Remediation of Sb-Contaminated Soil by Low Molecular Weight Organic Acids Washing: Efficiencies and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Preparation
2.2. Washing Experiments
2.3. Sequential Extraction of Sb from Soil
2.4. Analysis and Determination Methods
2.5. Quality Control and Statistical Analysis
3. Results and Discussion
3.1. Properties of Contaminated Soil Samples
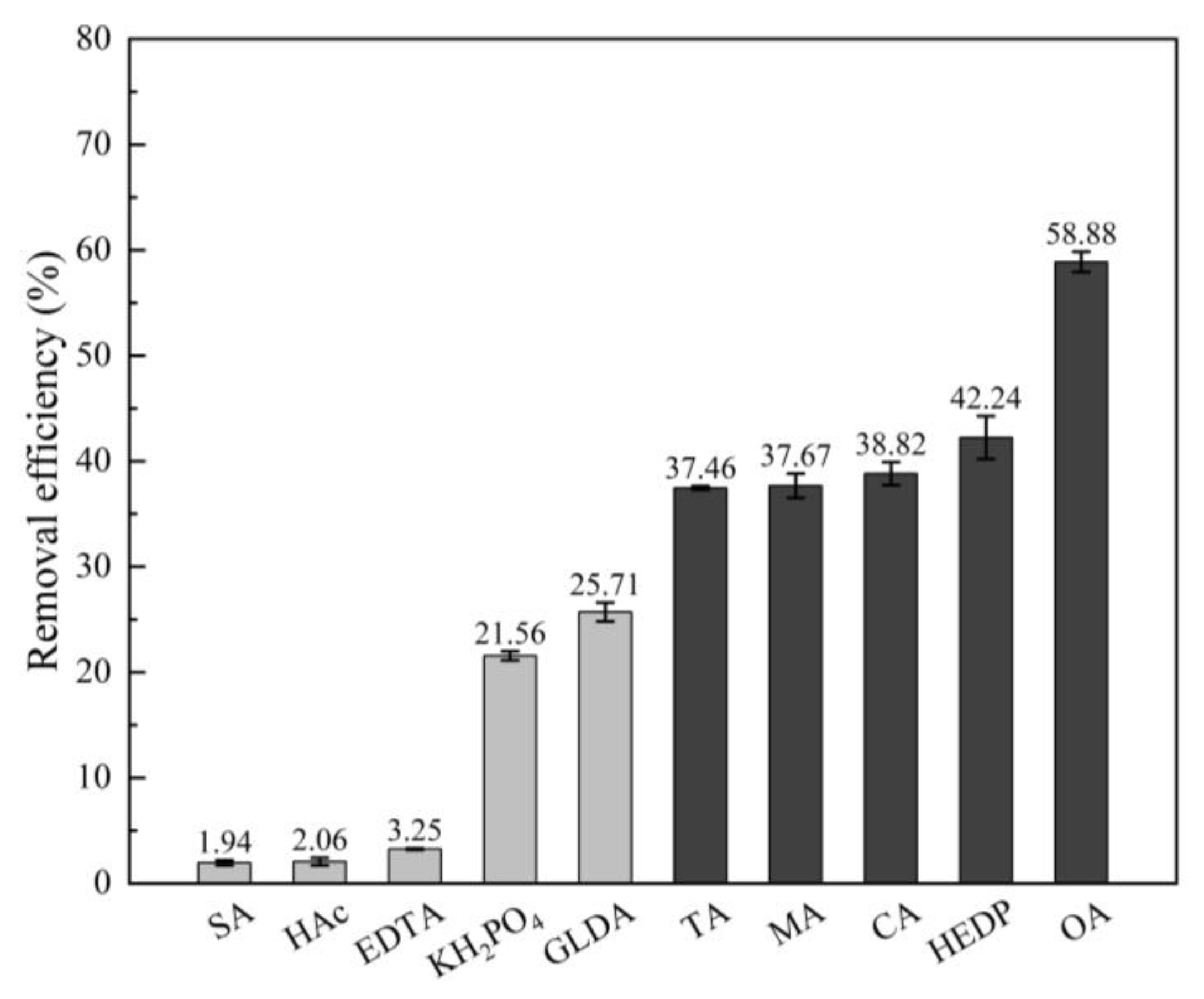
3.2. Washing Agents Screening
3.3. Optimization of Soil Washing Parameters
3.3.1. Concentrations of Washing Agents
3.3.2. L/S Ratio
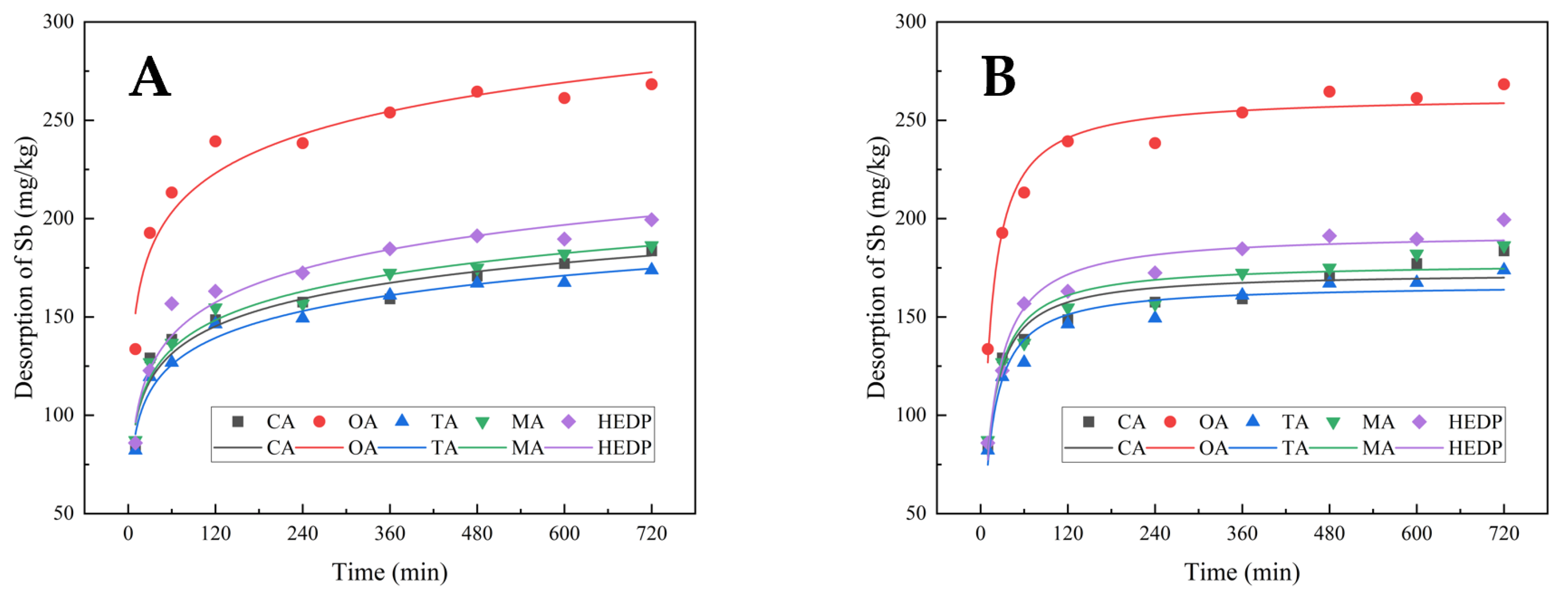
3.3.3. Washing Time and Desorption Kinetic
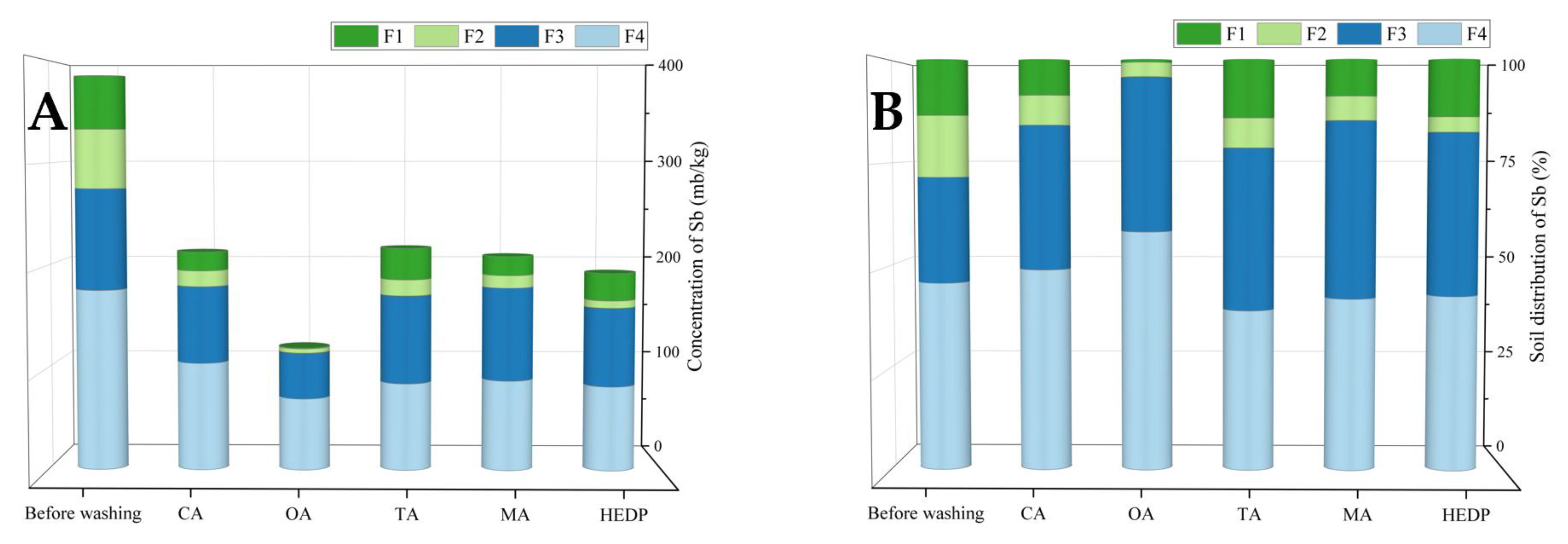
3.4. Sb Fractions in the Soil before and after Washing
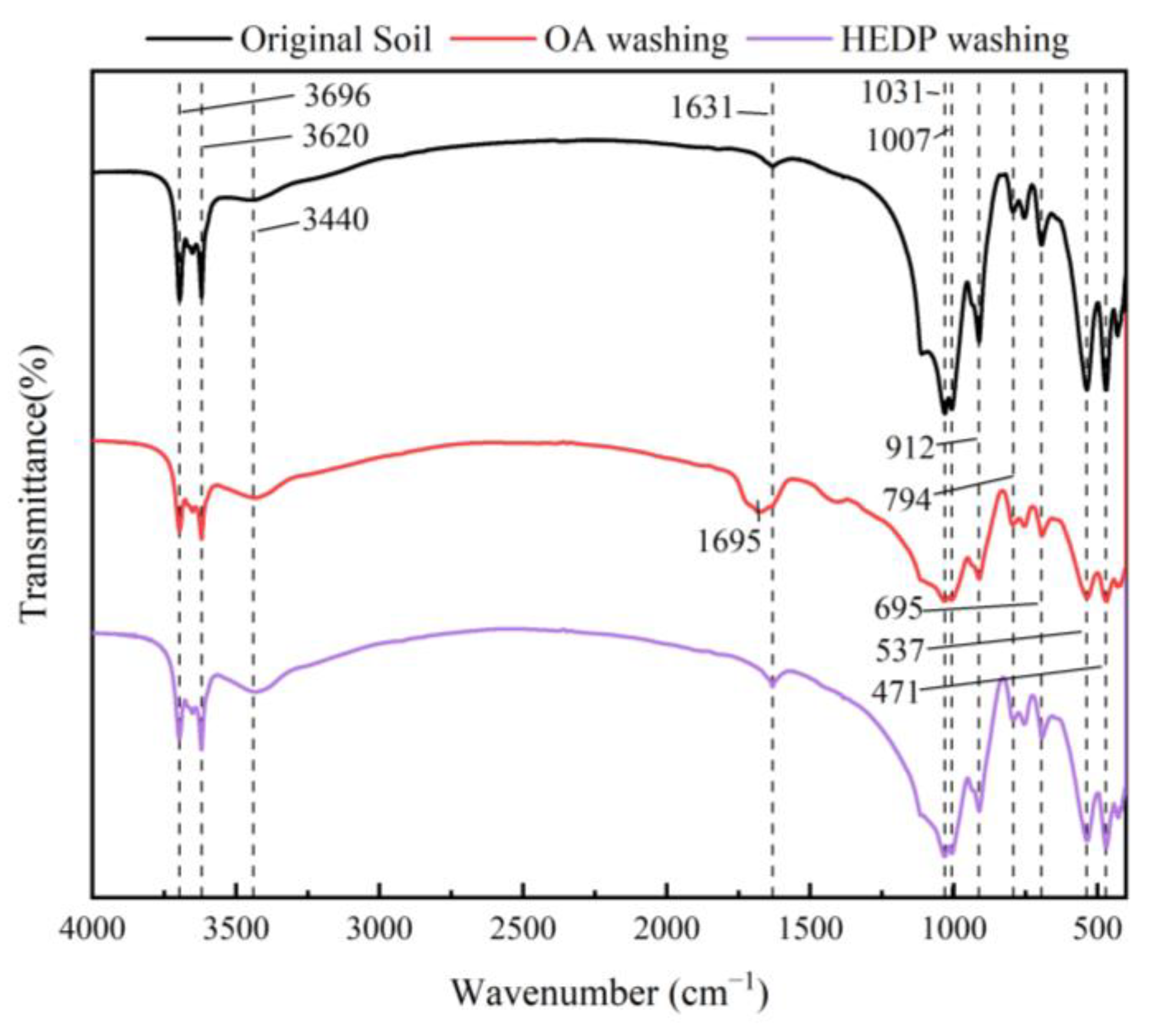
3.5. Analysis of Functional Groups in the Soil
3.6. The Mineral Structure of the Soil
3.7. Soil Surface Morphology and Element Distribution
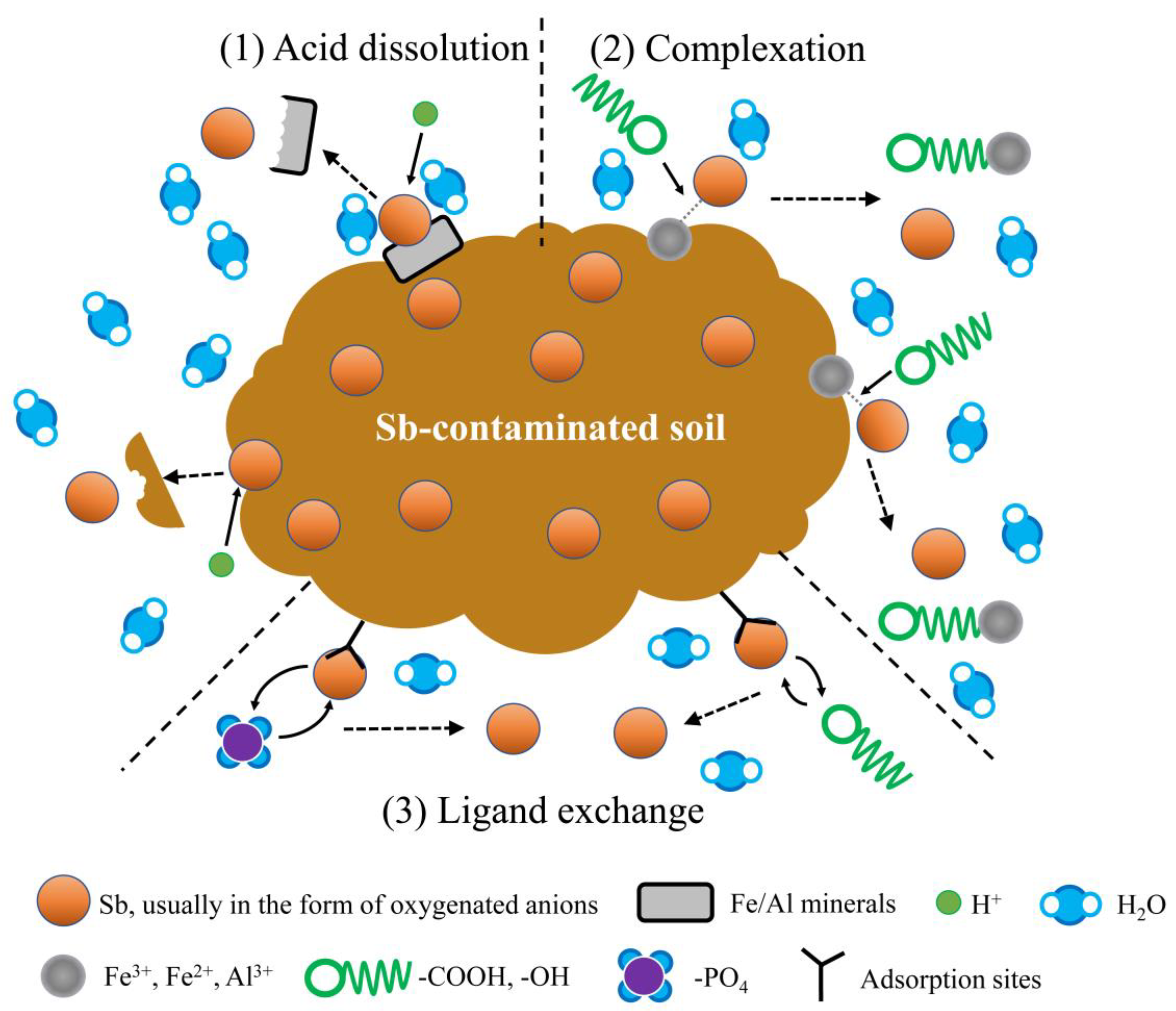
3.8. Potential Mechanism of Sb Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Nishad, P.A.; Bhaskarapillai, A. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. Chemosphere 2021, 277, 130252. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, C. Leaching remediation of antimony-naphthalene compound contaminated soil by carboxymethyl-β-cyclodextrin and its mechanism. Acta Sci. Circumstantiae 2022, 42, 1–12. [Google Scholar]
- Broomandi, P.; Guney, M.; Kim, J.R.; Karaca, F. Soil Contamination in Areas Impacted by Military Activities: A Critical Review. Sustainability 2020, 12, 9002. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, F.; Mo, C.; Deng, Q.; Meng, W.; Giesy, J.P. Comparison of arsenic and antimony biogeochemical behavior in water, soil and tailings from Xikuangshan, China. Sci. Total Environ. 2016, 539, 97–104. [Google Scholar] [CrossRef]
- Periferakis, A.; Caruntu, A.; Periferakis, A.; Scheau, A.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef]
- Long, J.; Zhou, D.; Li, B.; Zhou, Y.; Li, Y.; Lei, M. The effect of an antimony resistant bacterium on the iron plaque fraction and antimony uptake by rice seedlings. Environ. Pollut. 2020, 258, 113670. [Google Scholar] [CrossRef]
- He, M.; Wang, X.; Wu, F.; Fu, Z. Antimony pollution in China. Sci. Total Environ. 2012, 421–422, 41–50. [Google Scholar] [CrossRef]
- Ogawa, S.; Katoh, M.; Sato, T. Simultaneous lead and antimony immobilization in shooting range soil by a combined application of hydroxyapatite and ferrihydrite. Environ. Technol. 2015, 36, 2647–2656. [Google Scholar] [CrossRef]
- Sanderson, P.; Qi, F.; Seshadri, B.; Wijayawardena, A.; Naidu, R. Contamination, Fate and Management of Metals in Shooting Range Soils—A Review. Curr. Pollut. Rep. 2018, 4, 175–187. [Google Scholar] [CrossRef]
