environmental factors on seed germination of Anthyllis barbajovis L. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2008, 142, 275–286. [Google Scholar] [CrossRef]
- Ibañez, A.; Passera, C. Factors affecting the germination of albaida (Anthyllis cytisoides L.), a forage legume of the Mediterranean coast. J. Arid. Environ. 1997, 35, 225–231. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, J.M.; Baskin, C.C.; Benakashani, F. A meta-analysis of the effects of treatments used to break dormancy in seeds of the megagenus Astragalus (Fabaceae). Seed Sci. Res. 2020, 30, 224–233. [Google Scholar] [CrossRef]
- Vlachou, G.; Martini, A.Ν.; Dariotis, Ε.; Papafotiou, Μ. Comparative evaluation of seed germination of five Mediterranean sage species (Salvia sp.) native to Greece. Acta Hortic. 2020, 1298, 593–598. [Google Scholar] [CrossRef]
- Rolston, M.P. Water impermeable seed dormancy. Bot. Rev. 1978, 44, 365–396. [Google Scholar] [CrossRef]
- Kadis, C.; Georghiou, K. Seed dispersal and germination behavior of three threatened endemic labiates of Cyprus. Plant Species Biol. 2010, 25, 77–84. [Google Scholar] [CrossRef]
- Kanellou, E.; Vlachou, G.; Martini, A.N.; Bertsouklis, K.; Papafotiou, M. Seed germination of five sage species (Salvia sp.) of populations native to Greece. Acta Hortic. 2022, 1345, 439–444. [Google Scholar] [CrossRef]
- Chalupa, V. Micropropagation of European mountain ash (Sorbus aucuparia L.) and wild service tree [Sorbus torminalis (L.) Cr.]. In Biotechnology in Agriculture and Forestry 18; High-Tech and Micropropagation, II; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1992; pp. 211–226. [Google Scholar]
- Capuana, M.; Giannini, R. Micropropagation of young and adult plants of cypress (Cupressus sempervirens L.). J. Hortic. Sci. 1997, 72, 453–460. [Google Scholar] [CrossRef]
- Chang, S.-H.; Ho, C.-K.; Chen, Z.-Z.; Tsay, J.-Y. Micropropagation of Taxus mairei from mature trees. Plant Cell Rep. 2001, 20, 496–502. [Google Scholar] [CrossRef]
- Monteuuis, O. In vitro micropropagation and rooting of Acacia mangium microshoots from juvenile and mature origins. In Vitr. Cell. Dev. Biol. Plant 2004, 40, 102–107. [Google Scholar] [CrossRef]
- Kartsonas, E.; Papafotiou, M. Mother plant age and seasonal influence on in vitro propagation of Quercus euboica Pap., an endemic, rare and endangered oak species of Greece. Plant Cell Tissue Organ Cult. 2007, 90, 111–116. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Vemmos, S.N. Season and Explant Origin Affect Phenolic Content, Browning of Explants, and Micropropagation of ×Malosorbus florentina (Zucc.) Browicz. Hortscience 2013, 48, 102–107. [Google Scholar] [CrossRef]
