Tetracycline Removal from Water by Adsorption on Hydrochar and Hydrochar-Derived Activated Carbon: Performance, Mechanism, and Cost Calculation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tetracycline Characterization
2.2. Adsorbent Preparation
2.3. Adsorption Kinetics Experiment
2.4. Adsorption Equilibrium Experiment
2.5. Influence of pH Solution on TC Adsorption
2.6. Adsorption Data Analysis
3. Results
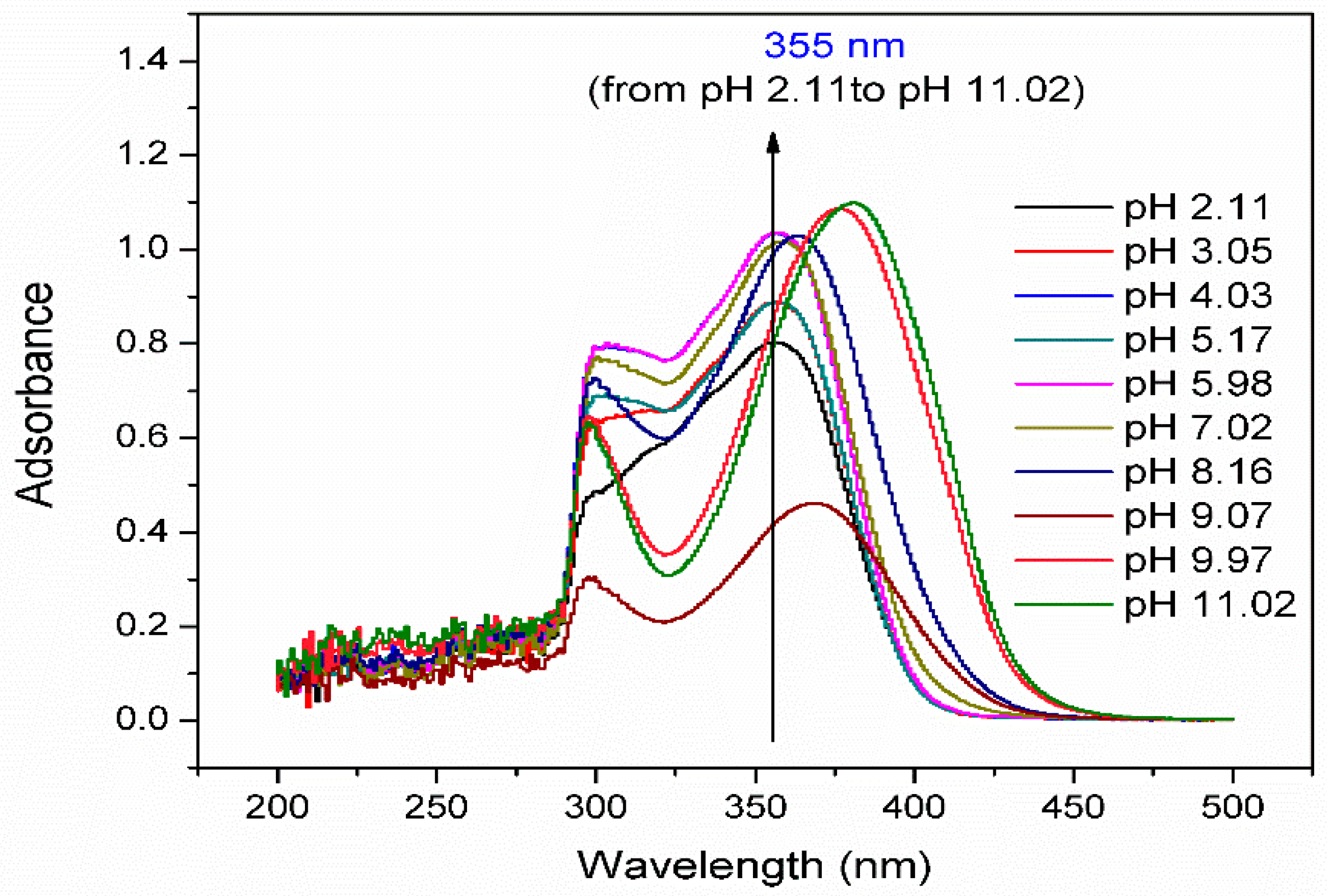
3.1. Effect of pH of the TC Solution on λmax Values
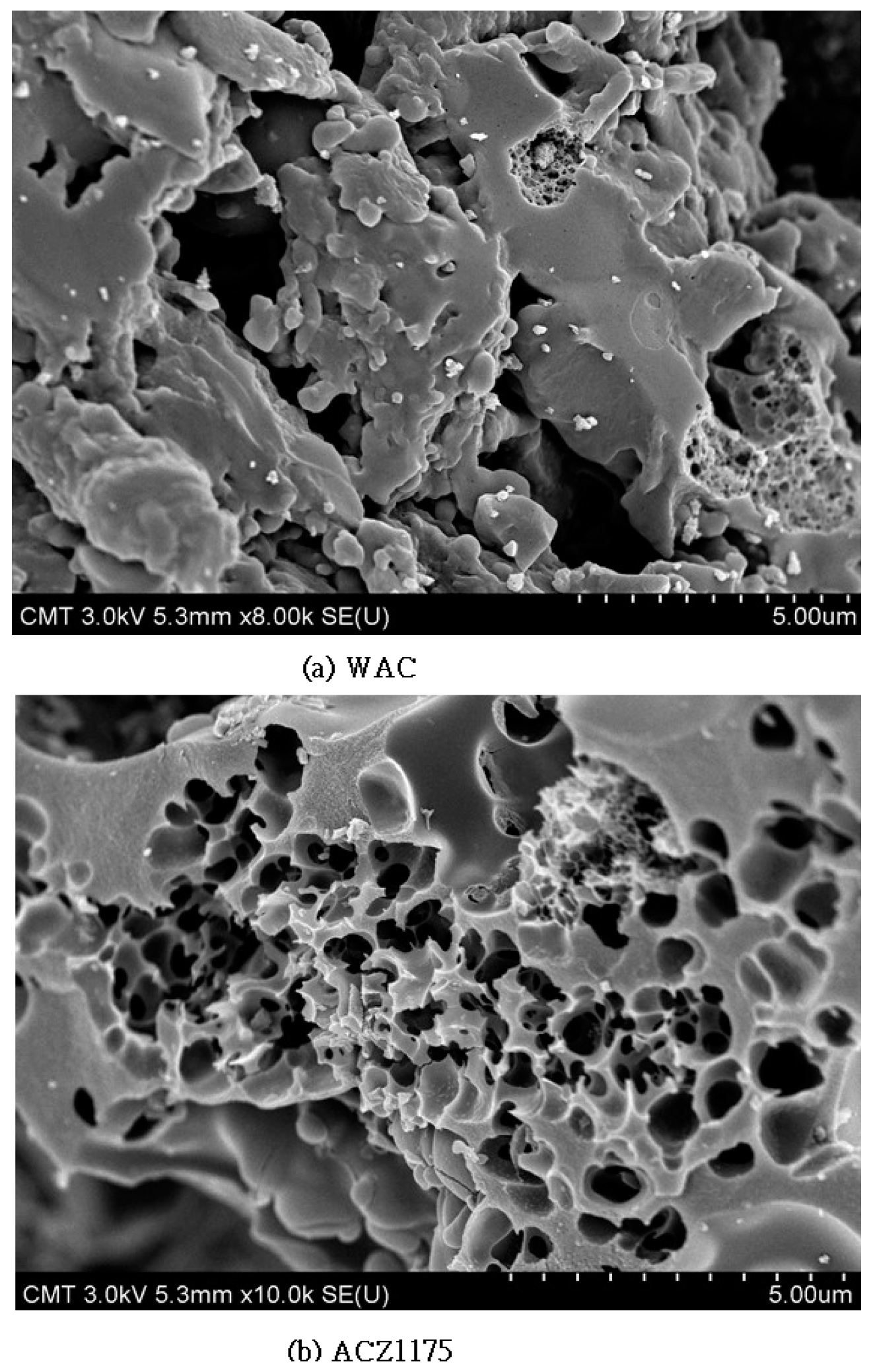
3.2. Morphological and Textural Properties of Synthetic Adsorbents
3.3. Characteristics of the Synthetic Adsorbents
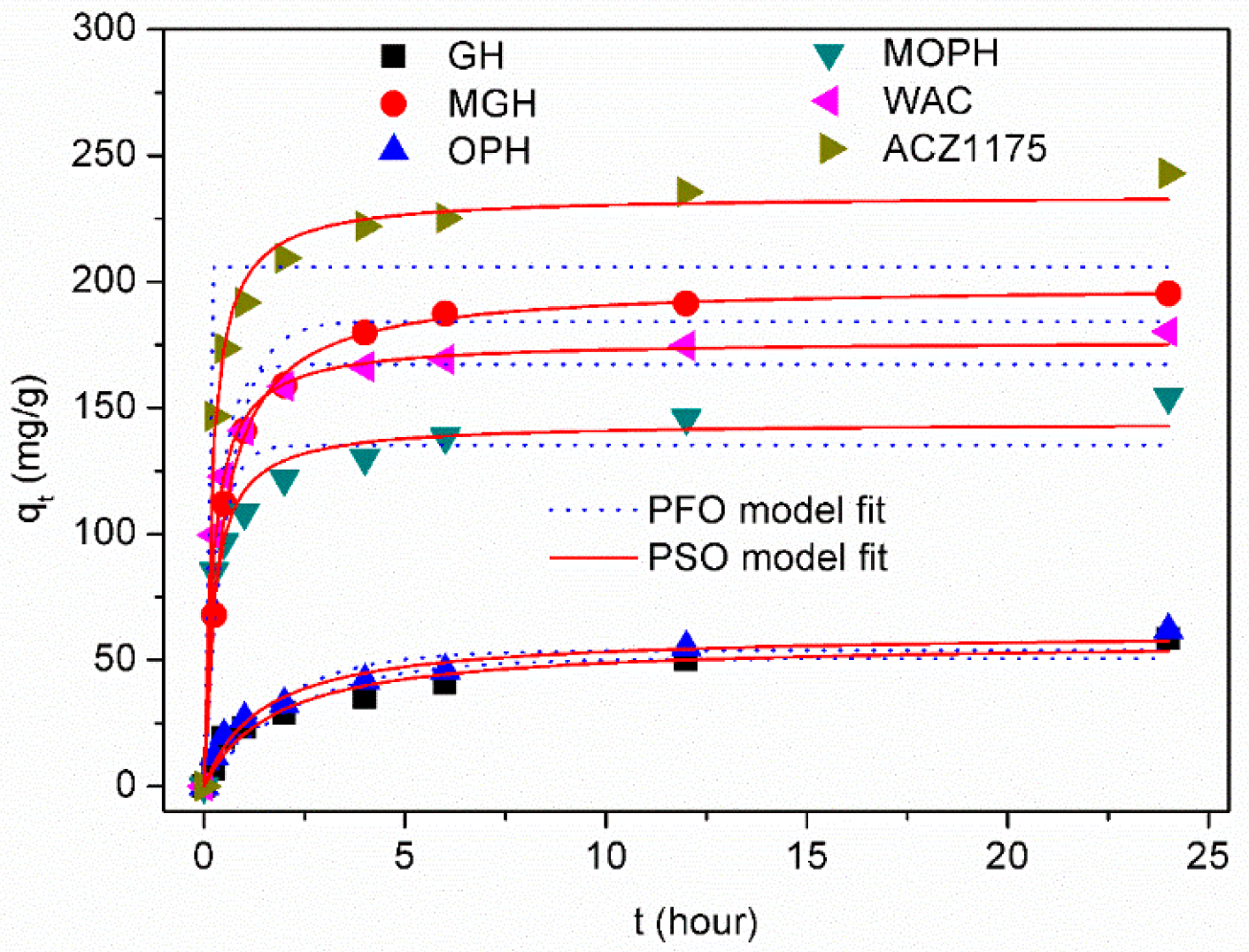
3.4. Adsorption Kinetic
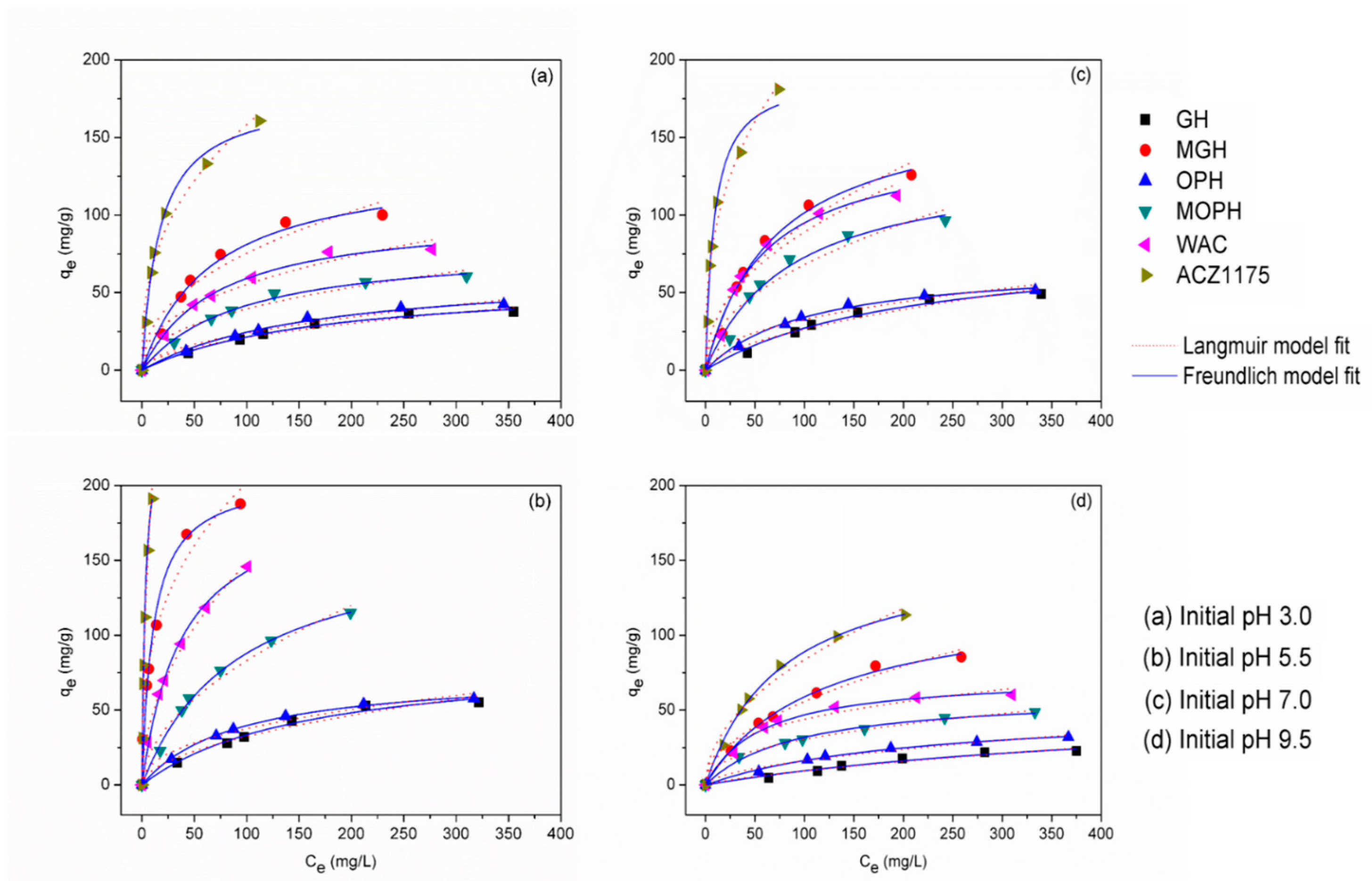
3.5. Adsorption Isotherms
3.6. Influence of Solution pH and Adsorption Mechanism
3.7. Estimation of Adsorbent Production Cost
3.8. Comparison of the Estimation of Adsorbent Production Cost
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| WAC | Activated carbon derived from teak-sawdust hydrochars (only high-temperature calcination without activation process) |
| ACZ1175 | AC derived from ZnCl2-activated teak-sawdust hydrochar (high-temperature, weight ratio of ZnCl2 to hydrochar = 1.75:1.0) |
| GH | Hydrochars derived from glucose solution through a hydrothermal process |
| OPH | Hydrochars derived from orange peel through a hydrothermal process |
