Stiffening and Toughening of Asphalt Mastic Induced by Bitumen–Mineral Selective Molecular Adsorption and Nanostructural Reconstruction
Abstract
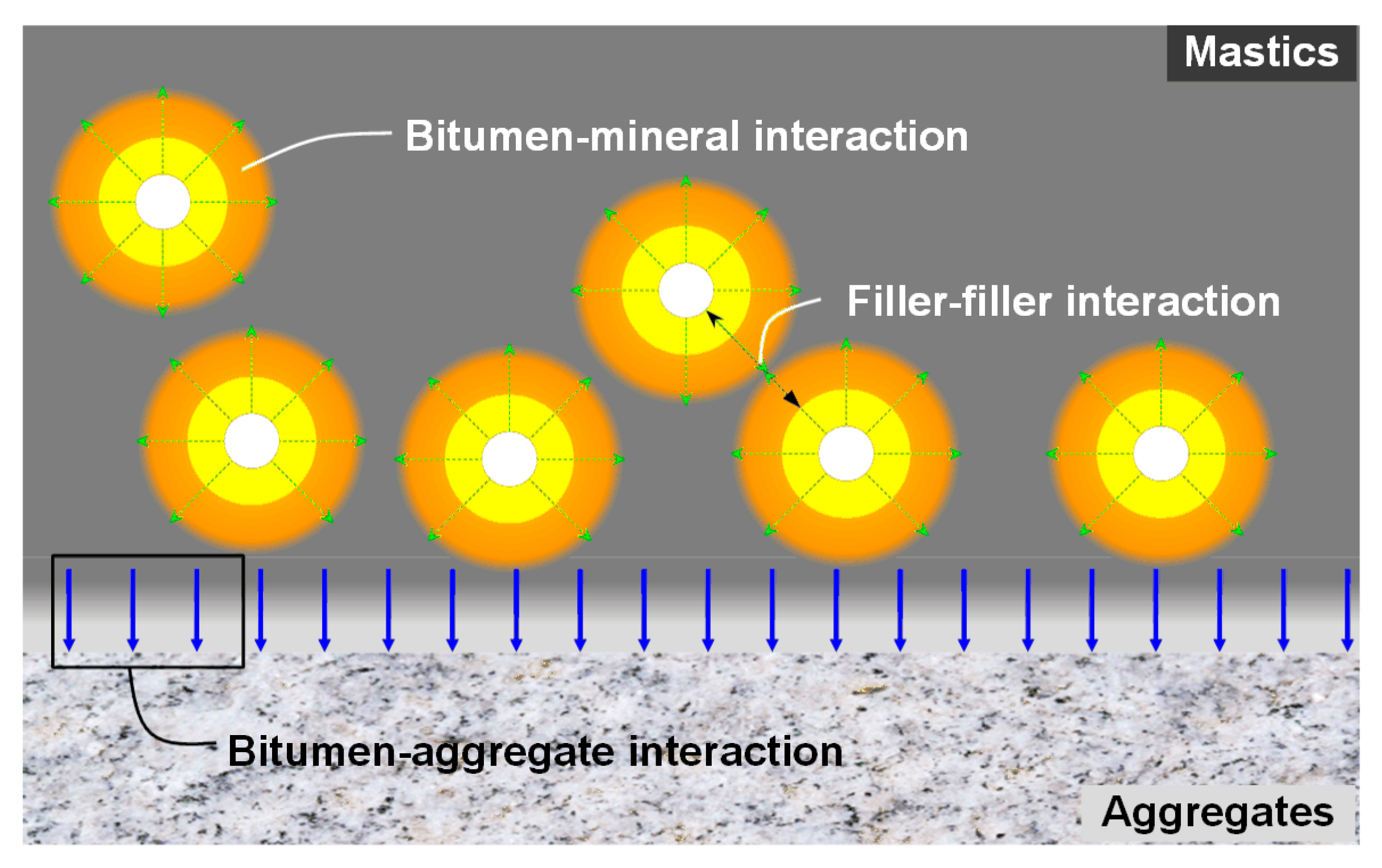
1. Introduction
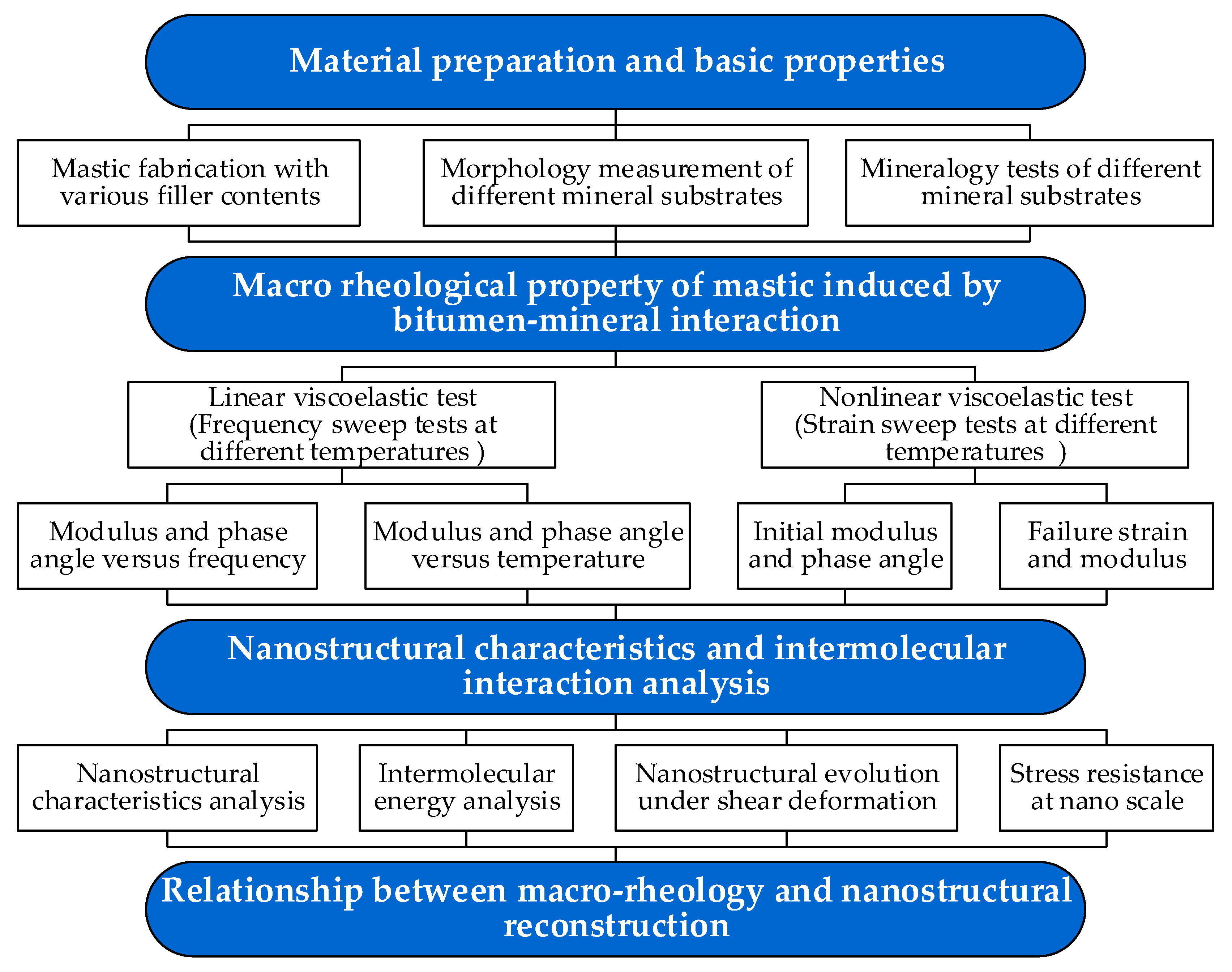
2. Materials and Methods
2.1. Materials and Sample Preparation
2.1.1. Bitumen and Asphalt Mastic
2.1.2. Mineral Substrates
2.2. Rheological Characteristic Tests
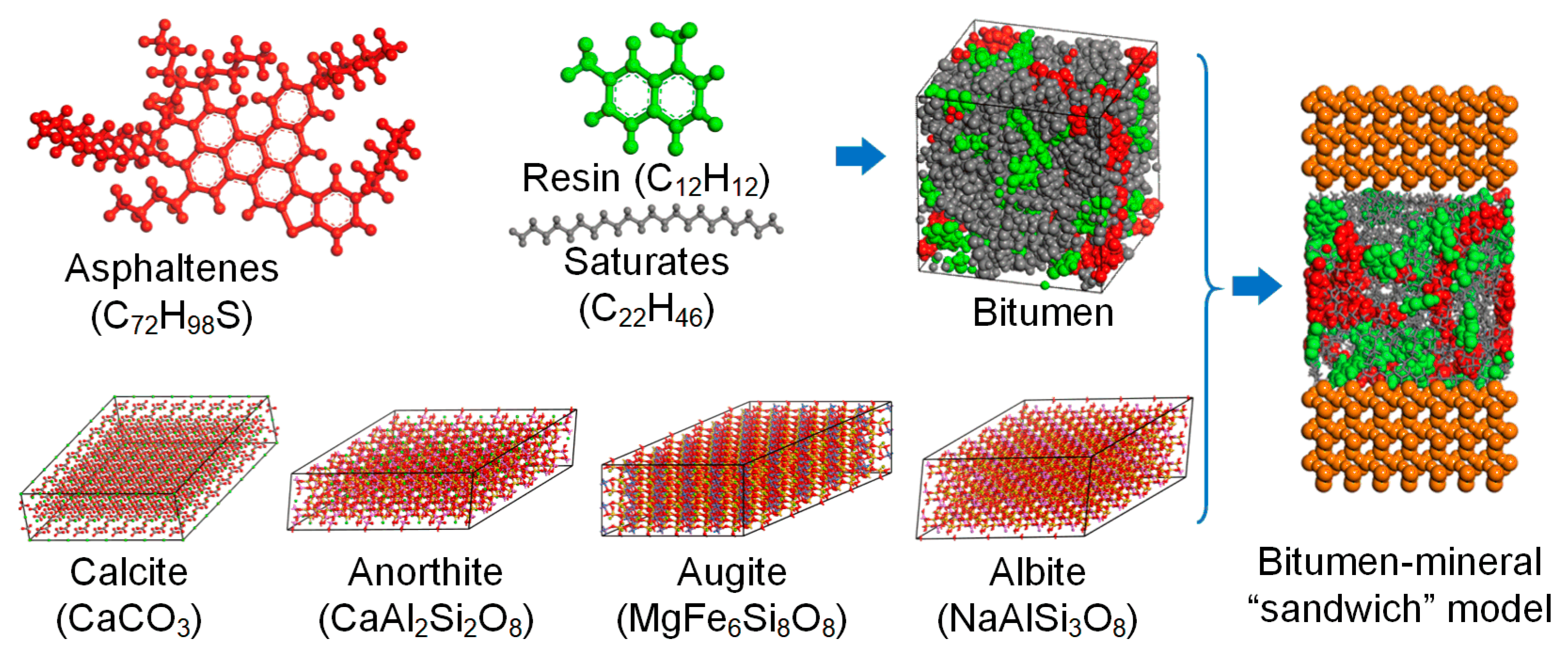