- Pierik, R.L.M. Rejuvenation and Micropropagation. In Progress in Plant Cellular and Molecular Biology. Current Plant Science and Biotechnology in Agriculture; Nijkamp, H.J.J., Van Der Plas, L.H.W., Van Aartrijk, J., Eds.; Springer: Dordrecht, The Netherlands, 1990; Volume 9, pp. 91–101. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. Εffects of plant growth regulators and environmental factors on in vitro propagation of ×Malosorbus florentina. Propag. Ornam. Plants 2013, 13, 112–122. [Google Scholar]
- Vlachou, G.; Trigka, M.; Papafotiou, M. Effect of plant growth regulators and agar concentration on shoot multiplication and hyperhydricity of Anthyllis barbajovis. Acta Hortic. 2020, 341–346. [Google Scholar] [CrossRef]
- Lai, C.-C.; Lin, H.-M.; Nalawade, S.M.; Fang, W.; Tsay, H.-S. Hyperhydricity in shoot cultures of Scrophularia yoshimurae can be effectively reduced by ventilation of culture vessels. J. Plant Physiol. 2004, 162, 355–361. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Studies on in vitro propagation of lithodora zahnii. Acta Hortic. 2009, 813, 465–470. [Google Scholar] [CrossRef]
- Kartsonas, E.; Papafotiou, M. Effect of culture vessels size and covering material on leaf morphological and anatomical characteristics of quercus euboica in vitro plantlets. Acta Hortic. 2010, 885, 191–196. [Google Scholar] [CrossRef]
- Lane, W.D. Micropropagation of apple (Malus domestica Borkh.). In Biotechnology in Agriculture and Forestry 18; High-Tech and Micropropagation, II; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1992; pp. 229–243. [Google Scholar]
- Liu, P.Z.; He, S.T.; Han, L.X.; Wang, J.S. Study on rooting techniques for apple in vitro. J. Fruit Sci. 1991, 8, 139–144. [Google Scholar]
- De Klerk, G.-J.; Van Der Krieken, W.; De Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. In Vitr. Cell. Dev. Biol. Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszki, J.; Hudák, I. Effect of cytokinin content of the regeneration media on in vitro rooting ability of adventitious apple shoots. Sci. Hortic. 2011, 129, 910–913. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]






| Pretreatment | Photoperiod | Germination Temperature (°C) | Germination (%) | T50 (Days) | Time for Full Germination (Days) |
|---|---|---|---|---|---|
| Seed collecting time: | A13/J14 | A13/J14 | A13/J14 | ||
| No scarification | 16 h light/ 8 h dark | 5 | 0.0 k Z/6.0 h | -/30 | -/33 |
| 10 | 50.0 h/42.0 cde | 10/10 | 19/27 | ||
| 15 | 35.0 i/40.0 de | 15/7 | 19/24 | ||
| 20 | 70.0 de/58.0 c | 9/10 | 37/27 | ||
| 25 | 50.0 h/35.0 def | 16/12 | 34/24 | ||
| 30 | 20.0 j/18.0 fgh | 9/9 | 10/21 | ||
| Continuous darkness | 5 | 0.0 k/12.0 gh | -/30 | -/33 | |
