Anthropogenic Nitrate Contamination Impacts Nitrous Oxide Emissions and Microbial Communities in the Marchica Lagoon (Morocco)
Abstract
:1. Introduction
2. Materials and Methods
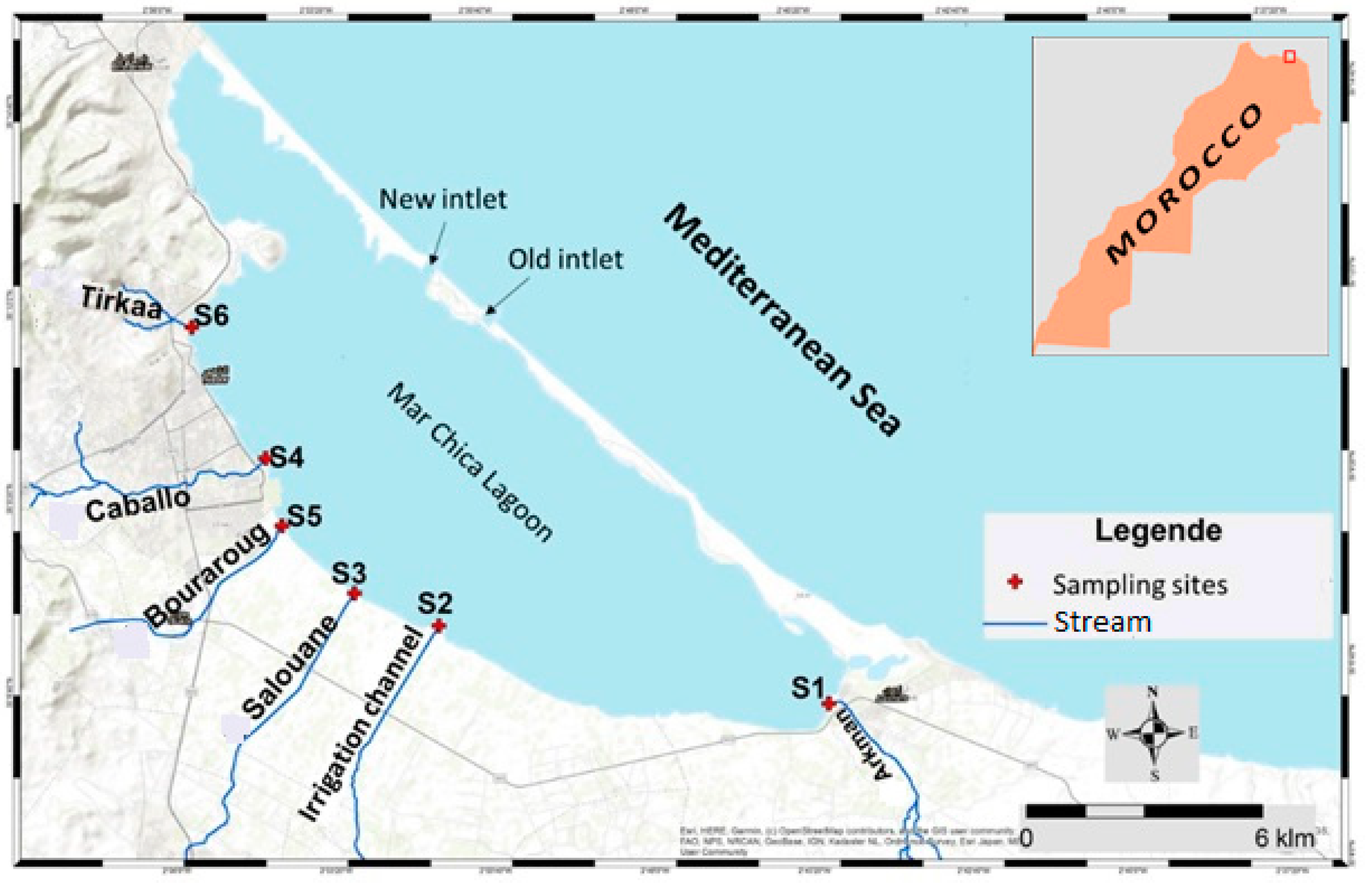
2.1. Sampling Sites
2.2. Sediment Physicochemical Properties and Enzymatic Analysis
2.3. Nitrous Oxide Emissions
2.4. Extraction of DNA and Quantification of N-Cycling Genes
2.5. Amplicon Sequencing and Data Processing
2.6. Bacterial Diversity
2.7. Statistical Analyses
3. Results
3.1. Physicochemical Properties of the Sediments
3.2. Enzymatic Activities in the Sediments
3.3. Nitrous Oxide Emission
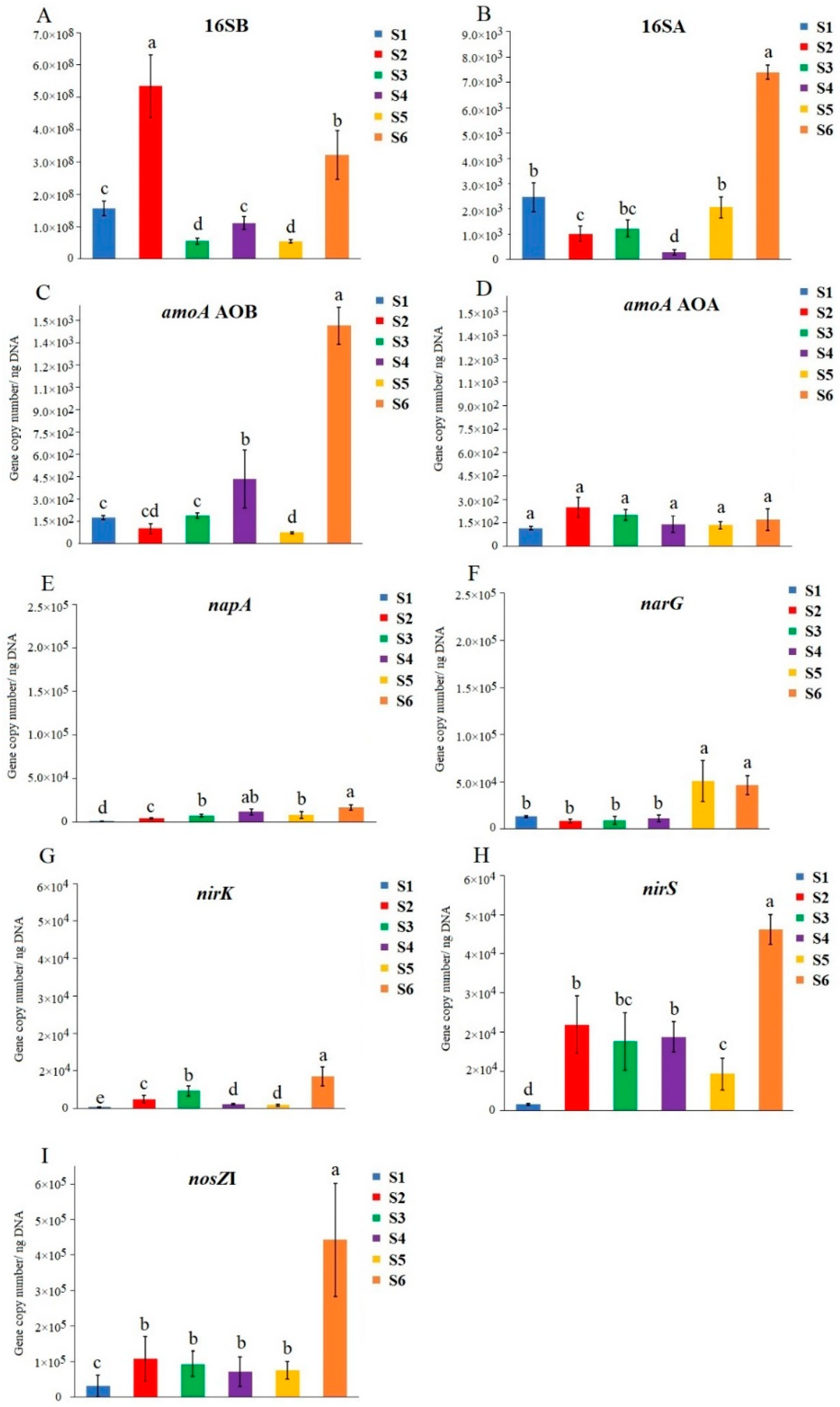
3.4. Quantification of the Total Communities and N-Cycling Genes
3.5. Linking Sediment Physicochemical Properties, Gene Abundance, and N2O Emissions
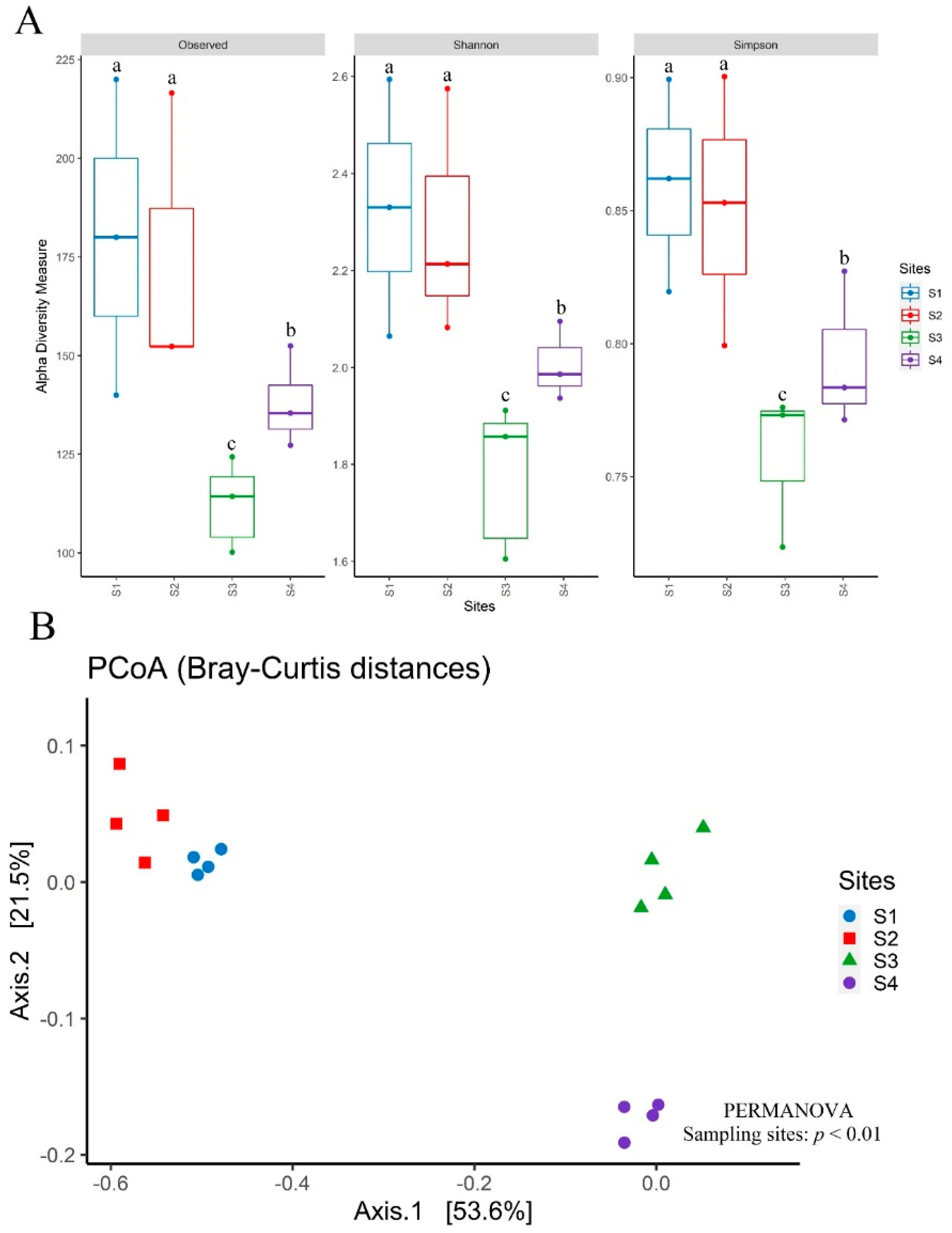
3.6. Diversity, Structure, and Composition of the Bacterial Community
3.7. Linking Sediment Physicochemical Properties with Bacterial Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Ruzafa, A.; Campillo, S.; Fernández-Palacios, J.M.; García-Lacunza, A.; García-Oliva, M.; Ibañez, H.; Navarro-Martínez, P.C.; Pérez-Marcos, M.; Pérez-Ruzafa, I.M.; Quispe-Becerra, J.I.; et al. Long-Term Dynamic in Nutrients, Chlorophyll a, and Water Quality Parameters in a Coastal Lagoon During a Process of Eutrophication for Decades, a Sudden Break and a Relatively Rapid Recovery. Front. Mar. Sci. 2019, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ruzafa, A.; Marcos, C.; Pérez-Ruzafa, I.M.; Pérez-Marcos, M. Coastal lagoons: “transitional ecosystems” between transitional and coastal waters. J. Coast. Conserv. 2011, 15, 369–392. [Google Scholar] [CrossRef]
- Galloway, J.N.; Leach, A.M.; Bleeker, A.; Erisman, J.W. A chronology of human understanding of the nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130120. [Google Scholar] [CrossRef] [Green Version]
- Erisman, J.W.; Galloway, J.N.; Dice, N.B.; Sutton, M.A.; Bleeker, A.; Grizzetti, B.; Leach, A.M.; de Vries, W. Nitrogen: Too Much of a Vital Resource: Science Brief; WWF: Zeist, The Netherlands, 2015. [Google Scholar]
- López-Aizpún, M.; Castellano-Hinojosa, A.; González-López, J.; Bedmar, E.J.; Loick, N.; Barrat, H.; Ma, Y.; Chadwick, D.; Cardenas, L.M. Nitrogen Cycle in Agriculture: Biotic and Abiotic Factors Regulating Nitrogen Losses, in Nitrogen Cycle; CRC Press: Boca Raton, FL, USA, 2021; pp. 34–59. [Google Scholar] [CrossRef]