- Zhou, S.; Du, Y.; Feng, Y.; Sun, H.; Xia, W.; Yuan, H. Stabilization of arsenic and antimony Co-contaminated soil with an iron-based stabilizer: Assessment of strength, leaching and hydraulic properties and immobilization mechanisms. Chemosphere 2022, 301, 134644. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, A.; Zhong, Q.; Rehman, S.A.U.; Ma, C.; Jiao, Y.; He, M. Immobilization and phytoavailability of antimony (Sb) in contaminated agricultural soils amended with composted manure. Sci. Total Environ. 2023, 856, 159213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; He, Y.; Wu, Y.; Dian, B.; Zhang, J.; Li, T.; Jiang, M. Solidification/stabilization of soil heavy metals by alkaline industrial wastes: A critical review. Environ. Pollut. 2022, 312, 120094. [Google Scholar] [CrossRef]
- Huang, H.; Fan, L.; Zhao, Y.; Jin, Q.; Yang, G.; Zhao, D.; Xu, Z. Integrating Broussonetia papyrifera and Two Bacillus Species to Repair Soil Antimony Pollutions. Front. Microbiol. 2022, 13, 871581. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Junaid, M.F.; Ijaz, N.; Khalid, U.; Ijaz, Z. Remediation methods of heavy metal contaminated soils from environmental and geotechnical standpoints. Sci. Total Environ. 2023, 867, 161468. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicol. Environ. Saf. 2020, 203, 110981. [Google Scholar] [CrossRef]
- Guo, X.; Wei, Z.; Wu, Q.; Li, C.; Qian, T.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419. [Google Scholar] [CrossRef]
- Serafimovska, J.M.; Arpadjan, S.; Stafilov, T.; Tsekova, K. Study of the antimony species distribution in industrially contaminated soils. J. Soil Sediments 2013, 13, 294–303. [Google Scholar] [CrossRef]
- Navarro, A.; Martínez, F. The use of soil-flushing to remediate metal contamination in a smelting slag dumping area: Column and pilot-scale experiments. Eng. Geol. 2010, 115, 16–27. [Google Scholar] [CrossRef]
- Tokunaga, S.; Park, S.W.; Ulmanu, M. Extraction behavior of metallic contaminants and soil constituents from contaminated soils. Environ. Technol. 2005, 26, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Guemiza, K.; Mercier, G.; Blais, J.F. Pilot-scale counter-current acid leaching process for Cu, Pb, Sb, and Zn from small-arms shooting range soil. J. Soil Sediments 2014, 14, 1359–1369. [Google Scholar] [CrossRef]
- Sun, H.; Xu, S.; Ren, H.; Chen, Q.; Xu, D.; An, Y. Leaching study on arsenic and antimony in the soil of antimony ore zone by tartaric and malic acid. Earth Environ. 2016, 44, 304–308. [Google Scholar]