2.3. Molecular Dynamics Simulation
2.3.1. Molecular Models
2.3.2. Molecular Dynamics
3. Results and Discussion
3.1. Linear Rheological Characteristics of Asphalt Mastic in Contact with Various Mineral Aggregates
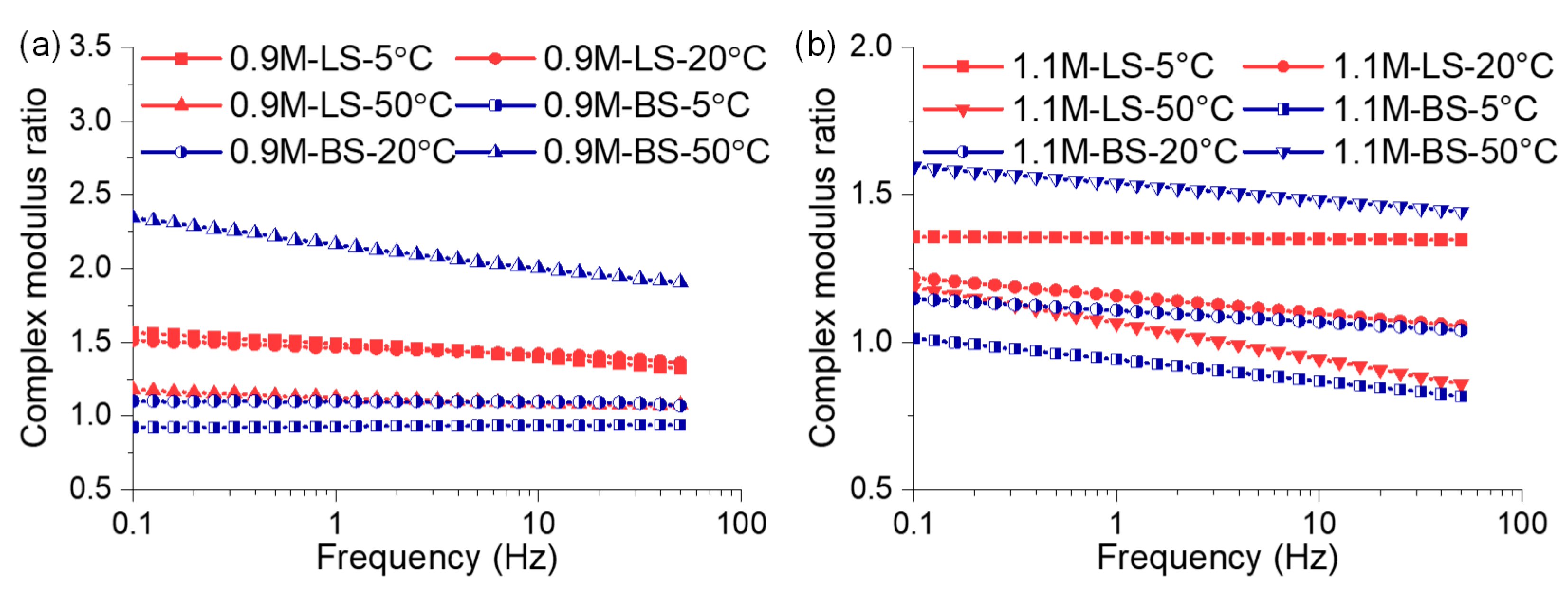
3.1.1. Modulus Analysis at Various Frequencies
3.1.2. Modulus Analysis at Different Temperatures
3.2. Nonlinear Rheological Properties of Asphalt Mastics Affected by the Mineral Aggregates
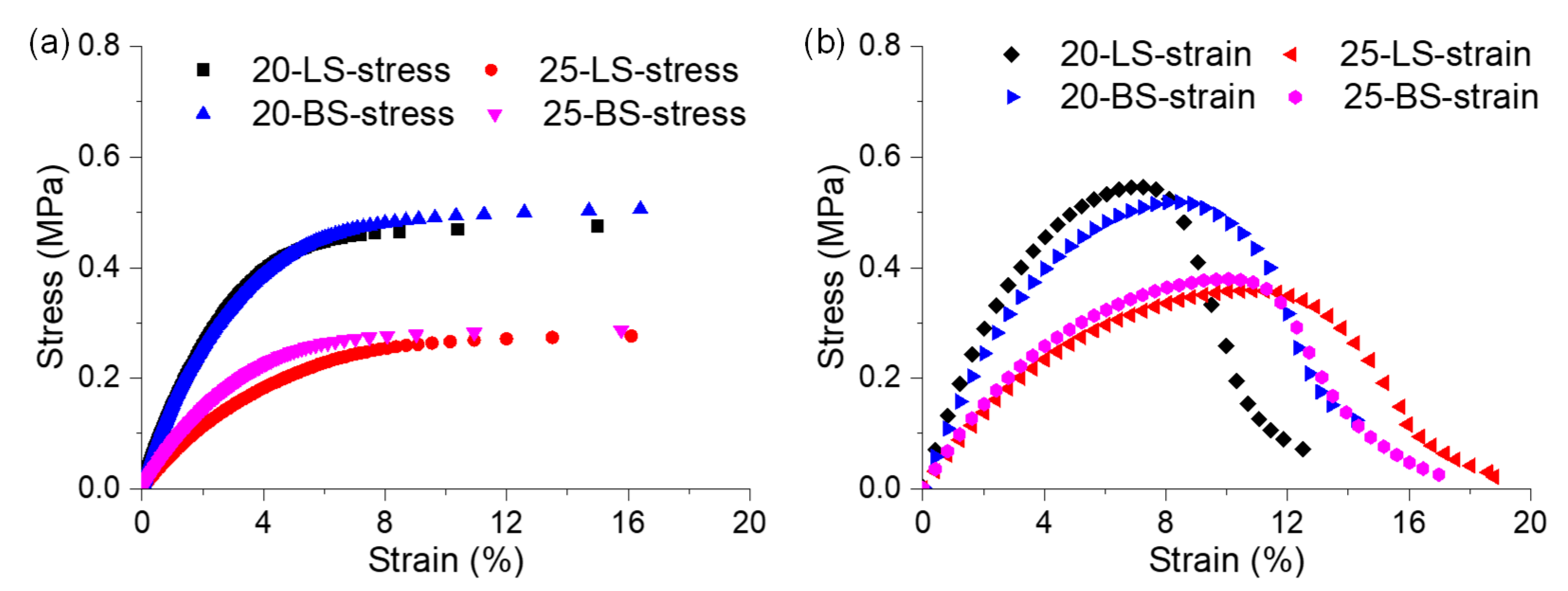