| 10 | 40.0 i/28.0 efg | 8/16 | 22/27 | ||
| 15 | 55.0 gh/44.0 cde | 8/7 | 28/27 | ||
| 20 | 50.0 h/46.0 cd | 8/5 | 19/27 | ||
| 25 | 25.0 j/40.0 de | 7/15 | 37/27 | ||
| 30 | 25.0 j/36.0 de | 4/12 | 31/27 | ||
| Mechanical scarification with sandpaper for 1 min | 16 h light/ 8 h dark | 5 | 0.0 k/40.0 de | -/28 | -/33 |
| 10 | 75.0 d/84.0 ab | 8/9 | 28/27 | ||
| 15 | 100.0 a/86.0 ab | 6/7 | 28/27 | ||
| 20 | 100.0 a/94.0 a | 6/7 | 37/15 | ||
| 25 | 85.0 c/76.0 b | 6/10 | 37/21 | ||
| 30 | 65.0 ef/46.0 cd | 6/9 | 19/30 | ||
| Continuous darkness | 5 | 0.0 k/30.0 def | -/28 | -/33 | |
| 10 | 95.0 ab/90.0 ab | 8/9 | 16/24 | ||
| 15 | 85.0 c/98.0 a | 6/5 | 22/15 | ||
| 20 | 90.0 bc/98.0 a | 4/5 | 16/12 | ||
| 25 | 90.0 bc/86.0 ab | 6/12 | 40/30 | ||
| 30 | 60.0 fg/44.0 cde | 6/10 | 40/30 | ||
| Significance § | |||||
| Fpretreatment | -/- | ||||
| Fphotoperiod | -/NS | ||||
| Ftemperature | -/- | ||||
| Fpretreatment × photoperiod | -/NS | ||||
| Fpretreatment × temperature | -/** | ||||
| Fphotoperiod × temperature | -/NS | ||||
| Fpretreatment × photoperiod × temperature | **/NS | ||||
| Fone-way ANOVA | **/** | ||||
| Cytokinin | Shooting (%) | Mean Shoot Number | Mean Shoot Length (cm) | Multiplication Index | |
|---|---|---|---|---|---|
| Type | Concentration | ||||
| Control # | 0.0 | 100.0 a Z | 1.5 h | 2.0 a | 4.9 |
| BA | 0.5 | 100.0 a | 17.3 b | 1.4 cdef | 40.9 |
| 1.0 | 100.0 a | 17.8 b | 1.4 cdef | 40.9 | |
| 2.0 | 100.0 a | 20.8 a | 1.3 f | 44.4 | |
| 4.0 | 100.0 a | 17.3 b | 0.9 g | 27.1 | |
| ZEA | 0.5 | 100.0 a | 7.0 e | 1.4 cdef | 16.3 |
| 1.0 | 100.0 a | 8.6 de | 1.5 cdef | 20.8 | |
| 2.0 | 100.0 a | 10.2 d | 1.5 cde | 26.2 | |
| 4.0 | 100.0 a | 14.9 c | 1.4 def | 33.8 | |
| KIN | 0.5 | 93.3 b | 1.3 h | 1.3 ef | 2.7 |
| 1.0 | 90.0 b | 1.6 gh | 1.6 bcd | 3.8 | |
| 2.0 | 93.3 b | 1.6 gh | 1.6 bc | 4.0 | |
| 4.0 | 100.0 a | 2.5 gh | 1.4 cdef | 6.8 | |
| 2iP | 0.5 | 100.0 a | 1.3 h | 1.8 ab | 3.9 |
| 1.0 | 100.0 a | 2.2 gh | 1.8 ab | 6.7 | |
| 2.0 | 100.0 a | 3.1 g | 1.6 bc | 9.2 | |
| 4.0 | 100.0 a | 5.0 f | 1.4 cdef | 11.9 | |
| Significance § | |||||
| Fcytokinin type | ** | - | - | ||
| Fcytokinin concentration | NS | - | - | ||
| Fcytokinin type × concetration | NS | ** | * | ||
| Fone-way ANOVA | * | ** | ** | ||
| Microshoot Origin | ΙΒA (mg L−1) | Callus (%) | Rooting (%) | Mean Root Number | Mean Root Length (cm) |
|---|---|---|---|---|---|
| 6 w/1 w | 6 w/1 w | 6 w/1 w | 6 w/1 w | ||
| Adult native plants | 0.0 | 4.8 b Z/0.0 c | 48.8 abc/15.0 b | 1.9 c/3.7 ab | 3.4 bc/4.8 bcd |
| 0.5 | 6.7 b/0.0 c | 39.1 c/25.0 b | 2.7 bc/1.6 bc | 3.4 bc/2.0 d | |
| 1.0 | 6.7 b/5.0 bc | 42.3 c/20.0 b | 2.6 bc/1.3 c | 4.2 abc/3.0 cd | |
