Adsorption Characteristics of Cd2+ Ions in Aqueous Solution on Modified Straw Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Material
2.2. Preparation of Modified Biochar
2.2.1. Preparation of the Straw Biochar
2.2.2. Preparation of Fe-Modified Biochar
2.2.3. Preparation of Mn-Modified Biochar
2.2.4. Preparation of Fe-Mn Modified Biochar
2.3. Structural Characterization of Biochar
2.4. Sorption Experiments
2.4.1. Sorption Kinetics
2.4.2. Sorption Isotherms
2.5. Data Processing
3. Results and Analysis
3.1. Characterization of Biochar
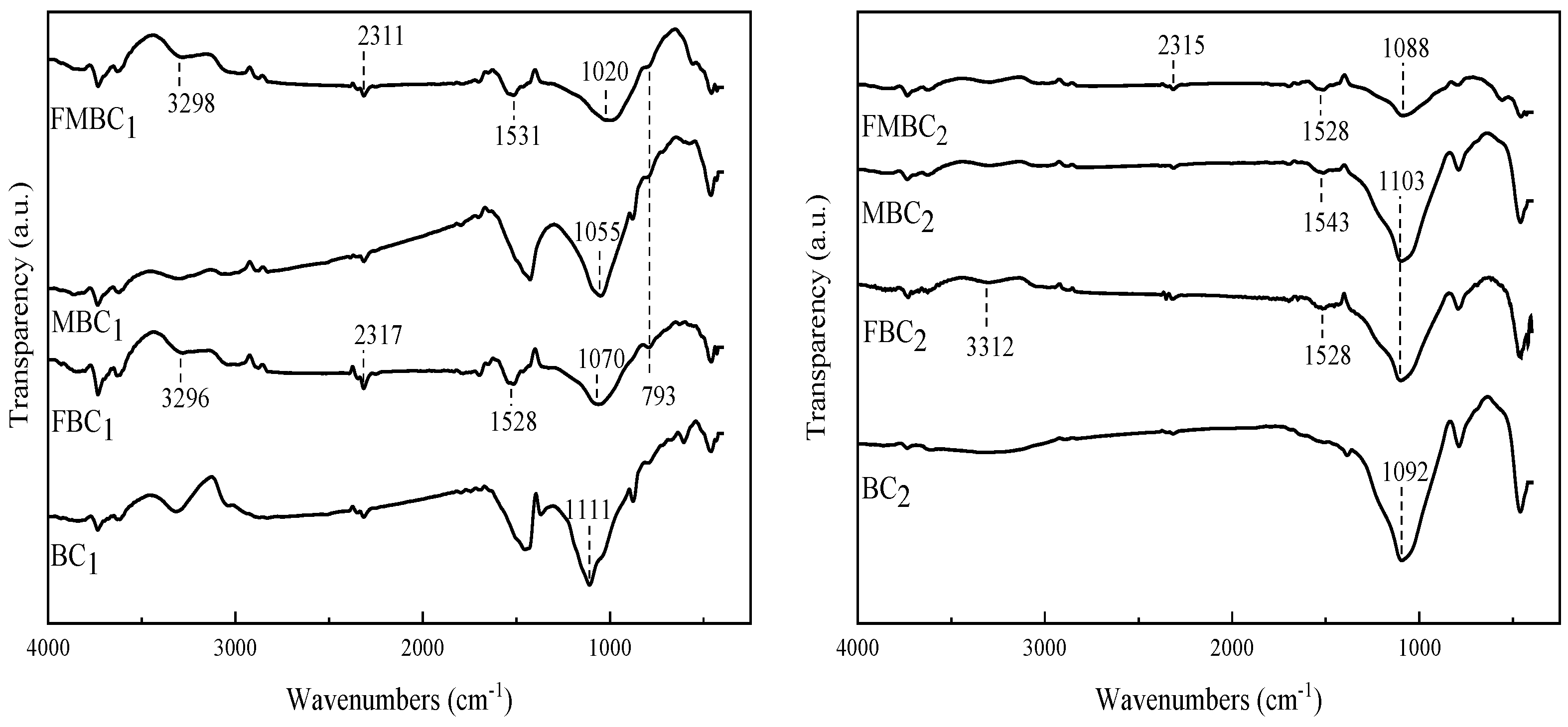
3.1.1. FT-IR Characterization
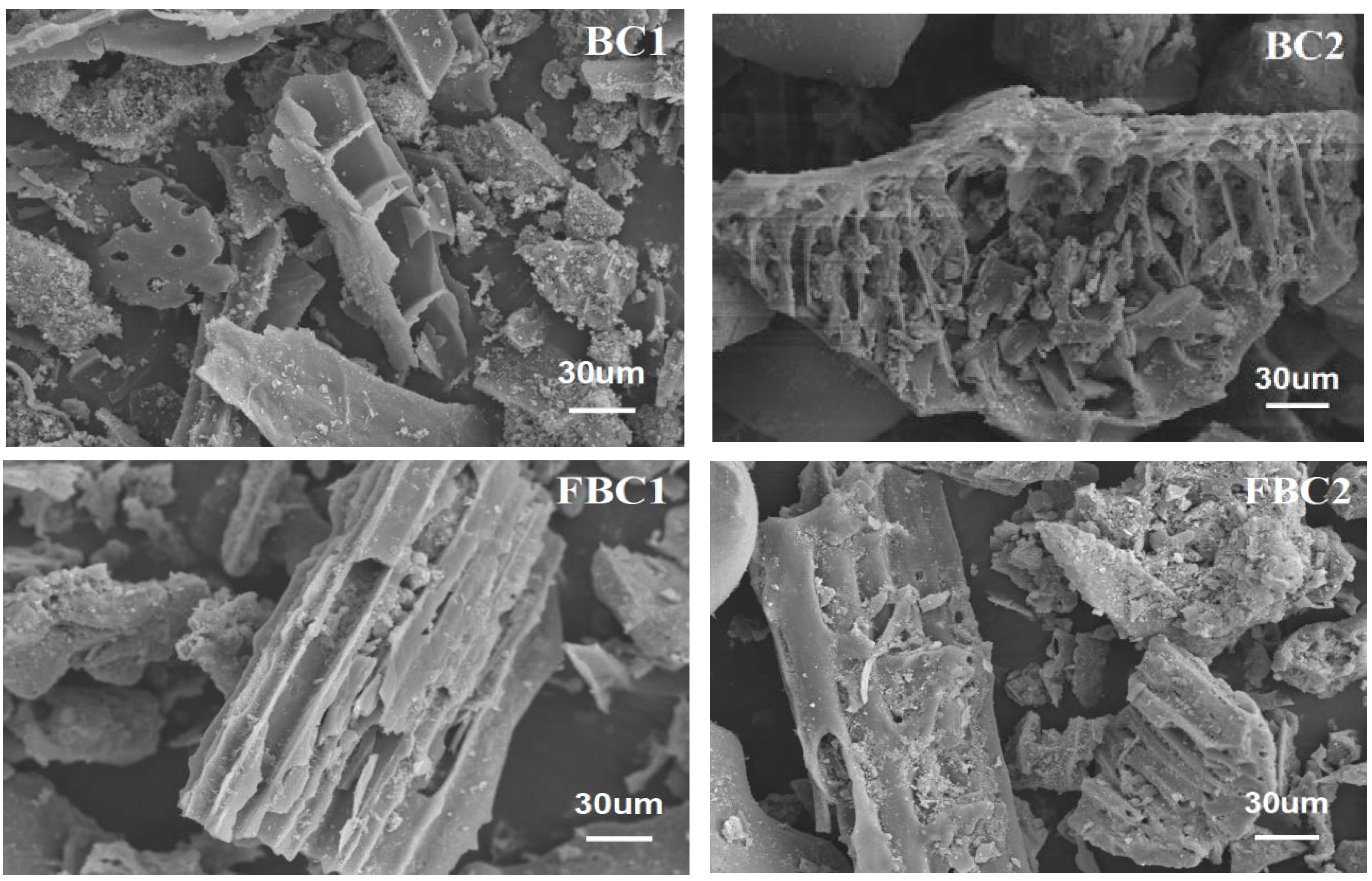
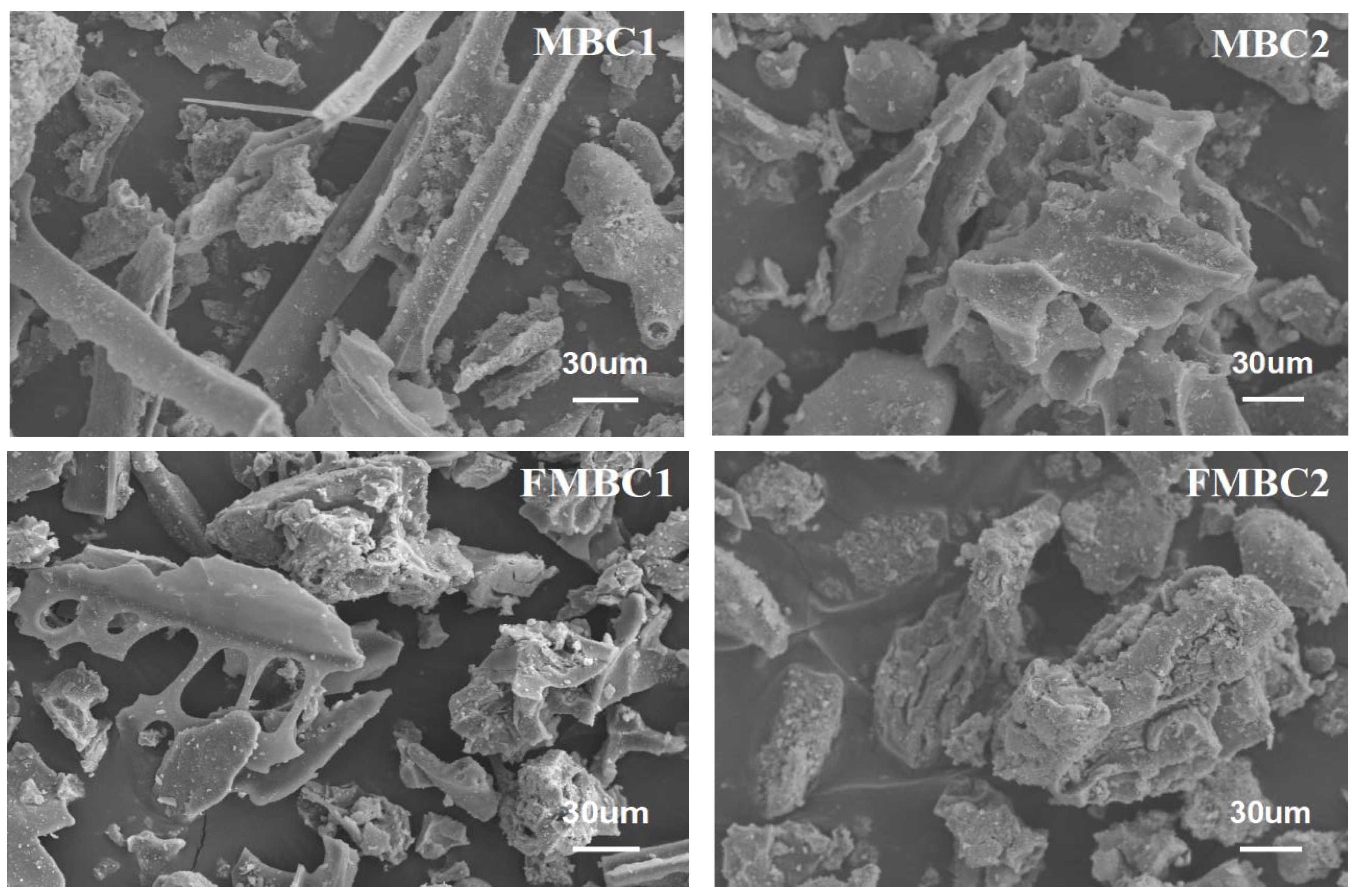
3.1.2. SEM Characterization
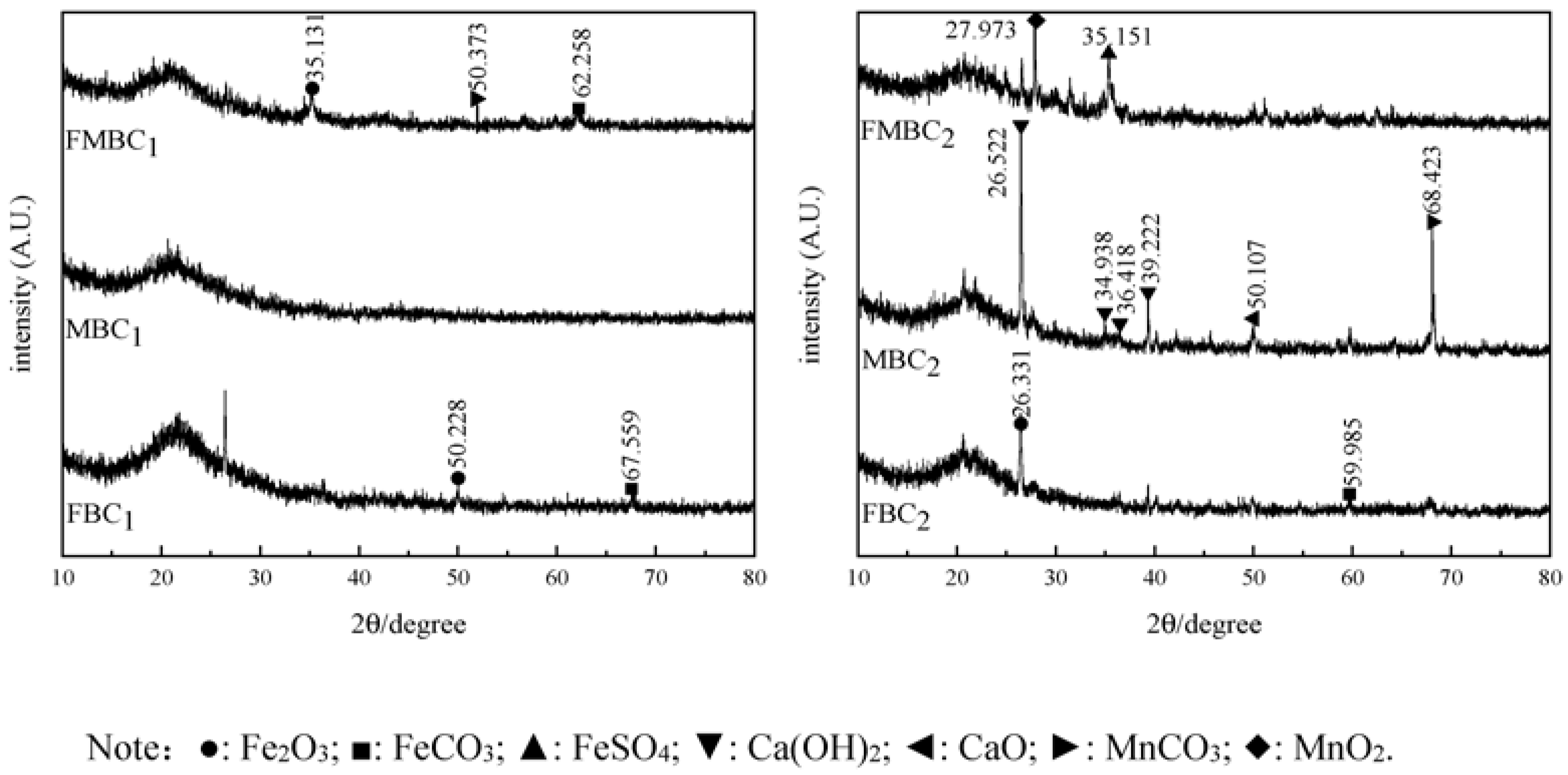
3.1.3. XRD Characterization
3.2. Absorption Experiment
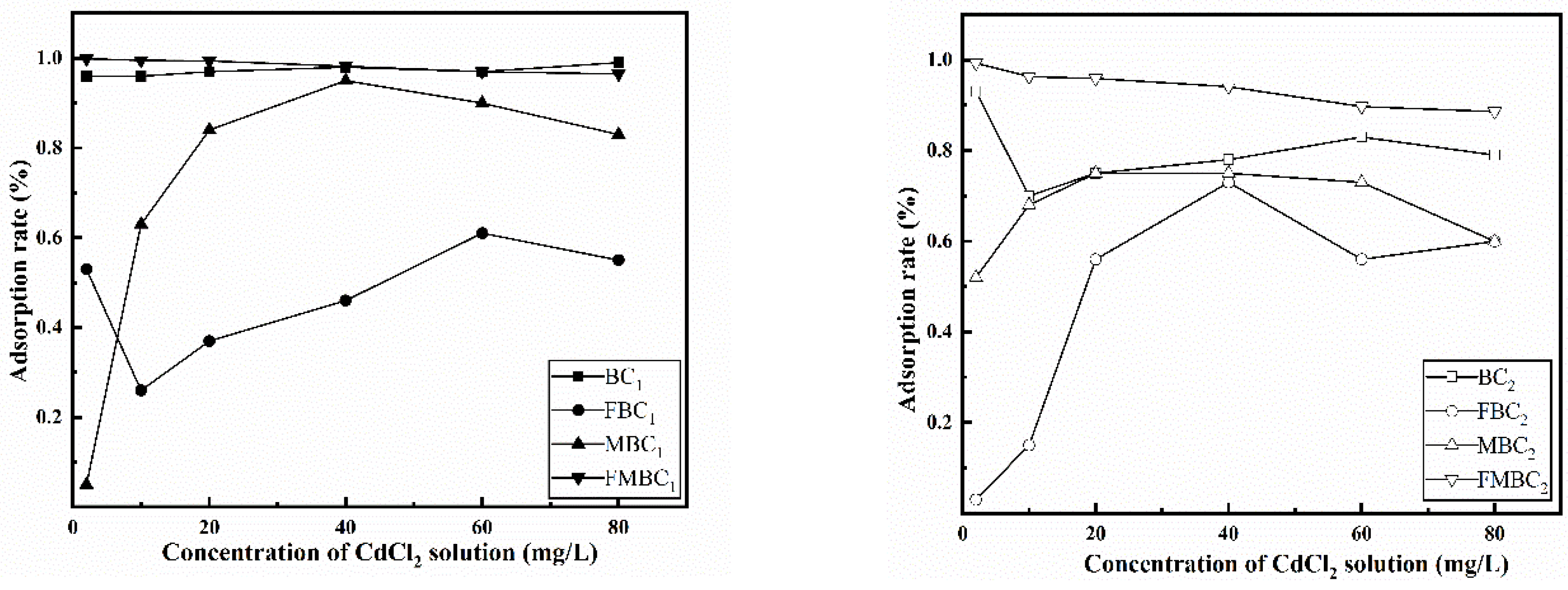
3.2.1. Effect of Concentration ofCdCl2 Solution on Adsorption Rate
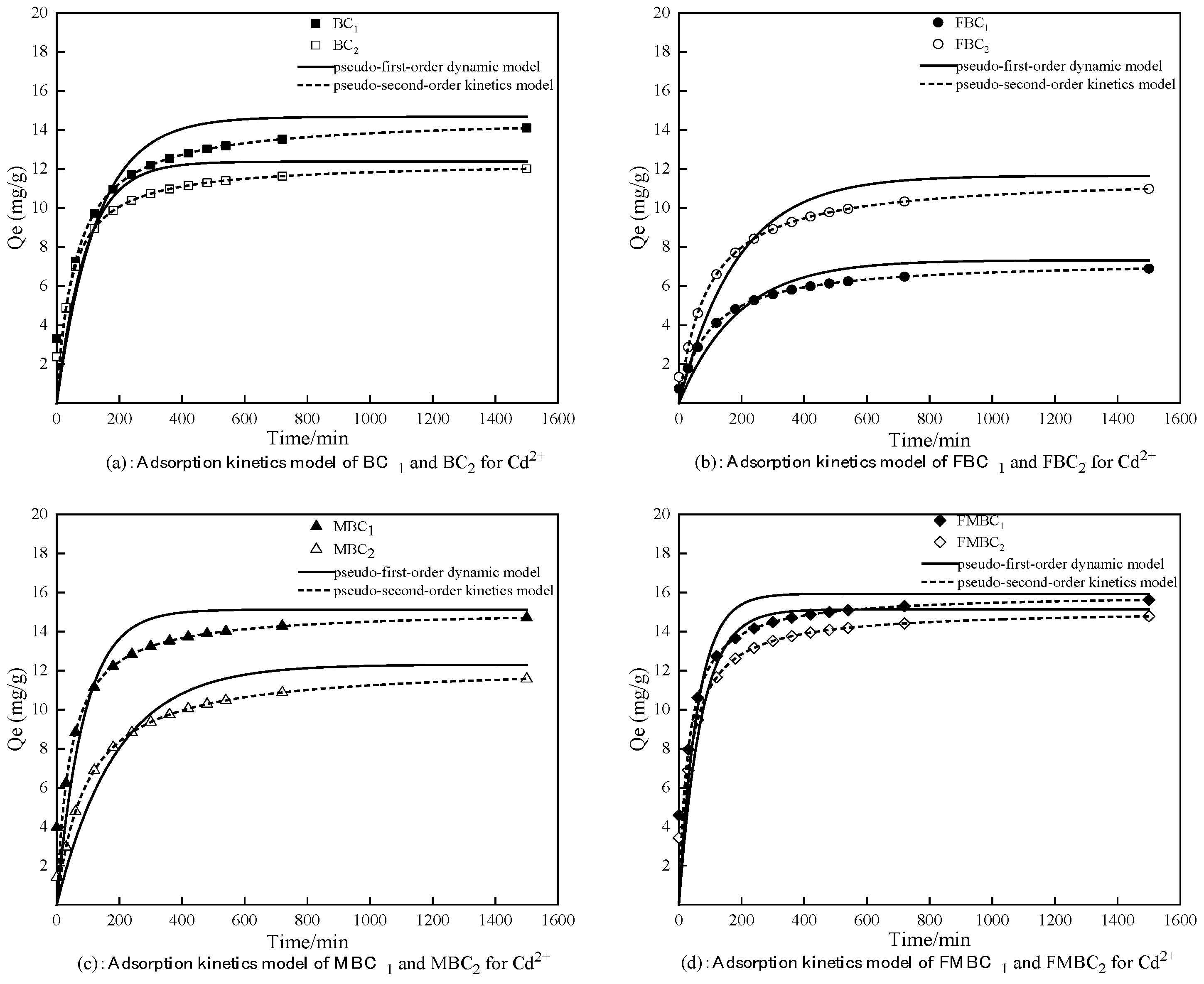
3.2.2. Adsorption Kinetics
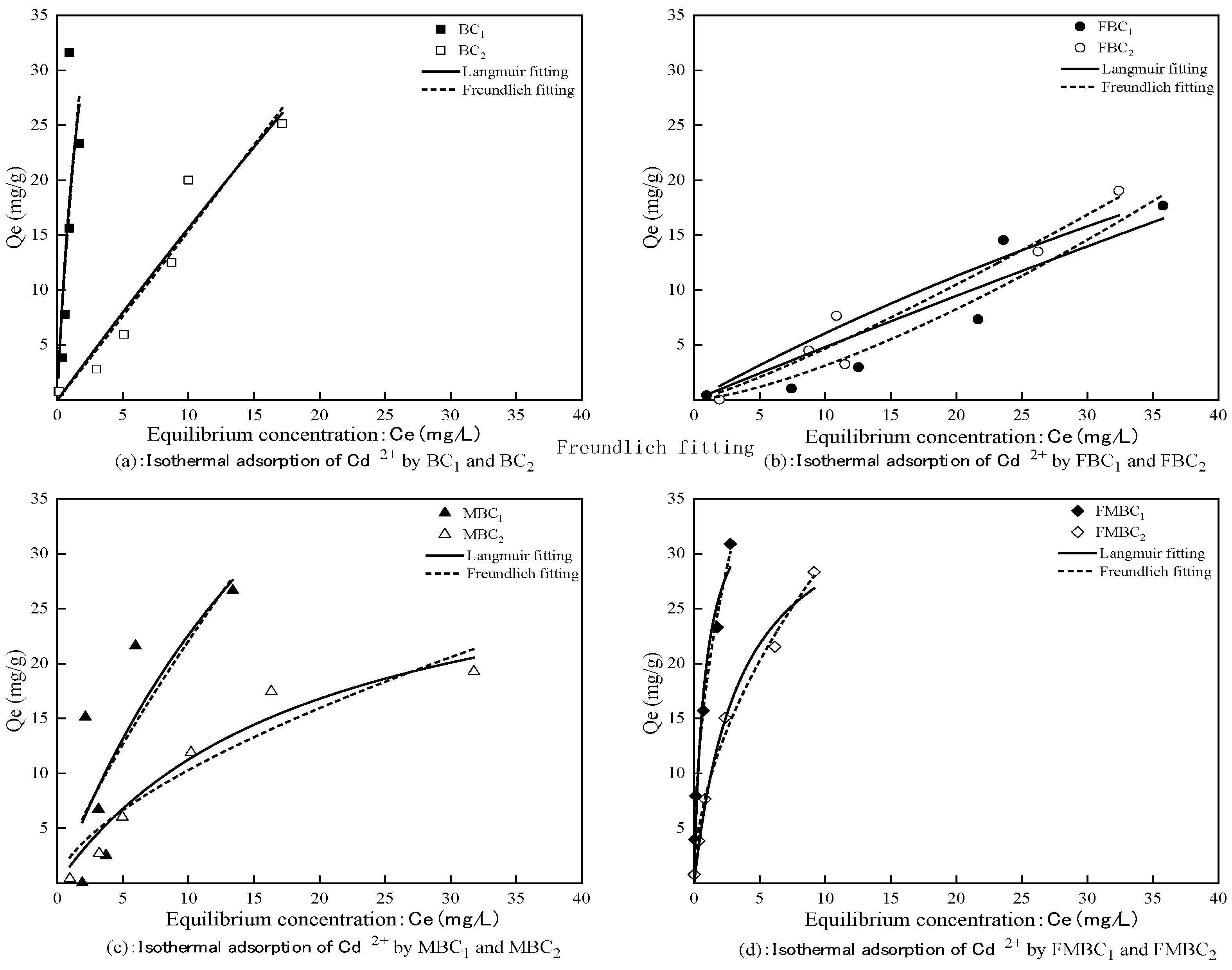