- Bueno, E.; Mesa, S.; Bedmar, E.J.; Richardson, D.J.; Delgado, M.J.; Schut, G.J.; Zadvornyy, O.; Wu, C.-H.; Peters, J.W.; Boyd, E.S.; et al. Bacterial Adaptation of Respiration from Oxic to Microoxic and Anoxic Conditions: Redox Control. Antioxid. Redox Signal. 2012, 16, 819–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Pan, B.; Zheng, X.; Yu, J.; Ding, H.; Zhang, Y. Pathways of soil N2O uptake, consumption, and its driving factors: A review. Environ. Sci. Pollut. Res. 2022, 29, 30850–30864. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Z.; Zhang, Q.; Cheng, X.; Lu, J.; Liu, G. Sediment denitrification and nitrous oxide production in Chinese plateau lakes with varying watershed land uses. Biogeochemistry 2015, 123, 379–390. [Google Scholar] [CrossRef]
- Liu, W.; Yao, L.; Jiang, X.; Guo, L.; Cheng, X.; Liu, G. Sediment denitrification in Yangtze lakes is mainly influenced by environmental conditions but not biological communities. Sci. Total Environ. 2018, 616, 978–987. [Google Scholar] [CrossRef]
- Correa-Galeote, D.; Tortosa, G.; Moreno, S.; Bru, D.; Philippot, L.; Bedmar, E.J. Spatio-Temporal Variations in the Abundance and Structure of Denitrifier Communities in Sediments Differing in Nitrate Content. Curr. Issues Mol. Biol. 2017, 24, 71–102. [Google Scholar] [CrossRef]
- Chang, Y.; Yin, G.; Hou, L.; Liu, M.; Zheng, Y.; Han, P.; Dong, H.; Liang, X.; Gao, D.; Liu, C. Nitrogen removal processes coupled with nitrification in coastal sediments off the north East China Sea. J. Soils Sediments 2021, 21, 3289–3299. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Hergoualc’h, K.; Akiyama, H.; Bernoux, M.; Chirinda, N.; Prado, A.D.; Kasimir, Å.; MacDonald, J.D.; Ogle, S.M.; Regina, K.; Weerden, T.J. IPCC N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application. In Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories Prepared by the National Greenhouse Gas Inventories Programme IGES; Intergovernmental Panel on Climate Change: Hayama, Japan, 2019. [Google Scholar]
- Karim, H.; Ahmed, D.; Mohammed, I.; Benyounes, D. Circulation marine de la lagune de Nador (Maroc) par modélisation hydrodynamique. Eur. Sci. J. 2015, 11, 418–428. [Google Scholar]
- Selfati, M.; El Ouamari, N.; Franco, A.; Lenfant, P.; Lecaillon, G.; Mesfioui, A.; Boissery, P.; Bazairi, H. Fish assemblages of the Marchica lagoon (Mediterranean, Morocco): Spatial patterns and environmental drivers. Reg. Stud. Mar. Sci. 2019, 32, 100896. [Google Scholar] [CrossRef]