| MGH | Hydrochars derived from glucose solution through a hydrothermal process, and then were activated by HNO3 |
| MOPH | Hydrochars derived from orange peel through a hydrothermal process, and then were activated by HNO3 |
References
- Chen, Z.; Zhang, W.; Wang, G.; Zhang, Y.; Gao, Y.; Boyd, S.A.; Teppen, B.J.; Tiedje, J.M.; Zhu, D.; Li, H. Bioavailability of soil-sorbed tetracycline to Escherichia coli under unsaturated conditions. Environ. Sci. Technol. 2017, 51, 6165–6173. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.-R.; Wang, Y.-Y.; Liu, W.-J.; Wang, Y.-K.; Jiang, H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem. Eng. J. 2014, 248, 168–174. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Wan, Y.; Yu, Q.; Xie, X. Effects of soil/solution ratios and cation types on adsorption and desorption of tetracycline in soils. Soil Sci. Soc. Am. J. 2010, 74, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Lian, F.; Song, Z.; Liu, Z.; Zhu, L.; Xing, B. Mechanistic understanding of tetracycline sorption on waste tire powder and its chars as affected by Cu2+ and pH. Environ. Pollut. 2013, 178, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Ma, Y.-L.; Yang, J.; Wang, L.-Q.; Lv, J.-M.; Ren, C.-J. Aqueous tetracycline degradation by H2O2 alone: Removal and transformation pathway. Chem. Eng. J. 2017, 307, 15–23. [Google Scholar] [CrossRef]