3.2.3. Isothermal Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.X.; Xu, Z.y.; Xu, L.F.; Faeiza, B.; Tay, C.; Chay, Z.L.; Cai, Y.W.; Hu, B.W.; Zhu, Y.L.; Wang, X.K. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8–29. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.Q.; Gao, W.H.; Zhou, J.R. Hazards of Characteristic Pollutants in Electroplating Wastewater and Researching Progress on Method of Disposal. Plat. Finish. 2020, 9, 31–34. [Google Scholar]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Liu, Y.; Lin, X.; Wang, J.; Zhang, Z.; Li, N.; Li, Y.; Zhang, Y. KOH modification effectively enhances the Cd and Pb adsorption performance of N-enriched biochar derived from waste chicken feathers. Waste Manag. 2021, 130, 82–92. [Google Scholar] [CrossRef]
- Chakravarty, R.; Banerjee, P.C. Mechanism of cadmium binding on the cell wall of an acidophilic bacterium. Bioresour. Technol. 2012, 108, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.Y.; Deng, J.H.; Huang, G.F.; Li, K.; Cai, K.Z.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Kang, C.Y.; Li, Q.Y.; Liu, J.Y. Effect of biochar at different pyrolysis temperatures on the adsorption of Cd2+. Ind. Water Treat. 2021, 41, 68–72. [Google Scholar]