- Umgiesser, G.; Ferrarin, C.; Cucco, A.; De Pascalis, F.; Bellafiore, D.; Ghezzo, M.; Bajo, M. Comparative hydrodynamics of 10 Mediterranean lagoons by means of numerical modeling. J. Geophys. Res. Ocean. 2014, 119, 2212–2226. [Google Scholar] [CrossRef]
- Coelho, S.; Pérez-Ruzafa, A.; Gamito, S. Phytoplankton community dynamics in an intermittently open hypereutrophic coastal lagoon in southern Portugal. Estuar. Coast. Shelf Sci. 2015, 167, 102–112. [Google Scholar] [CrossRef]
- Scanes, P.; Ferguson, A.; Potts, J. Estuary form and function: Implications for palaeoecological studies, in Applications of paleoenvironmental techniques in estuarine studies. Springer 2017, 20, 9–44. [Google Scholar] [CrossRef]
- Oujidi, B.; El Bouch, M.; Tahri, M.; Layachi, M.; Boutoumit, S.; Bouchnan, R.; Ouahidi, H.; Bounakhla, M.; El Ouamari, N.; Maanan, M.; et al. Seasonal and Spatial Patterns of Ecotoxicological Indices of Trace Elements in Superficial Sediments of the Marchica Lagoon Following Restoration Actions during the Last Decade. Diversity 2021, 13, 51. [Google Scholar] [CrossRef]
- Scofield, V.; Jacques, S.; Guimarães, J.R.D.; Farjalla, V.F. Potential changes in bacterial metabolism associated with increased water temperature and nutrient inputs in tropical humic lagoons. Front. Microbiol. 2015, 6, 310. [Google Scholar] [CrossRef] [Green Version]
- Highton, M.P.; Roosa, S.; Crawshaw, J.; Schallenberg, M.; Morales, S.E. Physical Factors Correlate to Microbial Community Structure and Nitrogen Cycling Gene Abundance in a Nitrate Fed Eutrophic Lagoon. Front. Microbiol. 2016, 7, 1691. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, W.; Wu, D.; Wang, X.; Zhu, G. Differentiation of nitrogen and microbial community in the littoral and limnetic sediments of a large shallow eutrophic lake (Chaohu Lake, China). J. Soils Sediments 2019, 19, 1005–1016. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Zhang, H.; Wang, L.; Zhang, W.; Niu, L.; Wang, P.; Wang, C. The responses of bacterial community and N2O emission to nitrogen input in lake sediment: Estrogen as a co-pollutant. Environ. Res. 2019, 179, 108769. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Li, Q.; Gu, P.; Gu, X.; Wu, L.; Gao, Y.; Shan, J.; Zheng, Z.; Zhang, W. Effects of Nutrient Levels on Microbial Diversity in Sediments of a Eutrophic Shallow Lake. Front. Ecol. Evol. 2022, 10, 412. [Google Scholar] [CrossRef]