- Guan, R.; Yuan, X.; Wu, Z.; Jiang, L.; Zhang, J.; Li, Y.; Zeng, G.; Mo, D. Efficient degradation of tetracycline by heterogeneous cobalt oxide/cerium oxide composites mediated with persulfate. Sep. Purif. Technol. 2019, 212, 223–232. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Yan, M.; Hua, Y.; Zhu, F.; Gu, W.; Jiang, J.; Shen, H.; Shi, W. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 pn junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B Environ. 2017, 202, 518–527. [Google Scholar] [CrossRef]
- Leng, Y.; Bao, J.; Chang, G.; Zheng, H.; Li, X.; Du, J.; Snow, D.; Li, X. Biotransformation of tetracycline by a novel bacterial strain Stenotrophomonas maltophilia DT1. J. Hazard. Mater. 2016, 318, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254 nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M. Occurrence and removal of antibiotic resistance genes in municipal wastewater and rural domestic sewage treatment systems in eastern China. Environ. Int. 2013, 55, 9–14. [Google Scholar] [CrossRef]
- Liu, M.; Hou, L.-a.; Yu, S.; Xi, B.; Zhao, Y.; Xia, X. MCM-41 impregnated with A zeolite precursor: Synthesis, characterization and tetracycline antibiotics removal from aqueous solution. Chem. Eng. J. 2013, 223, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Ye, S.; Wu, H.; Xiao, R.; Zeng, G.; Liang, J.; Zhang, C.; Yu, J.; Fang, Y.; Song, B. Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution. Sci. Total Environ. 2019, 648, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Chen, W.-R.; Huang, C.-H. Adsorption and transformation of tetracycline antibiotics with aluminum oxide. Chemosphere 2010, 79, 779–785. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Kang, J.; Fan, M.; Qu, J. Removal of tetracycline from water by Fe-Mn binary oxide. J. Environ. Sci. 2012, 24, 242–247. [Google Scholar] [CrossRef]
- Parolo, M.E.; Avena, M.J.; Pettinari, G.R.; Baschini, M.T. Influence of Ca2+ on tetracycline adsorption on montmorillonite. J. Colloid Interface Sci. 2012, 368, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.A.; Islam, M.T.; Hernandez, C.; Castro, E.; Katla, S.K.; Kim, H.; Lin, Y.; Curry, M.L.; Gardea-Torresdey, J.; Noveron, J.C. Biomass conversion of saw dust to a functionalized carbonaceous materials for the removal of Tetracycline, Sulfamethoxazole and Bisphenol A from water. J. Environ. Chem. Eng. 2018, 6, 4329–4338. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Rodríguez, A.; Ovejero, G.; García, J. Comparative adsorption performance of ibuprofen and tetracycline from aqueous solution by carbonaceous materials. Chem. Eng. J. 2016, 283, 936–947. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Gómez-Pacheco, C.V.; Sánchez-Polo, M.; López-Peñalver, J.J.; Ocampo-Pérez, R. Tetracycline removal from water by adsorption/bioadsorption on activated carbons and sludge-derived adsorbents. J. Environ. Manag. 2013, 131, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sayğılı, H.; Güzel, F. Effective removal of tetracycline from aqueous solution using activated carbon prepared from tomato (Lycopersicon esculentum Mill.) industrial processing waste. Ecotoxicol. Environ. Saf. 2016, 131, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Wan, Y.; Zheng, S.; Zhu, D. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: Implication for the relative importance of black carbon to soil sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Zhou, C.; Luo, G.; Zhang, S.; Chen, J. A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline. Carbon 2014, 77, 627–636. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Tran, H.N.; Chao, H.-P.; Lin, C.-C. Effect of nitric acid oxidation on the surface of hydrochars to sorb methylene blue: An adsorption mechanism comparison. Adsorpt. Sci. Technol. 2019, 37, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Duy Nguyen, H.; Nguyen Tran, H.; Chao, H.-P.; Lin, C.-C. Activated carbons derived from teak sawdust-hydrochars for efficient removal of methylene blue, copper, and cadmium from aqueous solution. Water 2019, 11, 2581. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.; Huang, F.-C.; Lee, C.-K.; Chao, H.-P. Activated carbon derived from spherical hydrochar functionalized with triethylenetetramine: Synthesis, characterizations, and adsorption application. Green Process. Synth. 2017, 6, 565–576. [Google Scholar] [CrossRef]
- Corbett, J.F. Pseudo first-order kinetics. J. Chem. Educ. 1972, 49, 663. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Freundlich, H. Uber die adsorption in losungen. Z. Fur Phys. Chem.-Leipz. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Insight into adsorption mechanism of cationic dye onto agricultural residues-derived hydrochars: Negligible role of π-π interaction. Korean J. Chem. Eng. 2017, 34, 1708–1720. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Boehm, H.; Tereozki, B.; Schanz, K. Blocking of pores in porous carbons by chemisorption. Stud. Surf. Sci. Catal. 1982, 10, 395–401. [Google Scholar]
- Vo, A.T.; Nguyen, V.P.; Ouakouak, A.; Nieva, A.; Doma, B.T., Jr.; Tran, H.N.; Chao, H.-P. Efficient removal of Cr (VI) from water by biochar and activated carbon prepared through hydrothermal carbonization and pyrolysis: Adsorption-coupled reduction mechanism. Water 2019, 11, 1164. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, X.; Sun, K.; He, F.; Zhao, Y.; Lin, C. Competitive Sorption of Metsulfuron-Methyl and Tetracycline on Corn Straw Biochars. J. Environ. Qual. 2012, 41, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, P.; Liu, J.; Rehman, S.; Ahmed, Z.; Ruan, B.; Zhu, N. Batch interaction of emerging tetracycline contaminant with novel phosphoric acid activated corn straw porous carbon: Adsorption rate and nature of mechanism. Environ. Res. 2020, 181, 108899. [Google Scholar] [CrossRef]
- Banerjee, M.; Basu, R.K.; Das, S.K. Cr (VI) adsorption by a green adsorbent walnut shell: Adsorption studies, regeneration studies, scale-up design and economic feasibility. Process Saf. Environ. Prot. 2018, 116, 693–702. [Google Scholar] [CrossRef]