- Reddy, D.H.K.; Lee, S.M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloid Surf. A Physicochem. Eng. Asp. 2019, 454, 96–103. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du, L.G. Use of natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Mondal, M.M.; Weichgrebe, D. Biochar from co-pyrolysis of urban organic wastes—Investigation of carbon sink potential using ATR-FTIR and TGA. Biomass Convers. Biorefnery 2019, 12, 4729–4743. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinentto itspotential use inremediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, H.; Yu, R.; Hu, G.; Chen, F. Removal of aquatic cadmium Ions using thiourea modified poplar biochar. Water 2020, 12, 1117. [Google Scholar] [CrossRef] [Green Version]
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Luo, J.P.; Fang, R.; Shi, J.J.; Zhang, X.Z.; Tan, Z.Z.; Meng, Y.Y. Adsorption performance of tetracycline on nitric acid-modified rape biochar. Environ. Sci. Technol. 2019, 32, 17–23. [Google Scholar]
- Uchimiya, M.; Lima, I.M.; Thomas, K.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agric. Food Chem. 2012, 60, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.R.; Ji, L.Y.; Gao, C.C. Preparation of modified biochar and its application in environmental remediation. J. Agro-Environ. Sci. 2021, 40, 913–925. [Google Scholar]
- Cui, Z.W.; Ren, Y.F.; Wang, W. Adsorption Characteristics and Mechanism of Cadmium in Water by Alkali and Magnetic Composite Modified Wheat Straw Biochar. Environ. Sci. 2020, 41, 3315–3325. [Google Scholar]
- Liu, W.; Wang, Y.; Sik, O.K.; Daniel, C.W. New trends in biochar pyrolysis and modification strategies: Feedstock, pyrolysis conditions, sustainability concerns and implications for soil amendment. Soil Use Manag. 2020, 36, 358–386. [Google Scholar]
- Fang, L.; Li, J.; Donatello, S. Use of Mg/Ca modified biochars to take up phosphorus from acid-extract of incinerated sewage sludge ash(ISSA)for fertilizer application. J. Clean. Prod. 2020, 244, 118–853. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.M.; Zhang, Q.; Gong, X.M.; Xu, P. Synergistic removal of copper and tetracycline from aqueous solution by steam-activated bamboo-derived biochar. J. Hazard Mater. 2020, 384, 121470. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Jiang, X.T.; Chi, J. Phosphorus adsorption by and forms in Fe-modified biochar. J. Agro-Environ. Sci. 2014, 33, 1817–1822. [Google Scholar]