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na 2 EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dai, H.; Skuza, L.; Xu, J.; Shi, J.; Wei, S. Co-high-efficiency washing agents for simultaneous removal of Cd, Pb and As from smelting soil with risk assessment. Chemosphere 2022, 300, 134581. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, H.; Chai, L.; Mirza, N.; Yang, Z.; Pervez, A.; Tariq, M.; Shaheen, S.; Mahmood, Q. Antimony (Sb)—Pollution and removal techniques—Critical assessment of technologies. Toxicol. Environ. Chem. 2015, 97, 1296–1318. [Google Scholar] [CrossRef]
- Qian, J.; Li, Y.; Su, F.; Wu, J.; Sun, J.; Huang, T. Citric acid-based deep eutectic solvent (CA-DES) as a new soil detergent for the removal of cadmium from coking sites. Environ. Sci. Pollut. Res. 2022, 30, 2118–2127. [Google Scholar] [CrossRef]
- Chen, C.; Tian, T.; Wang, M.K.; Wang, G. Release of Pb in soils washed with various extractants. Geoderma 2016, 275, 74–81. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T. Extraction of Arsenic and Heavy Metals from Contaminated Mine Tailings by Soil Washing. Soil Sediment Contam. 2011, 20, 631–648. [Google Scholar] [CrossRef]
- Gluhar, S.; Kaurin, A.; Finžgar, N.; Gerl, M.; Kastelec, D.; Lestan, D. Demonstrational gardens with EDTA-washed soil. Part I: Remediation efficiency, effect on soil properties and toxicity hazards. Sci. Total Environ. 2021, 792, 149060. [Google Scholar] [CrossRef]
- Huang, K.; Shen, Y.; Wang, X.; Song, X.; Yuan, W.; Xie, J.; Wang, S.; Bai, J.; Wang, J. Choline-based deep eutectic solvent combined with EDTA-2Na as novel soil washing agent for lead removal in contaminated soil. Chemosphere 2021, 279, 130568. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Stein, M.; Seidel-Morgenstern, A.; Pant, K.K.; Nigam, K.D.P. The thermodynamics and biodegradability of chelating agents upon metal extraction. Chem. Eng. Sci. 2015, 137, 768–785. [Google Scholar] [CrossRef]
- Ash, C.; Tejnecký, V.; Borůvka, L.; Drábek, O. Different low-molecular-mass organic acids specifically control leaching of arsenic and lead from contaminated soil. J. Contam. Hydrol. 2016, 187, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.; Guo, E.; Yang, X. Path analysis of phosphorus activation capacity as induced by low-molecular-weight organic acids in a black soil of Northeast China. J. Soil Sediments 2019, 19, 840–847. [Google Scholar] [CrossRef]