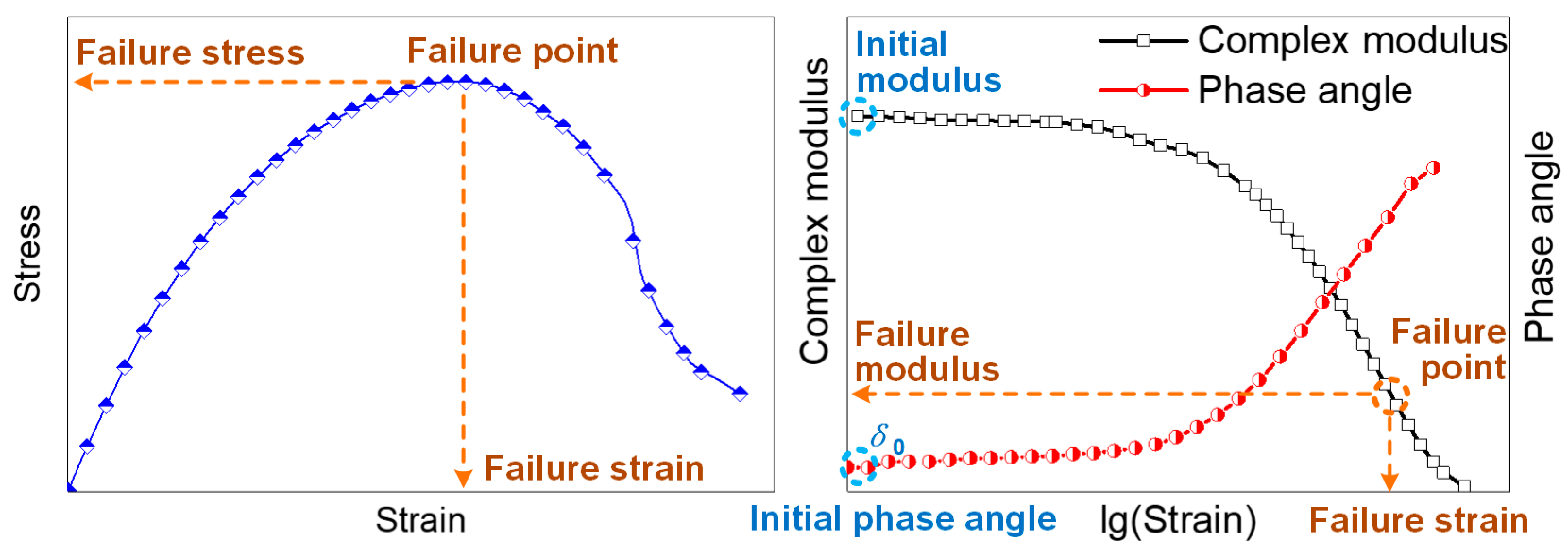
3.2.1. Nonlinear Rheological Characterization of the Mastic
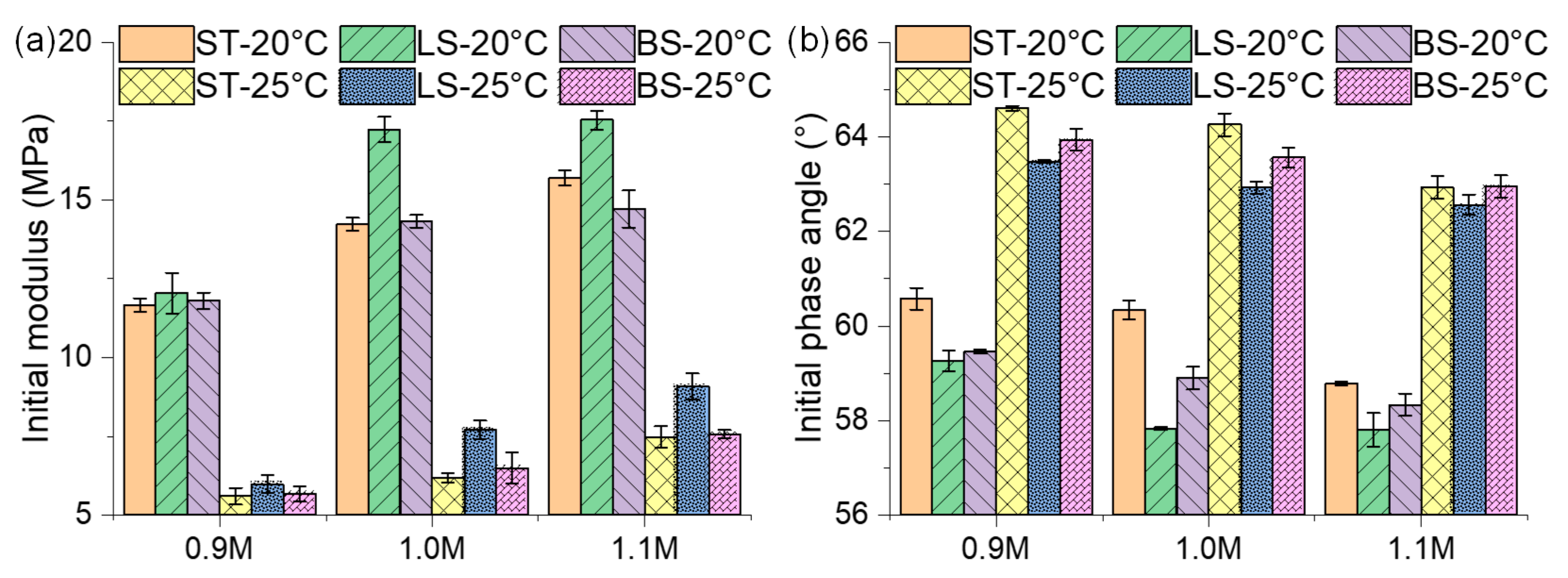
3.2.2. Initial Modulus and Phase Angle of Mastic on Different Mineral Substrates
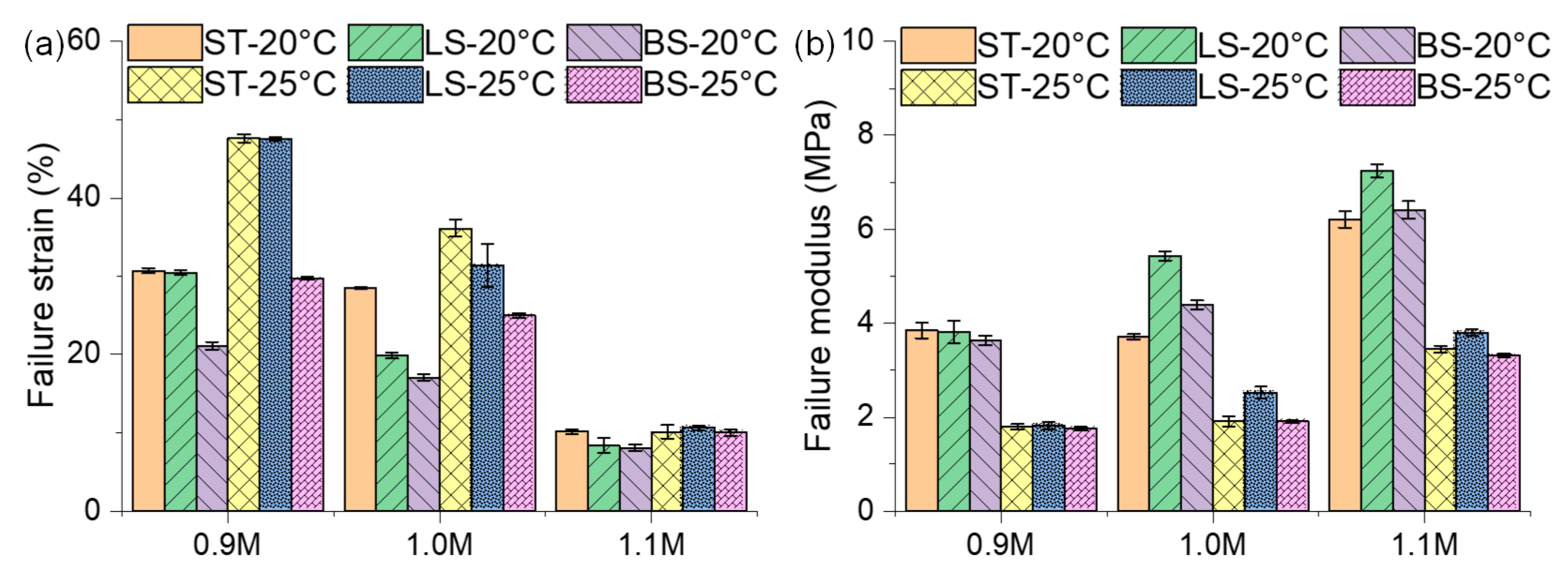
3.2.3. Failure Strain and Failure Modulus of Mastic on Different Mineral Substrates
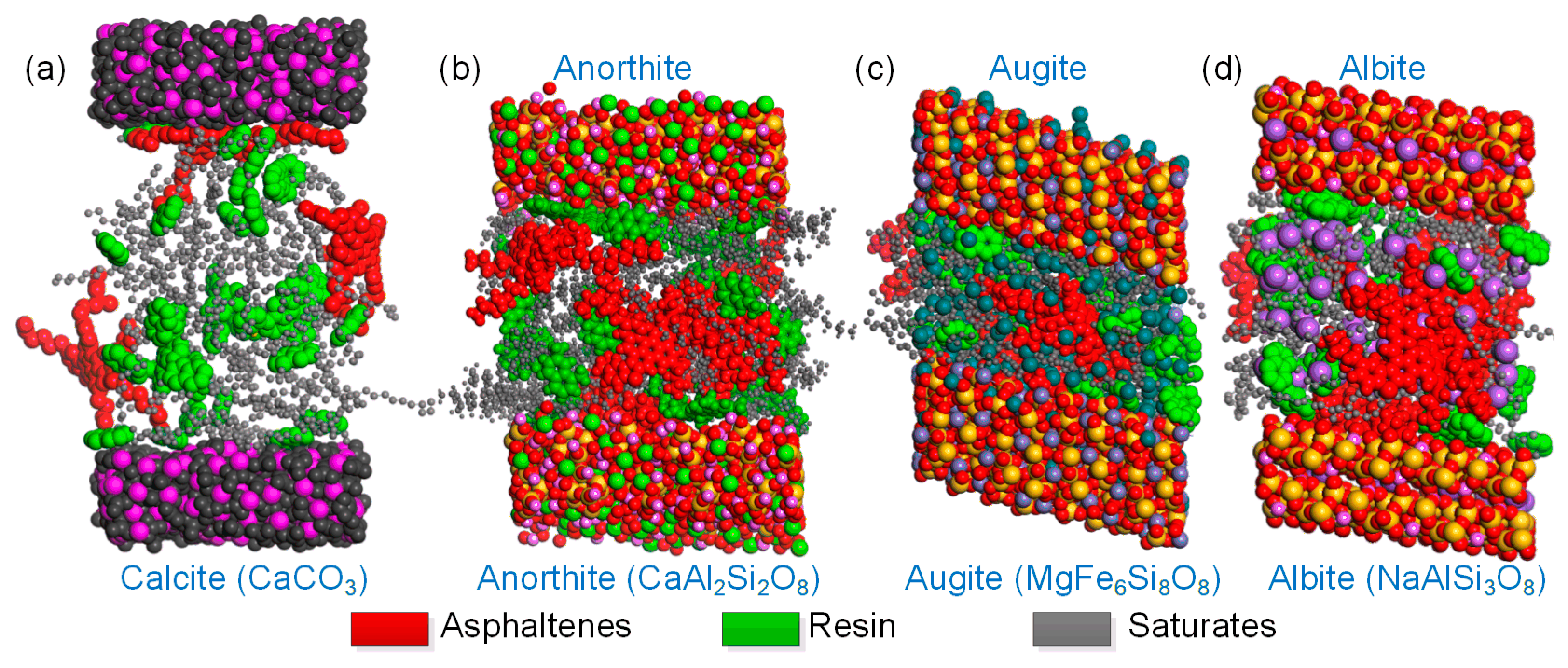
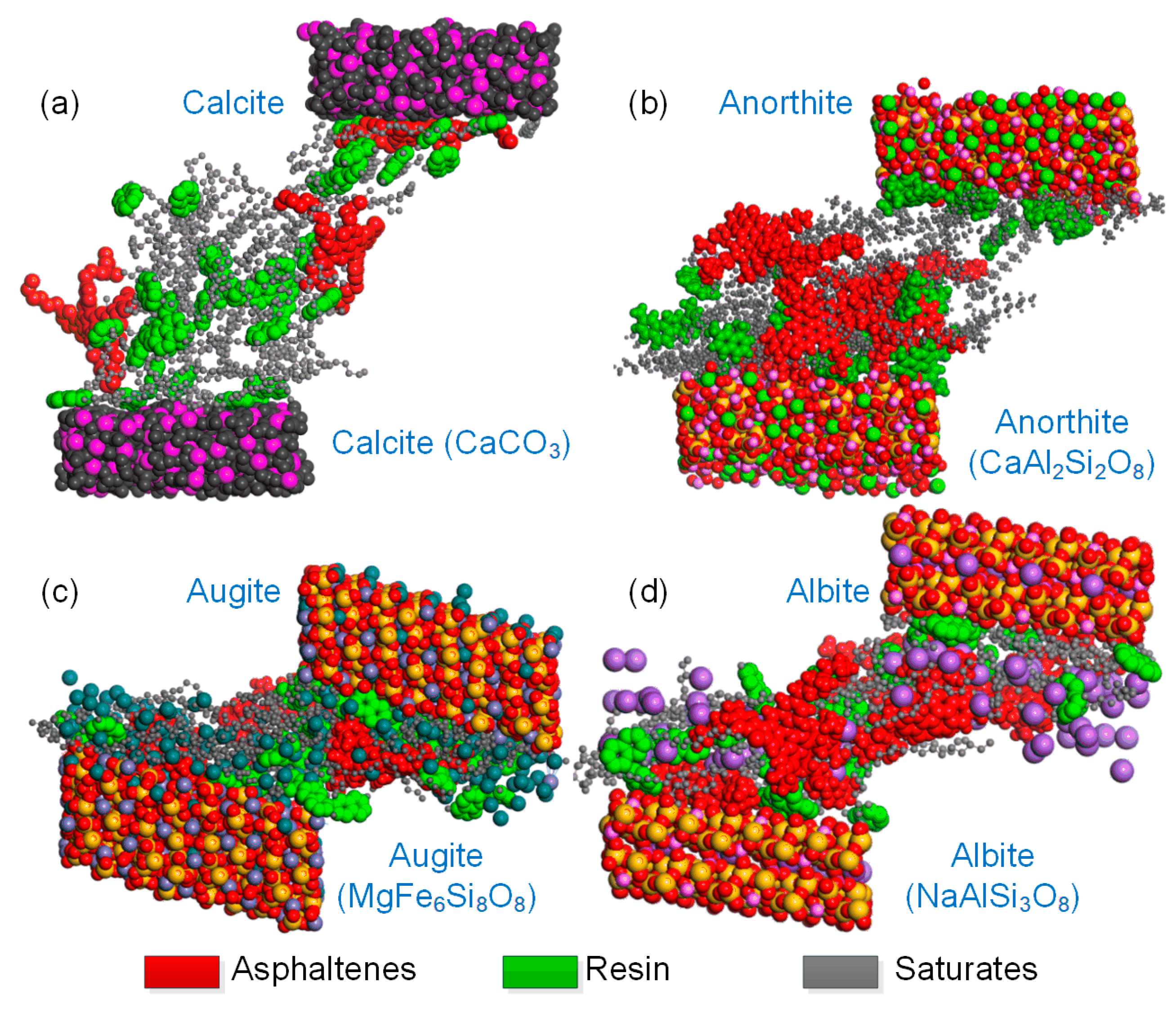