- Bashir, S.; Zhu, J.; Fu, Q. Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 11875–11883. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Li, C.; Xie, S.; You, F.; Cao, Z.; Xu, Z.; Yu, G.; Wang, Y. Preparation of biochar via pyrolysis at laboratory and pilot scales to remove antibiotics and immobilize heavy metals in livestock feces. J. Soils Sediments 2019, 19, 2891–2902. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Preparation, modification and environmental application of biochar: A review. J. Clean Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Chen, L.; Zhi, J. Enhanced adsorption activity of manganese oxide-modified biochar for the removal of tetracycline from aqueous solution. J. Agro-Environ. Sci. 2021, 40, 194–201. [Google Scholar]
- Tan, X.; Wei, W.; Xu, C. Manganese-modified biochar for highly efficient sorption of cadmium. Environ. Sci. Pollut. Res. 2020, 7, 9126–9134. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef]
- Wang, X.X.; Yang, T.; Xiao, L.R. Study on the adsorption performance of rice straw biomass charcoal to heavy metal Cd2+. Acta Sci. Circumstantiae 2021, 41, 2691–2699. [Google Scholar]
- Tan, W.T.; Zhou, H.; Tang, S.F.; Zeng, P.; Gu, J.F.; Liao, B.H. Enhancing Cd(II) adsorption on rice straw biochar by modification of iron and manganese oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef]