- Debela, F.; Arocena, J.M.; Thring, R.W.; Whitcombe, T. Organic acids inhibit the formation of pyromorphite and Zn-phosphate in phosphorous amended Pb- and Zn-contaminated soil. J. Environ. Manag. 2013, 116, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, J.; Dong, C.; Wu, L.; Hu, P. Remediation of metal-contaminated paddy soils by chemical washing with FeCl3 and citric acid. J. Soil Sediments 2018, 18, 1020–1028. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, L.; Zhang, S.; Li, T.; Xu, X.; Wang, G.; Li, Y. Mechanism and Optimization of Polyepoxysuccinic Acid in Washing Cd-, Pb-, and Zn-Contaminated Soils. Water Air Soil Pollut. 2021, 409, 232. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, G.; Zhang, G.; He, Q.; Wei, Z.; Zheng, W.; Qian, T.; Wu, Q. Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties. Chemosphere 2018, 209, 776–782. [Google Scholar] [CrossRef]
- Wang, G.; Pan, X.; Zhang, S.; Zhong, Q.; Zhou, W.; Zhang, X.; Wu, J.; Vijver, M.G.; Peijnenburg, W.J.G.M. Remediation of heavy metal contaminated soil by biodegradable chelator–induced washing: Efficiencies and mechanisms. Environ. Res. 2020, 186, 109554. [Google Scholar] [CrossRef]
- Fazle Bari, A.S.M.; Lamb, D.; MacFarlane, G.R.; Rahman, M.M. Soil washing of arsenic from mixed contaminated abandoned mine soils and fate of arsenic after washing. Chemosphere 2022, 296, 134053. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Yang, S.; Sun, Z.; Liu, Y. Experimental study on washing of arsenic-contaminated soil with different washing agents. Saf. Environ. Eng. 2021, 28, 182–187. [Google Scholar]
- Okkenhaug, G.; Zhu, Y.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Soil—Determination of pH—Potentiometry (HJ 962-2018). Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201808/t20180815_451430.shtml (accessed on 14 February 2023).
- Ministry of Agriculture of the People’s Republic of China. Soil Testing Part 6: Method for Determination of Soil Organic Matter (NY/T 1121.6-2006). Available online: https://hbba.sacinfo.org.cn/attachment/onlineRead/8c5969f13e0acfbaf685a468a20e6f92b028b4c34fce864c83d6916c47287abe (accessed on 14 February 2023).
- Ministry of Ecology and Environment of the People’s Republic of China. Soil Quality—Determination of Cation Exchange Capacity (CEC)—Hexamminecobalt Trichloride Solution-Spectrophotometric Method (HJ 889-2017). Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201712/t20171220_428288.shtml (accessed on 14 February 2023).
- Ministry of Ecology and Environment of the People’s Republic of China. Soil and Sediment—Determination of Mercury, Arsenic, Selenium, Bismuth, Antimony—Microwave Dissolution/Atomic Fluorescence Spectrometry (HJ 680-2013). Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201312/t20131203_264304.shtml (accessed on 14 February 2023).