3.3. Nanostructural Characteristics and Intermolecular Interaction Analysis between the Bitumen and Minerals
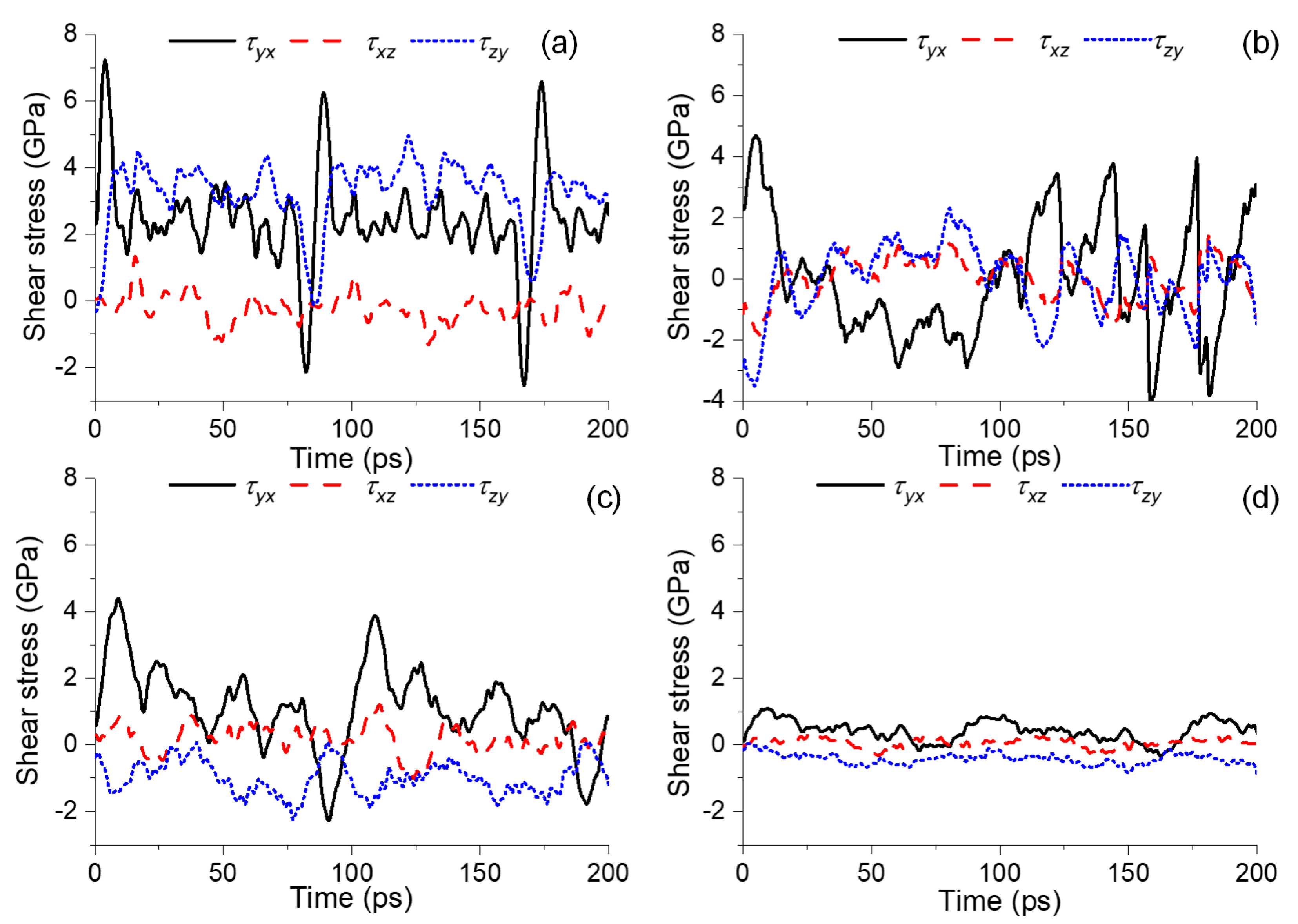
3.4. Nanomechanical Characteristics of the Bitumen–Mineral Interface under Shearing Displacement
3.5. Relationship between Macrorheological Characteristics and Nanomolecular Interactions
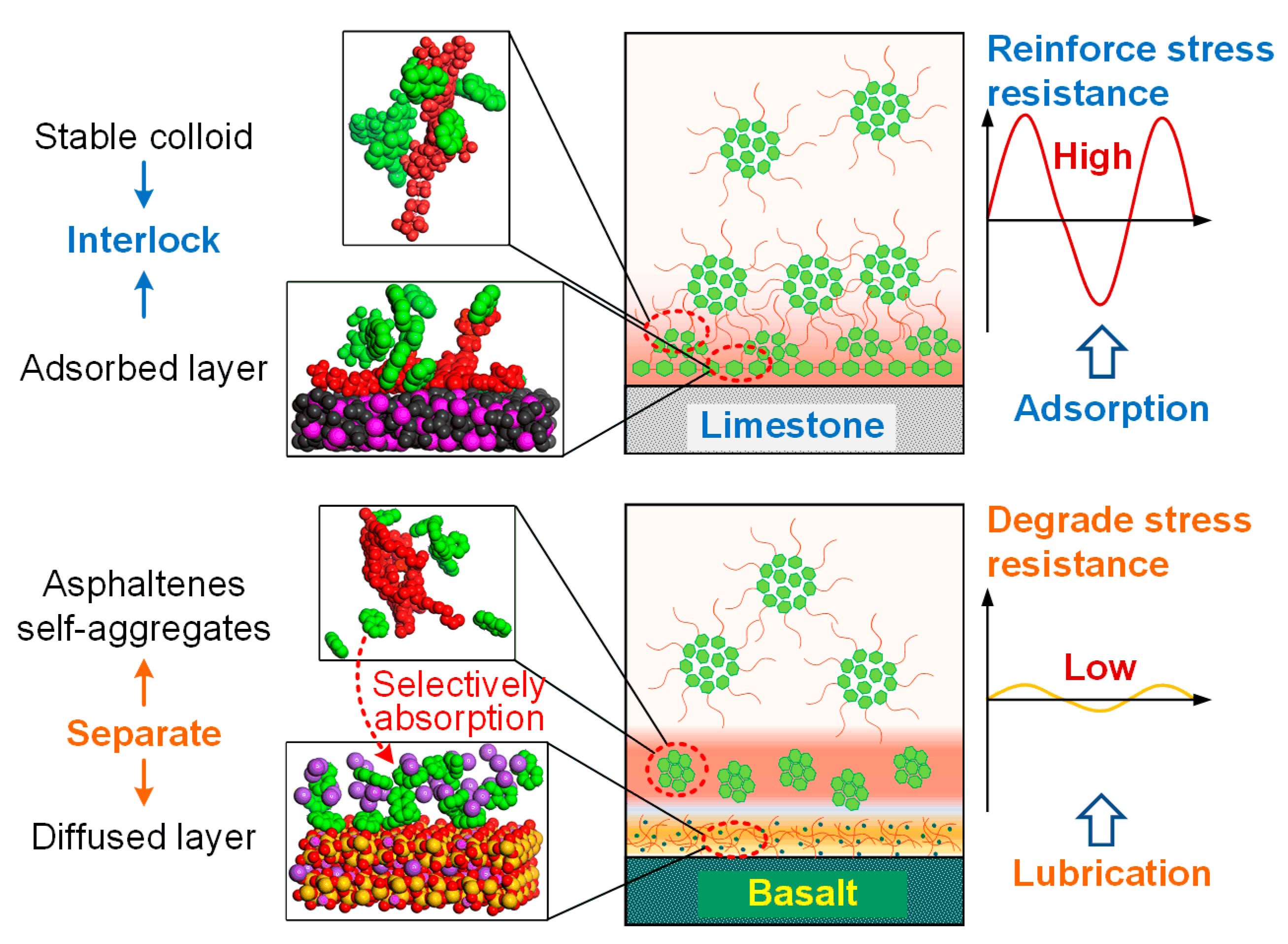
4. Conclusions and Summary
- The mineral aggregates increased the modulus of the mastics but decreased their phase angles, thereby strengthening the stiffness and toughness of the mastics compared with the mastic on the steel plate.
- The mastic on the limestone substrate had a higher modulus but a lower phase angle than that on the basalt substrate within the linear region at a temperature below 50 °C. The mastic on limestone had a higher modulus than that on basalt, and its ratio reached up to 1.18. This was attributed to the coexistence of the adsorbed bitumen layer and internal colloidal nanostructure in the bitumen–calcite system.
- However, the modulus of the mastic on the basalt exceeded that on the limestone, as the temperature was higher than 50 °C, and the maximum ratio reached 2.17. The stable bitumen nanostructure, containing dispersed metal ions in the bitumen–augite and bitumen–albite systems at high temperatures, was responsible for the high modulus on the basalt.