- Banerjee, S.; Barman, S.; Halder, G. Sorptive elucidation of rice husk ash derived synthetic zeolite towards deionization of coalmine waste water: A comparative study. Groundw. Sustain. Dev. 2017, 5, 137–151. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Chakraborty, P.; Show, S.; Banerjee, S.; Halder, G. Mechanistic insight into sorptive elimination of ibuprofen employing bi-directional activated biochar from sugarcane bagasse: Performance evaluation and cost estimation. J. Environ. Chem. Eng. 2018, 6, 5287–5300. [Google Scholar] [CrossRef]
- Yihunu, E.W.; Minale, M.; Abebe, S.; Limin, M. Preparation, characterization and cost analysis of activated biochar and hydrochar derived from agricultural waste: A comparative study. SN Appl. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Aikat, K.; Halder, G. Biosorptive uptake of ibuprofen by chemically modified Parthenium hysterophorus derived biochar: Equilibrium, kinetics, thermodynamics and modeling. Ecol. Eng. 2016, 92, 158–172. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Xu, T. Which is more competitive for production of organic acids, ion-exchange or electrodialysis with bipolar membranes? J. Membr. Sci. 2011, 374, 150–156. [Google Scholar] [CrossRef]

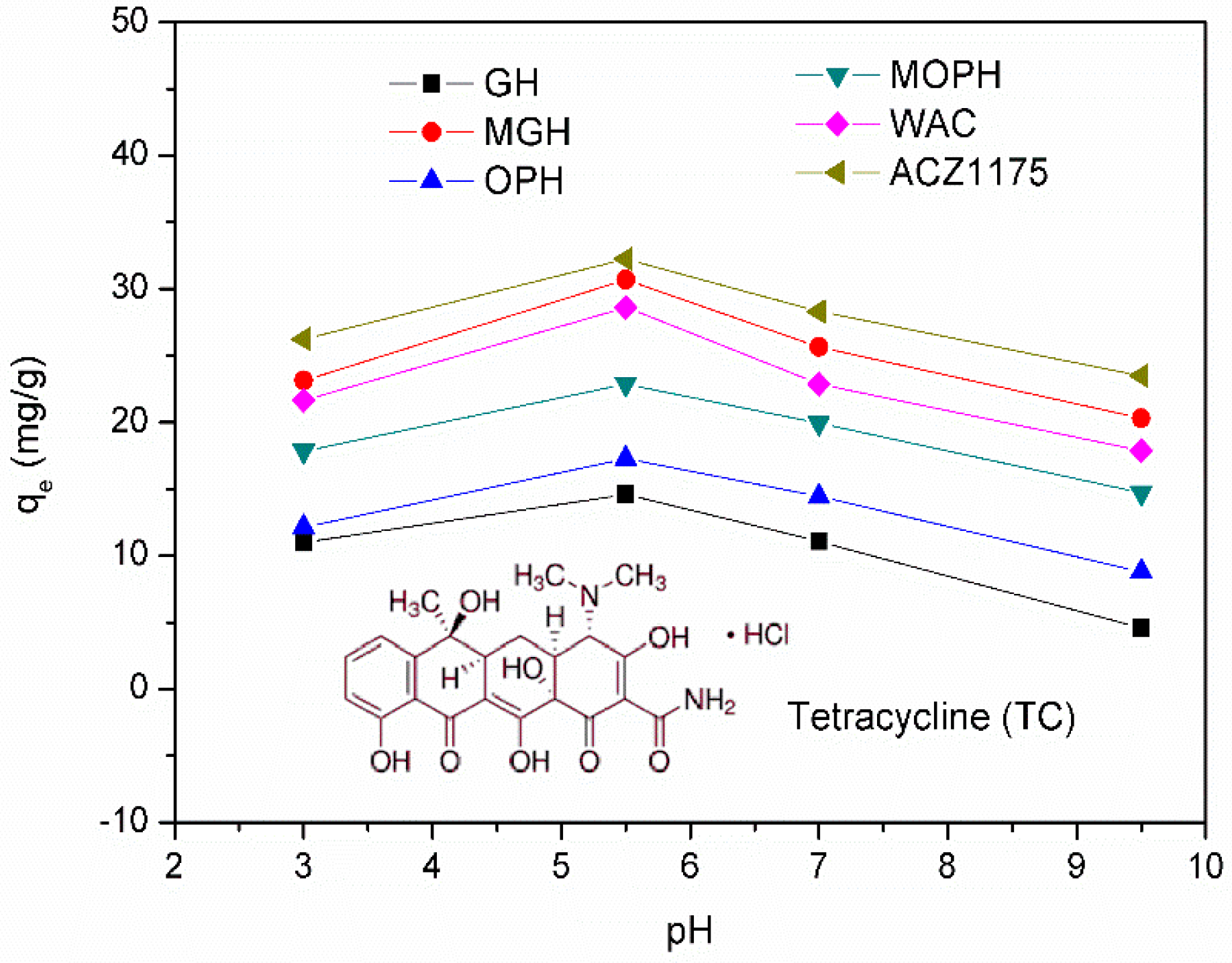
| Name of Samples | SBET (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| GH | 7.33 | 0.015 |
| MGH | 4.49 | 0.012 |
| OPH | 34.06 | 0.047 |