- Yan, D.; Guo, Z.; Xiao, X.; Peng, C.; He, Y.; Yang, A.; Wang, X.; Hu, Y.; Li, Z. Cleanup of arsenic, cadmium, and lead in the soil from a smelting site using N,N-bis(carboxymethyl)-L-glutamic acid combined with ascorbic acid: A lab-scale experiment. J. Environ. Manag. 2021, 296, 113174. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Sun, L. Washing kinetics and mechanism of removing Pb and Cd from the contaminated soil with the organic acids. J. Saf. Environ. 2015, 15, 261–266. [Google Scholar]
- Ji, M.; Wang, X.; Ma, H.; Zhang, C.; Ruan, W.; Ren, H.; Deng, Y. Removal of Cd from contaminated soil using amino acid salt. J. Agro-Environ. Sci. 2021, 40, 329–337. [Google Scholar]
- Wei, F.; Zheng, C.; Chen, J.; Wu, Y. Study on the backgroud contents on 61 elements of soils in China. Environ. Sci. 1991, 12, 12–19. [Google Scholar]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Dai, S.; Li, Y.; Zhou, T.; Zhao, Y. Reclamation of heavy metals from contaminated soil using organic acid liquid generated from food waste: Removal of Cd, Cu, and Zn, and soil fertility improvement. Environ. Sci. Pollut. Res. 2017, 24, 15260–15269. [Google Scholar] [CrossRef]
- Fox, T.R.; Comerford, N.B. Low-Molecular-Weight Organic Acids in Selected Forest Soils of the Southeastern USA. Soil Sci. Soc. Am. J. 1990, 54, 1139–1144. [Google Scholar] [CrossRef]
- Nguyen Van, T.; Osanai, Y.; Do Nguyen, H.; Kurosawa, K. Arsenic Speciation and Extraction and the Significance of Biodegradable Acid on Arsenic Removal—An Approach for Remediation of Arsenic-Contaminated Soil. Int. J. Environ. Res. Public Health 2017, 14, 990. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab. J. Chem. 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Jho, E.H.; Im, J.; Yang, K.; Kim, Y.; Nam, K. Changes in soil toxicity by phosphate-aided soil washing: Effect of soil characteristics, chemical forms of arsenic, and cations in washing solutions. Chemosphere 2015, 119, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Bohan, L.; Ming, L.; Yong, Z.; Qingru, Z.; Bin, O. Arsenic removal from contaminated soil using phosphoric acid and phosphate. J. Environ. Sci.-China 2008, 20, 75–79. [Google Scholar]
- Kim, E.J.; Jeon, E.; Baek, K. Role of reducing agent in extraction of arsenic and heavy metals from soils by use of EDTA. Chemosphere 2016, 152, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Xu, X.; Yao, P.; Li, T.; Wang, G.; Gong, G.; Li, Y.; Deng, O. Effects of surfactants on low-molecular-weight organic acids to wash soil zinc. Environ. Sci. Pollut. Res. 2016, 23, 4629–4638. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.N.; Hizaddin, H.F.; Hayyan, A.; Hasikin, K.; Abdul Razak, S.; Mokhtar, M.I.; Azizan, M.M. Deep eutectic solvents for the removal of lead contaminants in mangrove soil. J. Environ. Chem. Eng. 2022, 10, 107264. [Google Scholar] [CrossRef]