- Raji, O.; Dezileau, L.; Von Grafenstein, U.; Niazi, S.; Snoussi, M.; Martinez, P. Extreme sea events during the last millennium in the northeast of Morocco. Nat. Hazards Earth Syst. Sci. 2015, 15, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.; Driss, N.; Nadia, B.; Roberto, P.; Abdeljaouad, L.; Nor-Dine, R. Characterization of the New Status of Nador Lagoon (Morocco) after the Implementation of the Management Plan. J. Mar. Sci. Eng. 2017, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Oujidi, B.; Tahri, M.; Layachi, M.; Abid, A.; Bouchnan, R.; Selfati, M.; Bounakhla, M.; El Bouch, M.; Maanan, M.; Bazairi, H.; et al. Effects of the watershed on the seasonal variation of the surface water quality of a post-restoration coastal wetland: The case of the Nador lagoon (Mediterranean sea, Morocco). Reg. Stud. Mar. Sci. 2020, 35, 101127. [Google Scholar] [CrossRef]
- Lee, S.-A.; Lee, J.; Han, Y.; Kim, G. Biogeochemical alteration and fluxes of dissolved organic matter and nutrients in coastal bays. Estuar. Coast. Shelf Sci. 2020, 245, 106992. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Rodriguez-Sanchez, A.; Garcia-Ruiz, M.J.; Palazon, B.M.; Cortes-Lorenzo, C.; Osorio, F.; Vahala, R. Performance and bacterial community dynamics of a CANON bioreactor acclimated from high to low operational temperatures. Chem. Eng. J. 2016, 287, 557–567. [Google Scholar] [CrossRef]
- Tortosa, G.; Correa, D.; Sánchez-Raya, A.J.; Delgado, A.; Sánchez-Monedero, M.A.; Bedmar, E.J. Effects of nitrate contamination and seasonal variation on the denitrification and greenhouse gas production in La Rocina Stream (Doñana National Park, SW Spain). Ecol. Eng. 2011, 37, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Castellano-Hinojosa, A.; Correa-Galeote, D.; Carrillo, P.; Bedmar, E.J.; Medina-Sánchez, J.M. Denitrification and Biodiversity of Denitrifiers in a High-Mountain Mediterranean Lake. Front. Microbiol. 2017, 8, 1911. [Google Scholar] [CrossRef] [Green Version]
- Yoshinari, T.; Knowles, R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem. Biophys. Res. Commun. 1976, 69, 705–710. [Google Scholar] [CrossRef]
- Correa-Galeote, D.; Tortosa, G.; Bedmar, E.J. Quantification of functional microbial nitrogen cycle genes in environmental samples. In Metagenomics of the Microbial Nitrogen Cycle: Theory, Methods and Applications; Caister Academic Press: Norwich, UK, 2014; pp. 65–85. [Google Scholar]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellano-Hinojosa, A.; Strauss, S.L. Insights into the taxonomic and functional characterization of agricultural crop core rhizobiomes and their potential microbial drivers. Sci. Rep. 2021, 11, 10068. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 2018, 6, e27295v1. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Warnes, M.G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W. Package ‘gplots’. Various R Programming Tools for Plotting Data. 2016. Available online: https://cran.microsoft.com/snapshot/2016-03-30/web/packages/gplots/gplots.pdf (accessed on 15 October 2022).
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Castellano-Hinojosa, A.; Correa-Galeote, D.; González-López, J.; Bedmar, E.J. Effect of nitrogen fertilisers on nitrous oxide emission, nitrifier and denitrifier abundance and bacterial diversity in closed ecological systems. Appl. Soil Ecol. 2020, 145, 103380. [Google Scholar] [CrossRef]
- Chen, N.; Wu, J.; Zhou, X.; Chen, Z.; Lu, T. Riverine N2O production, emissions and export from a region dominated by agriculture in Southeast Asia (Jiulong River). Agric. Ecosyst. Environ. 2015, 208, 37–47. [Google Scholar] [CrossRef]
- Lin, J.; Chen, N.; Yuan, X.; Tian, Q.; Hu, A.; Zheng, Y. Impacts of human disturbance on the biogeochemical nitrogen cycle in a subtropical river system revealed by nitrifier and denitrifier genes. Sci. Total. Environ. 2020, 746, 141139. [Google Scholar] [CrossRef]
- Ruiz, F.; Abad, M.; Olías, M.; Galán, E.; González, I.; Aguilá, E.; Hamoumi, N.; Pulido, I.; Cantano, M. The present environmental scenario of the Nador Lagoon (Morocco). Environ. Res. 2006, 102, 215–229. [Google Scholar] [CrossRef]
- Aknaf, A.; Akodad, M.; Moumen, A.; Chekroun, K.B.; Elhamouti, C.; Bailal, A.; Baghour, M. Impact of the new pass on the eutrophication of the lagoon Marchica: Study of the two sites Bou Areg and Mohandis. J. Mater. Environ. Sci. 2015, 6, 2939–2943. [Google Scholar]
- Mostarih, M.M.M.; Madani, F.E.; Ali, H.S. Evaluation physico-chimique de la qualité de l’eau de la lagune de Nador-Nord du Maroc oriental après l’ouverture de la nouvelle passe. J. Mater. Environ. Sci. 2016, 7, 4795–5809. [Google Scholar]
- Zhang, H.-H.; He, P.-J.; Shao, L.-M. Ammonia volatilization, N2O and CO2 emissions from landfill leachate-irrigated soils. Waste Manag. 2010, 30, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, L.; Zhang, Y.; Xu, H.; Jiang, X. Denitrification occurring on suspended sediment in a large, shallow, subtropical lake (Poyang Lake, China). Environ. Pollut. 2016, 219, 501–511. [Google Scholar] [CrossRef]