| 2.0 | 16.3 ab/10.0 ab | 45.9 bc/30.0 b | 3.0 bc/1.2 c | 3.3 c/5.9 bc | |
| 4.0 | 16.5 ab/15.0 a | 67.0 ab/35.0 b | 2.6 bc/1.9 bc | 5.3 a/6.1 bc | |
| In vitro grown seedlings | 0.0 | 0.0 b/0.0 c | 33.0 c/33.3 b | 2.5 bc/3.2 ab | 5.1 ab/9.9 a |
| 0.5 | 21.8 ab/0.0 c | 35.1 c/50.0 ab | 2.7 bc/3.1 ab | 4.9 abc/7.6 ab | |
| 1.0 | 15.2 ab/0.0 c | 39.3 c/42.9 ab | 3.1 bc/3.5 a | 4.9 abc/7.1 b | |
| 2.0 | 30.6 ab/0.0 c | 46.0 bc/68.6 a | 3.4 b/3.7 a | 4.4 abc/6.8 b | |
| 4.0 | 41.1 a/0.0 c | 71.8 a/70.0 a | 4.8 a/3.7 a | 5.4 a/7.1 b | |
| Significance § | |||||
| Fmicroshoot origin | ΝS/- | NS/** | */** | **/** | |
| FΙΒA concentration | NS/- | **/* | **/NS | */NS | |
| Fmicroshoot origin ×ΙΒA concentration | NS/* | NS/NS | NS/NS | NS/NS | |
| Fone-way ANOVA | */** | */** | **/** | */** | |
| Cytokinin Type (mg L−1) | ΙΒA (mg L−1) | Callus (%) | Rooting (%) | Mean Root Number | Mean Root Length (cm) |
|---|---|---|---|---|---|
| A/J | A/J | A/J | A/J | ||
| 1.0 ΒA | 0.0 | 0.0 a Z/0.0 a | 46.7 abc/33.0 d | 2.0 cd/2.5 d | 3.8 a/5.1 a |
| 0.5 | 0.0 a/19.6 a | 33.3 c/35.1 d | 3.4 ab/2.7 d | 4.0 a/4.9 a | |
| 1.0 | 0.0 a/15.2 a | 26.7 c/39.3 cd | 4.0 ab/3.1 cd | 3.2 abc/4.9 a | |
| 2.0 | 0.0 a/30.6 a | 46.7 abc/46.0 bcd | 3.7 ab/3.4 cd | 3.4 ab/4.4 a | |
| 4.0 | 0.0 a/41.1 a | 40.0 bc/71.8 ab | 4.2 a/4.8 ab | 3.5 ab/5.4 a | |
| 1.0 ΖΕA | 0.0 | 0.0 a/2.1 a | 71.7 a/44.3 bcd | 3.1 ab/2.7 d | 3.0 abc/6.2 a |
| 0.5 | 0.0 a/15.2 a | 71.7 a/66.1 abc | 3.8 a/3.7 bcd | 2.6 bcd/5.3 a | |
| 1.0 | 0.0 a/21.7 a | 66.7 ab/57.5 abcd | 1.5 d/4.5 abc | 1.9 d/6.6 a | |
| 2.0 | 0.0 a/26.3 a | 50.0 abc/74.0 a | 2.9 bc/4.7 abc | 2.4 bcd/6.0 a | |
| 4.0 | 0.0 a/26.3 a | 71.7 a/72.2 ab | 3.3 ab/5.3 a | 2.3 cd/5.2 a | |
| Significance § | |||||
| Fcytokinin in shoot multipl. medium | NS | **/** | -/* | **/* | |
| FΙΒA concentration in rooting medium | NS | NS/* | -/** | NS/NS | |
| Fcytokinin multipl. ×ΙΒA concentration | NS | NS/NS | **/NS | NS/NS | |
| Fone-way ANOVA | NS | **/* | **/** | **/NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, A.N.; Papafotiou, M. In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L. Sustainability 2023, 15, 4025. https://doi.org/10.3390/su15054025
Martini AN, Papafotiou M. In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L. Sustainability. 2023; 15(5):4025. https://doi.org/10.3390/su15054025
Chicago/Turabian StyleMartini, Aikaterini N., and Maria Papafotiou. 2023. "In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L." Sustainability 15, no. 5: 4025. https://doi.org/10.3390/su15054025
APA StyleMartini, A. N., & Papafotiou, M. (2023). In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L. Sustainability, 15(5), 4025. https://doi.org/10.3390/su15054025