- Fanali, C.; Gallo, V.; Della Posta, S.; Dugo, L.; Mazzeo, L.; Cocchi, M.; Piemonte, V.; De Gara, L. Choline Chloride–Lactic Acid-Based NADES As an Extraction Medium in a Response Surface Methodology-Optimized Method for the Extraction of Phenolic Compounds from Hazelnut Skin. Molecules 2021, 26, 2652. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry and the Environment—Providing a “Green” Link between Chemistry, Physics and Engineering. Ultrason. Sonochem. 2007, 14, 476–483. [Google Scholar] [CrossRef]
- Yang, T.; Hodson, M.E. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci. Total Environ. 2019, 647, 290–300. [Google Scholar] [CrossRef]
- Yip, T.C.M.; Tsang, D.C.W.; Ng, K.T.W.; Lo, I.M.C. Kinetic Interactions of EDDS with Soils. 1. Metal Resorption and Competition under EDDS Deficiency. Environ. Sci. Technol. 2009, 43, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tsang, D.C.W.; Chen, H.; Huang, L. Remediation of an electroplating contaminated soil by EDTA flushing: Chromium release and soil dissolution. J. Soil Sediments 2013, 13, 354–363. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Zhang, W.; Lo, I.M.C. Copper extraction effectiveness and soil dissolution issues of EDTA-flushing of artificially contaminated soils. Chemosphere 2007, 68, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, S.; Xu, X.; Li, T.; Li, Y.; Deng, O.; Gong, G. Efficiency of nanoscale zero-valent iron on the enhanced low molecular weight organic acid removal Pb from contaminated soil. Chemosphere 2014, 117, 617–624. [Google Scholar] [CrossRef]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. TrAC Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Diquattro, S.; Castaldi, P.; Ritch, S.; Juhasz, A.L.; Brunetti, G.; Scheckel, K.G.; Garau, G.; Lombi, E. Insights into the fate of antimony (Sb) in contaminated soils: Ageing influence on Sb mobility, bioavailability, bioaccessibility and speciation. Sci. Total Environ. 2021, 770, 145354. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Zhong, Q.; Peijnenburg, W.J.G.M.; Vijver, M.G. Feasibility of Chinese cabbage (Brassica bara) and lettuce (Lactuca sativa) cultivation in heavily metals−contaminated soil after washing with biodegradable chelators. J Clean Prod 2018, 197, 479–490. [Google Scholar] [CrossRef]
- Lee, J.; Kim, E.J.; Kim, H.; Baek, K. Oxalate-based remediation of arsenic bound to amorphous Fe and Al hydrous oxides in soil. Geoderma 2016, 270, 76–82. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Wijesekara, H.; Weerarathne, N.; Ok, Y.S. Effects of soil type and fertilizer on As speciation in rice paddy contaminated with As-containing pesticide. Environ. Earth Sci. 2014, 71, 837–847. [Google Scholar] [CrossRef]