- The mastic on the limestone substrate had a better nonlinear rheological performance than that on the basalt substrate. The mastic in contact with the limestone had a higher failure strain and failure modulus than that in contact with the basalt, the ratios of which reached up to 1.60 and 1.32, respectively. Sufficient calcite in the limestone increased the stiffness of the mastic, whereas the augite and albite in the basalt impaired the mastic’s performance.
- The bitumen–calcite system had a higher shear stress than the bitumen–augite and bitumen–albite systems and presented a stronger resistance to the substrate shear deformation. The ratio of shear stress of the bitumen-calcite to the bitumen-albite reached up to 6.8. The non-bond energy of the bitumen-calcite was 14.15% higher than that of the bitumen-albite. The destruction and reconstruction of the bitumen colloidal structure in the bitumen–calcite system implies abundant work from external shearing, while small resin and diffused metal ions in the bitumen–augite and bitumen–albite systems form a lubrication layer between the asphaltene self-aggregates and mineral substrates and hardly constrain substrate shearing.
- The chemistry or mineral composition makes an essential contribution to the adhesion and interaction of the mastic–aggregate interface. Considering the influence of mineral aggregates on the rheology of mastic and bitumen is significant for the accurate characterization and prediction of the macroperformance of asphaltic paving material in the field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, D. Guidelines on the Use of Baghouse Fines; National Asphalt Paving Association: Greenbelt, MD, USA, 1987. [Google Scholar]
- Craus, J.; Ishai, I.; Sides, A. Some Physiochemical Aspects of the Effect and Role of the Filler in Bituminous Paving Mixtures. J. Assoc. Asph. Paving Technol. 1978, 47, 558–588. [Google Scholar]
- Buttlar, W.G.; Bozkurt, D.; Al-Khateeb, G.G.; Waldhoff, A.S. Understanding Asphalt Mastic Behavior through Micromechanics. Transp. Res. Rec. J. Transp. Res. Board 1999, 1681, 157–169. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, X.; Wang, H. Backcalculation of damage density of in-service asphalt pavements using artificial intelligence-based finite element model updating. Fatigue Fract. Eng. Mater. Struct. 2022, 45, 671–686. [Google Scholar] [CrossRef]
- Ma, X.; Quan, W.; Dong, Z.; Dong, Y.; Si, C. Dynamic response analysis of vehicle and asphalt pavement coupled system with the excitation of road surface unevenness. Appl. Math. Model. 2022, 104, 421–438. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, X.; Kou, Y.; Chen, J.; Shi, Q. Optimized design of asphalt concrete pavement containing phase change materials based on rutting performance. J. Clean. Prod. 2022, 380, 134787. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, X. An Accurate, Reproducible and Robust Model to Predict the Rutting of Asphalt Pavement: Neural Networks Coupled with Particle Swarm Optimization. IEEE Trans. Intell. Transp. Syst. 2022, 23, 22063–22072. [Google Scholar] [CrossRef]
- Delaporte, B.; DiBenedetto, H.; Chevrot, P.; Gauthier, G. Linear Viscoelastic Properties of Bituminous Materials: From Binders to Mastics. J. Assoc. Asph. Paving Technol. 2007, 76, 445–494. [Google Scholar]
- Liu, Z.; Dong, Z.; Zhou, T.; Cao, L. Water vapor diffusion models in asphalt mortar considering adsorption and capillary condensation. Constr. Build. Mater. 2021, 308, 125049. [Google Scholar] [CrossRef]
- Rigden, P.J. The use of fillers in bituminous road surfacings. A study of filler-binder systems in relation to filler characteristics. J. Soc. Chem. Ind. 1947, 66, 299–309. [Google Scholar] [CrossRef]
- Anderson, D.; Bahia, H.; Dongre, R. Rheological Properties of Mineral Filler-Asphalt Mastics and Its Importance to Pavement Performance. In Effects of Aggregates and Mineral Fillers on Asphalt Mixture Performance; ASTM International: West Conshohocken, PA, USA, 1992; pp. 131–153. [Google Scholar]
- Chen, J.-S. Rheological Properties of Asphalt-Mineral Filler Mastics. J. Mater. Concr. Struct. Pavements 1997, 36, 269–277. [Google Scholar] [CrossRef]
- Cooley, L.A.; Stroup-Gardinder, M.; Brown, E.R.; Hanson, D.I.; Fletcher, M.O. Characterization of Asphalt-Filler Mortars with Superpave Binder Tests. In Proceedings of the Journal of the Association of Asphalt Paving Technologists, Boston, MA, USA, 16–18 March 1998; Volume 67. [Google Scholar]
- Buttlar, W.G.; Roque, R. Evaluation of Empirical and Theoretical Models to Determine Asphalt Mixture Stiffnesses at Low Temperatures. In Proceedings of the 1996 Conference of the Association of Asphalt Paving Technologies, Baltimore, MD, USA, 18–20 March 1996; Volume 65, pp. 99–141. [Google Scholar]
- Shashidhar, N.; Romero, P. Factors Affecting the Stiffening Potential of Mineral Fillers. Transp. Res. Rec. 1998, 1638, 94–100. [Google Scholar] [CrossRef]
- Shashidhar, N.; Shenoy, A. On using micromechanical models to describe dynamic mechanical behavior of asphalt mastics. Mech. Mater. 2002, 34, 657–669. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Little, D.N. Linear Viscoelastic Analysis of Asphalt Mastics. J. Mater. Civ. Eng. 2004, 16, 122–132. [Google Scholar] [CrossRef]