- Palacin-Lizarbe, C.; Camarero, L.; Hallin, S.; Jones, C.M.; Cáliz, J.; Casamayor, E.O.; Catalan, J. The DNRA-Denitrification Dichotomy Differentiates Nitrogen Transformation Pathways in Mountain Lake Benthic Habitats. Front. Microbiol. 2019, 10, 1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhu, S.; Liu, X.; Yao, P.; Ge, T.; Zhang, X.-H. Spatiotemporal dynamics of the archaeal community in coastal sediments: Assembly process and co-occurrence relationship. ISME J. 2020, 14, 1463–1478. [Google Scholar] [CrossRef] [Green Version]
- Hug, L.A.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Hug, L.A.; Brown, C.T.; Wilkins, M.J.; Frischkorn, K.R.; Tringe, S.G.; Singh, A.; Markillie, L.M.; et al. Genomic Expansion of Domain Archaea Highlights Roles for Organisms from New Phyla in Anaerobic Carbon Cycling. Curr. Biol. 2015, 25, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Baxter, A.M.; Johnson, L.; Edgerton, J.; Royer, T.; Leff, L.G. Structure and function of denitrifying bacterial assemblages in low-order Indiana streams. Freshw. Sci. 2012, 31, 304–317. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Strauss, S.L.; González-López, J.; Bedmar, E.J. Changes in the diversity and predicted functional composition of the bulk and rhizosphere soil bacterial microbiomes of tomato and common bean after inorganic N-fertilization. Rhizosphere 2021, 18, 100362. [Google Scholar] [CrossRef]
- Pavloudi, C.; Oulas, A.; Vasileiadou, K.; Sarropoulou, E.; Kotoulas, G.; Arvanitidis, C. Salinity is the major factor influencing the sediment bacterial communities in a Mediterranean lagoonal complex (Amvrakikos Gulf, Ionian Sea). Mar. Genom. 2016, 28, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, F.; Ben Said, O.; Cravo-Laureau, C.; Mahmoudi, E.; Bru, N.; Monperrus, M.; Duran, R. Bacterial community assemblages in sediments under high anthropogenic pressure at Ichkeul Lake/Bizerte Lagoon hydrological system, Tunisia. Environ. Pollut. 2019, 252, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Bourhane, Z.; Lanzén, A.; Cagnon, C.; Ben Said, O.; Mahmoudi, E.; Coulon, F.; Atai, E.; Borja, A.; Cravo-Laureau, C.; Duran, R. Microbial diversity alteration reveals biomarkers of contamination in soil-river-lake continuum. J. Hazard. Mater. 2022, 421, 126789. [Google Scholar] [CrossRef] [PubMed]