- Gao, R.L.; Xiang, L.; Hu, H.Q.; Fu, Q.L.; Zhu, J.; Liu, Y.H.; Huang, G.Y. High-efficiency removal capacities and quantitative sorption mechanisms of Pb by oxidized rape straw biochars. Sci. Total Environ. 2019, 9, 1344. [Google Scholar]
- Hmed, M.B.; Zhou, J.L.; Ngo, H.H. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar]
- Ren, J.Q.; Wang, C.X.; Zhang, F. Adsorption of Cd2+ from aqueous solution by modified rice Husk -derived biochars. J. Ecol. Rural. Environ. 2021, 37, 73–79. [Google Scholar]
- Mohd, I.; Ahmed, A.; Mohammed, A.A.; Tansir, A.; Akhalakur, R.A.; Mohammad, S.; Dinesh, L.; Afzal, K. UV light enabled photocatalytic activity of a-Fe2O3 nanoparticles synthesized via phase transformation. Mater. Lett. 2020, 258, 126748. [Google Scholar]
- Mohammadi, Z.A.; Hosseiny, D.S.S.; Maleka, A.M.; Sarparast, M. Designing an asymmetric device based on graphene wrapped yolk−double shell NiGa2S4hollow microspheres and graphene wrapped FeS2−FeSe2core−shell cratered spheres with outstanding energy density. J. Mater. Chem. A Mater. 2019, 7, 10282−10292. [Google Scholar]