- Zhou, Q.; Ouyang, S.; Ao, Z.; Sun, J.; Liu, G.; Hu, X. Integrating Biolayer Interferometry, Atomic Force Microscopy, and Density Functional Theory Calculation Studies on the Affinity between Humic Acid Fractions and Graphene Oxide. Environ. Sci. Technol. 2019, 53, 3773–3781. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Hartley, N.R. Metal distribution and spectroscopic analysis after soil washing with chelating agents and humic substances. Environ. Sci. Pollut. Res. 2014, 21, 3987–3995. [Google Scholar] [CrossRef] [PubMed]
- Krivoshein, P.K.; Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. FTIR photoacoustic spectroscopy for identification and assessment of soil components: Chernozems and their size fractions. Photoacoustics 2020, 18, 100162. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, R.; Rajkumar, P. Characterization of minerals in air dust particles in the state of Tamilnadu, India through FTIR, XRD and SEM analyses. Infrared Phys. Technol. 2014, 67, 30–41. [Google Scholar] [CrossRef]
- Wei, M.; Chen, J. Influence of multi-step washing using Na2EDTA, oxalic acid and phosphoric acid on metal fractionation and spectroscopy characteristics from contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 23123–23133. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, J.; Baek, K. Abiotic reductive extraction of arsenic from contaminated soils enhanced by complexation: Arsenic extraction by reducing agents and combination of reducing and chelating agents. J. Hazard. Mater. 2015, 283, 454–461. [Google Scholar] [CrossRef]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef]
- Johnston, S.G.; Bennett, W.W.; Doriean, N.; Hockmann, K.; Karimian, N.; Burton, E.D. Antimony and arsenic speciation, redox-cycling and contrasting mobility in a mining-impacted river system. Sci. Total Environ. 2020, 710, 136354. [Google Scholar] [CrossRef]
- Bolan, N.; Kumar, M.; Singh, E.; Kumar, A.; Singh, L.; Kumar, S.; Keerthanan, S.; Hoang, S.A.; El-Naggar, A.; Vithanage, M.; et al. Antimony contamination and its risk management in complex environmental settings: A review. Environ. Int. 2022, 158, 106908. [Google Scholar] [CrossRef]
- Cerqueira, B.; Arenas-Lago, D.; Andrade, M.L.; Vega, F.A. Using time of flight secondary ion mass spectrometry and field emission scanning electron microscopy with energy dispersive X-ray spectroscopy to determine the role of soil components in competitive copper and cadmium migration and fixation in soils. Geoderma 2015, 251–252, 65–77. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Wang, Q.; Fang, L.; Xue, Q.; Cheeseman, C.R.; Donatello, S.; Liu, L.; Poon, C.S. Change in re-use value of incinerated sewage sludge ash due to chemical extraction of phosphorus. Waste Manag. 2018, 74, 404–412. [Google Scholar] [CrossRef]