- Manh, H.D.; Matsuo, Y.; Katsuta, A.; Matsuda, S.; Shizuri, Y.; Kasai, H. Robiginitalea myxolifaciens sp. nov., a novel myxol-producing bacterium isolated from marine sediment, and emended description of the genus Robiginitalea. Int. J. Syst. Evol. Microbiol. 2008, 58, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Choi, S.; Jung, H.; Scow, K.M.; Park, W. Primers for amplification of nitrous oxide reductase genes associated with Firmicutes and Bacteroidetes in organic-compound-rich soils. Microbiology 2013, 159, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-C.; Giovannoni, S.J. Robiginitalea biformata gen. nov., sp. nov., a novel marine bacterium in the family Flavobacteriaceae with a higher G+C content. Int. J. Syst. Evol. Microbiol. 2004, 54, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Sanford, R.A.; Wagner, D.D.; Wu, Q.; Chee-Sanford, J.C.; Thomas, S.H.; Cruz-García, C.; Rodríguez, G.; Massol-Deyá, A.; Krishnani, K.K.; Ritalahti, K.M.; et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl. Acad. Sci. USA 2012, 109, 19709–19714. [Google Scholar] [CrossRef] [Green Version]
- Mania, D.; Heylen, K.; van Spanning, R.J.M.; Frostegård, Å. The nitrate-ammonifying and nosZ-carrying bacterium Bacillus vireti is a potent source and sink for nitric and nitrous oxide under high nitrate conditions. Environ. Microbiol. 2014, 16, 3196–3210. [Google Scholar] [CrossRef]
- Van Grinsven, S.; Damsté, J.S.S.; Villanueva, L. Assessing the Effect of Humic Substances and Fe(III) as Potential Electron Acceptors for Anaerobic Methane Oxidation in a Marine Anoxic System. Microorganisms 2020, 8, 1288. [Google Scholar] [CrossRef]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochsenreiter, T.; Selezi, D.; Quaiser, A.; Bonch-Osmolovskaya, L.; Schleper, C. Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ. Microbiol. 2003, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [Green Version]
- Tourna, M.; Freitag, T.E.; Nicol, G.W.; Prosser, J.I. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol. 2008, 10, 1357–1364. [Google Scholar] [CrossRef]
- Bru, D.; Sarr, A.; Philippot, L. Relative abundance of the membrane bound and periplasmic nitrate reductase. Appl. Environ. Microbiol. 2007, 73, 5971–5974. [Google Scholar] [CrossRef] [Green Version]
- Henry, S.; Baudouin, E.; López-Gutiérrez, J.C.; Martin-Laurent, F.; Brauman, A.; Philippot, L. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J. Microbiol. Methods. 2004, 59, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Braker, G.; Tiedje, J.M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl. Environ. Microbiol. 2003, 69, 3476–3483. [Google Scholar] [CrossRef] [Green Version]