- Muhammad, A.R.; Naseem, I.; Tayyaba, N.; Zahid, A.G. Hierarchical flower-like NiMn-LDH@MnCo2S4grown on nickle foam as a high-specific capacity faradaic electrode. Energy Fuels 2023, 37, 1310−1317. [Google Scholar]
- Abbasi, L.; Arvand, M.; Moosavifard, S.E. Facile template-free synthesis of 3D hierarchical ravine-like interconnected MnCo2S4nanosheet arrays for hybrid energy storage device. Carbon 2020, 161, 299−308. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Xu, M.; Wu, J. Adsorption of Cd2+ by potassium ferrate/potassium permanganate-modified vinasse biochar. J. Agro-Environ. Sci. 2021, 40, 876–883. [Google Scholar]
- Wang, Y.; Liu, R. H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions. Sci. Total Environ. 2018, 628, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Li, D.Y.; Liu, S.T. Effects of Fe-Mn modified coconut shell biochar on cadmium speciation and accumulation in rice. Res. Environ. Sci. 2019, 32, 139–147. [Google Scholar]
| Biochar Species | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| KL/(L·mg−1) | Qm/(mg·g−1) | R2 | KF/(L·mg−1) | 1/n | R2 | |
| BC1 | 0.417 | 20.304 | 0.953 | 8.553 | 0.782 | 0.942 |
| BC2 | 0.004 | 30.371 | 0.921 | 1.503 | 1.010 | 0.920 |