- Druta, C. A Micromechanical Approach for Predicting the Complex Shear Modulus and Accumulated Shear Strain of Asphalt Mixtures from Binder and Mastics. Ph.D. Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2006. [Google Scholar] [CrossRef]
- Kumlai, S.; Jitsangiam, P.; Nikraz, H. Assessments of moisture damage resistance of asphalt concrete mixtures and asphalt mastic with various mineral fillers. Transp. Eng. 2022, 7, 100106. [Google Scholar] [CrossRef]
- Xu, O.; Xiang, S.; Yang, X.; Liu, Y. Estimation of the surface free energy and moisture susceptibility of asphalt mastic and aggregate system containing salt storage additive. Constr. Build. Mater. 2022, 318, 125814. [Google Scholar] [CrossRef]
- Scholz, T.V.; Brown, S.F. Rheological Characteristics of Bitumen in Contact with Mineral Aggregate (with Discussion). In Proceedings of the Journal of the Association of Asphalt Paving Technologists, Baltimore, MD, USA, 18–20 March 1996; pp. 357–384. [Google Scholar]
- Huang, S.-C.; Branthaver, J.F.; Robertson, R.E.; Kim, S.-S. Effect of Film Thickness on the Rheological Properties of Asphalts in Contact with Aggregate Surface. Transp. Res. Rec. 1998, 1638, 31–39. [Google Scholar] [CrossRef]
- Cho, D.-W.; Bahia, H.U. Effects of Aggregate Surface and Water on Rheology of Asphalt Films. Transp. Res. Rec. 2007, 1998, 10–17. [Google Scholar] [CrossRef]
- E, G.; Zhang, J.; Shen, Q.; Ji, P.; Wang, J.; Xiao, Y. Influence of Filler Type and Rheological Properties of Asphalt Mastic on the Asphalt Mastic–Aggregate Interaction. Materials 2023, 16, 574. [Google Scholar] [CrossRef]
- Huang, Q.; Qian, Z.; Hu, J.; Zheng, D.; Chen, L.; Zhang, M.; Yu, J. Investigation on the properties of aggregate-mastic interfacial transition zones (ITZs) in asphalt mixture containing recycled concrete aggregate. Constr. Build. Mater. 2021, 269, 121257. [Google Scholar] [CrossRef]
- Wang, P.; Zhai, F.; Dong, Z.-J.; Wang, L.-Z.; Liao, J.-P.; Li, G.-R. Micromorphology of Asphalt Modified by Polymer and Carbon Nanotubes through Molecular Dynamics Simulation and Experiments: Role of Strengthened Interfacial Interactions. Energy Fuels 2018, 32, 1179–1187. [Google Scholar] [CrossRef]
- Xu, M.; Yi, J.; Feng, D.; Huang, Y.; Wang, D. Analysis of Adhesive Characteristics of Asphalt Based on Atomic Force Microscopy and Molecular Dynamics Simulation. ACS Appl. Mater. Interfaces 2016, 8, 12393–12403. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Wang, P.; Gong, X. Nanostructure characterization of asphalt-aggregate interface through molecular dynamics simulation and atomic force microscopy. Fuel 2017, 189, 155–163. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, L.; Zhou, T.; Dong, Z. Multiscale Investigation of Moisture-Induced Structural Evolution in Asphalt–Aggregate Interfaces and Analysis of the Relevant Chemical Relationship Using Atomic Force Microscopy and Molecular Dynamics. Energy Fuels 2020, 34, 4006–4016. [Google Scholar] [CrossRef]
- Li, C.; Ma, F.; Fu, Z.; Dai, J.; Wen, Y.; Shi, K. Investigation of the solution effects on asphalt binder and mastic through molecular dynamics simulations. Constr. Build. Mater. 2022, 345, 128314. [Google Scholar] [CrossRef]
- MTPRC. Technical Specifications for Construction of Highway Asphalt Pavements (JTG F40-2004); China Communications Press: Beijing, China, 2004. [Google Scholar]
- Zhang, L.; Greenfield, M.L. Molecular Orientation in Model Asphalts Using Molecular Simulation. Energy Fuels 2007, 21, 1102–1111. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry for the Life Sciences, 2nd ed.; Oxford University Press: New York, NY, USA, 2015; ISBN 978-0-19-956428-6. [Google Scholar]
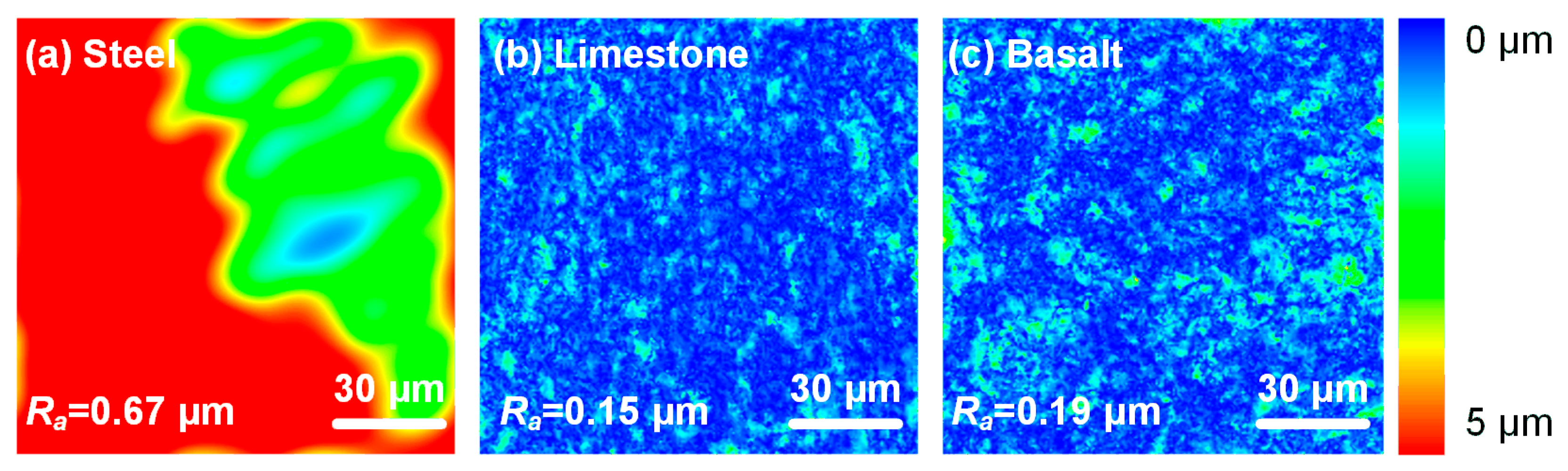



| Oxide Mass Concentration (%) | Primary Mineral Composition (%) | |||||
|---|---|---|---|---|---|---|
| Limestone | Basalt | Limestone | Basalt | |||
| SiO2 | 2.5 | 51.55 | Calcite (CaCO3) | 100 | Anorthite (CaAl2Si2O8) | 28.82 |
| CaO | 94.84 | 5.29 | ||||
| Al2O3 | 1.21 | 16.38 | ||||
| MgO | 0.42 | 9.77 | Augite (Mg6Fe6Si8O28) | 23.36 | ||
| Fe2O3 | 0.44 | 7.73 | ||||
| Na2O | 0.05 | 4.89 | Albite (NaAlSi3O8) | 47.83 | ||
| Other | 0.55 | 4.4 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, H.; Gong, X.; Cui, P.; Wei, H. Stiffening and Toughening of Asphalt Mastic Induced by Bitumen–Mineral Selective Molecular Adsorption and Nanostructural Reconstruction. Sustainability 2023, 15, 4398. https://doi.org/10.3390/su15054398
Liu Z, Wang H, Gong X, Cui P, Wei H. Stiffening and Toughening of Asphalt Mastic Induced by Bitumen–Mineral Selective Molecular Adsorption and Nanostructural Reconstruction. Sustainability. 2023; 15(5):4398. https://doi.org/10.3390/su15054398
Chicago/Turabian StyleLiu, Zhiyang, Haipeng Wang, Xiangbing Gong, Peng Cui, and Hongrui Wei. 2023. "Stiffening and Toughening of Asphalt Mastic Induced by Bitumen–Mineral Selective Molecular Adsorption and Nanostructural Reconstruction" Sustainability 15, no. 5: 4398. https://doi.org/10.3390/su15054398
APA StyleLiu, Z., Wang, H., Gong, X., Cui, P., & Wei, H. (2023). Stiffening and Toughening of Asphalt Mastic Induced by Bitumen–Mineral Selective Molecular Adsorption and Nanostructural Reconstruction. Sustainability, 15(5), 4398. https://doi.org/10.3390/su15054398