- Henry, S.; Bru, D.; Stres, B.; Hallet, S.; Philippot, L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006, 72, 5181–5189. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.M.; Graf, D.R.H.; Bru, D.; Philippot, L.; Hallin, S. The unaccounted yet abundant nitrous oxide-reducing microbial community: A potential nitrous oxide sink. ISME J. 2013, 7, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Site | pH | NH4+ (mg/L) | NO3− (mg/L) | TN (mg/g) | TC (mg/g) | TOC (mg/L) |
|---|---|---|---|---|---|---|
| S1 | 8.01 | 2.53 ± 0.93 a | 4.43 ± 1.46 e | 0.44 ± 0.17 c | 24.00 ± 2.95 a | 3.48 ± 0.76 c |
| S2 | 7.16 | 2.60 ± 1.20 a | 10.07 ± 1.15 d | 0.37 ± 0.12 c | 17.00 ± 6.19 a | 3.47 ± 1.17 c |
| S3 | 7.20 | 2.73 ± 2.17 a | 41.87 ± 9.02 a | 0.30 ± 0.09 c | 18.20 ± 3.69 a | 3.29 ± 1.28 c |
| S4 | 7.51 | 3.63 ± 1.62 a | 23.17 ± 1.74 b | 0.11 ± 0.02 d | 8.17 ± 1.12 b | 1.07 ± 0.13 d |
| S5 | 7.08 | 2.73 ± 0.86 a | 45.90 ± 6.56 a | 1.21 ± 0.30 b | 19.57 ± 3.55 a | 10.85 ± 1.16 b |
| S6 | 7.17 | 4.20 ± 1.93 a | 18.40 ± 1.85 c | 1.87 ± 0.16 a | 19.63 ± 2.75 a | 16.27 ± 2.84 a |
| Site | Nmol N2O/g Dry Sediment × h |
|---|---|
| S1 | 5.3 ± 1.9 d |
| S2 | 7.7 ± 1.7 d |
| S3 | 62.1 ± 4.8 a |
| S4 | 11.4 ± 2.6 c |
| S5 | 28.3 ± 2.4 b |
| S6 | 11.6 ± 2.1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hamouti, C.; Castellano-Hinojosa, A.; Mabrouki, Y.; Chaouni, B.; Ghazal, H.; Boukhatem, N.; Chahboune, R.; Bedmar, E.J. Anthropogenic Nitrate Contamination Impacts Nitrous Oxide Emissions and Microbial Communities in the Marchica Lagoon (Morocco). Sustainability 2023, 15, 4077. https://doi.org/10.3390/su15054077
El Hamouti C, Castellano-Hinojosa A, Mabrouki Y, Chaouni B, Ghazal H, Boukhatem N, Chahboune R, Bedmar EJ. Anthropogenic Nitrate Contamination Impacts Nitrous Oxide Emissions and Microbial Communities in the Marchica Lagoon (Morocco). Sustainability. 2023; 15(5):4077. https://doi.org/10.3390/su15054077
Chicago/Turabian StyleEl Hamouti, Chahrazade, Antonio Castellano-Hinojosa, Youness Mabrouki, Bouchra Chaouni, Hassan Ghazal, Noureddine Boukhatem, Rajaa Chahboune, and Eulogio J. Bedmar. 2023. "Anthropogenic Nitrate Contamination Impacts Nitrous Oxide Emissions and Microbial Communities in the Marchica Lagoon (Morocco)" Sustainability 15, no. 5: 4077. https://doi.org/10.3390/su15054077