| FBC1 | 0.002 | 50.273 | 0.859 | 0.124 | 1.401 | 0.884 |
| FBC2 | 0.008 | 93.748 | 0.911 | 0.316 | 1.170 | 0.934 |
| MBC1 | 0.039 | 40.893 | 0.974 | 3.482 | 0.802 | 0.946 |
| MBC2 | 0.052 | 33.018 | 0.947 | 2.418 | 0.630 | 0.887 |
| FMBC1 | 1.198 | 37.453 | 0.955 | 18.602 | 0.474 | 0.995 |
| FMBC2 | 0.281 | 37.284 | 0.983 | 8.507 | 0.538 | 0.989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, B.; Xu, H.; Song, F.; Liu, Z. Adsorption Characteristics of Cd2+ Ions in Aqueous Solution on Modified Straw Biochar. Sustainability 2023, 15, 4373. https://doi.org/10.3390/su15054373
Tang B, Xu H, Song F, Liu Z. Adsorption Characteristics of Cd2+ Ions in Aqueous Solution on Modified Straw Biochar. Sustainability. 2023; 15(5):4373. https://doi.org/10.3390/su15054373
Chicago/Turabian StyleTang, Bo, Haopu Xu, Fengmin Song, and Zhifeng Liu. 2023. "Adsorption Characteristics of Cd2+ Ions in Aqueous Solution on Modified Straw Biochar" Sustainability 15, no. 5: 4373. https://doi.org/10.3390/su15054373
APA StyleTang, B., Xu, H., Song, F., & Liu, Z. (2023). Adsorption Characteristics of Cd2+ Ions in Aqueous Solution on Modified Straw Biochar. Sustainability, 15(5), 4373. https://doi.org/10.3390/su15054373