| Fraction | Extractant | Extraction Conditions |
|---|---|---|
| Non-specifically adsorbed fraction (F1) | 0.05 mol·L−1 (NH4)2SO4 | 4 h shaking, 25 °C, L/S ratio 25:1 |
| Specifically adsorbed fraction (F2) | 0.05 mol·L−1 (NH4)H2SO4 | 16 h shaking, 25 °C, L/S ratio 25:1 |
| Amorphous and crystalline Fe and Al oxides bound fraction (F3) | 0.2 mol·L−1 NH4-oxalate buffer and 0.1 mol·L−1 ascorbic acid, pH = 3.25 | 0.5 h in a water basin at 96 ± 3 °C in the light, L/S ratio 25:1 |
| The residual fraction (F4) | Concentrated HCl, HNO3 in a ratio 3:1 | 180 °C, 30 min |
| Parameters | Values |
|---|---|
| pH | 8.63 |
| OM/(g·kg−1) | 3.155 |
| CEC/(cmol·kg−1) | 2.119 |
| Total Sb/(mg·kg−1) | 384.54 |
| Clay/% | 6.99 |
| Silt/% | 26.78 |
| Sand/% | 66.23 |
| Si/% | 25.88 |
| Al/% | 15.74 |
| Fe/% | 10.69 |
| K/% | 3.34 |
| Extractant | Elovich | Two-Constant | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | SE | R2 | SE | R2 | SE | R2 | SE | |
| CA | 0.933 | 0.550 | 0.903 | 0.567 | 0.779 | 0.322 | 0.922 | 0.401 |
| OA | 0.932 | 0.784 | 0.892 | 0.798 | 0.813 | 0.476 | 0.965 | 0.578 |
| TA | 0.971 | 0.539 | 0.939 | 0.558 | 0.744 | 0.331 | 0.929 | 0.399 |
| MA | 0.967 | 0.579 | 0.941 | 0.602 | 0.737 | 0.354 | 0.920 | 0.429 |
| HEDP | 0.951 | 0.670 | 0.910 | 0.689 | 0.836 | 0.469 | 0.963 | 0.520 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Pan, W.; Tong, L.; Hu, Y.; Zou, Y.; Huang, X. Remediation of Sb-Contaminated Soil by Low Molecular Weight Organic Acids Washing: Efficiencies and Mechanisms. Sustainability 2023, 15, 4147. https://doi.org/10.3390/su15054147
Li S, Pan W, Tong L, Hu Y, Zou Y, Huang X. Remediation of Sb-Contaminated Soil by Low Molecular Weight Organic Acids Washing: Efficiencies and Mechanisms. Sustainability. 2023; 15(5):4147. https://doi.org/10.3390/su15054147
Chicago/Turabian StyleLi, Sicheng, Weibin Pan, Lizhi Tong, Yuanyuan Hu, Yulin Zou, and Xiaojia Huang. 2023. "Remediation of Sb-Contaminated Soil by Low Molecular Weight Organic Acids Washing: Efficiencies and Mechanisms" Sustainability 15, no. 5: 4147. https://doi.org/10.3390/su15054147
APA StyleLi, S., Pan, W., Tong, L., Hu, Y., Zou, Y., & Huang, X. (2023). Remediation of Sb-Contaminated Soil by Low Molecular Weight Organic Acids Washing: Efficiencies and Mechanisms. Sustainability, 15(5), 4147. https://doi.org/10.3390/su15054147