| MOPH | 20.25 | 0.042 |
| WAC | 792 | 0.340 |
| ACZ1175 | 1757 | 1.020 |
| Kinetic Models | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Sample | qt,exp | qt,cal = qt(1 − e−k1t) | qt,cal = (k2 qt2t)/(1 + k2 qt2t) | ||||
| qt,cal | k1 | R2 | qt,cal | k2 | R2 | ||
| GH | 57.42 | 50.64 | 0.43 | 0.899 | 57.49 | 0.009 | 0.957 |
| MGH | 194.83 | 184.19 | 0.64 | 0.977 | 198.78 | 0.030 | 0.997 |
| OPH | 67.96 | 53.89 | 0.54 | 0.918 | 60.95 | 0.011 | 0.974 |
| MOPH | 154.74 | 135.21 | 0.88 | 0.912 | 144.14 | 0.021 | 0.972 |
| WAC | 180.19 | 167.17 | 0.91 | 0.965 | 176.63 | 0.035 | 0.997 |
| ACZ1175 | 243.08 | 205.89 | 0.94 | 0.807 | 234.29 | 0.037 | 0.992 |
| Temperature/Samples | Langmuir Parameters | Freundlich Parameters | |||||
|---|---|---|---|---|---|---|---|
| qe = (QomaxKLCe)/(1 + KLCe) | qe = KFCe1/nF | ||||||
| Qe,exp (mg/g) | Qo(max) (mg/g) | KL (L/mg) | R2 | KF (mg/g) | nF | R2 | |
| Initial pH = 3 | |||||||
| GH | 42.40 | 58.46 | 0.005 | 0.990 | 1.95 | 0.51 | 0.967 |
| MGH | 137.25 | 134.98 | 0.015 | 0.984 | 9.92 | 0.44 | 0.939 |
| OPH | 57.63 | 65.09 | 0.006 | 0.991 | 2.23 | 0.52 | 0.969 |
| MOPH | 85.57 | 81.08 | 0.011 | 0.993 | 5.42 | 0.43 | 0.962 |
| WAC | 97.97 | 101.09 | 0.014 | 0.994 | 8.34 | 0.41 | 0.965 |
| ACZ1175 | 160.74 | 178.32 | 0.060 | 0.993 | 28.29 | 0.37 | 0.972 |
| Initial pH = 5.5 | |||||||
| GH | 58.37 | 75.47 | 0.006 | 0.987 | 2.94 | 0.52 | 0.963 |
| MGH | 195.22 | 207.11 | 0.094 | 0.975 | 43.35 | 0.33 | 0.983 |
| OPH | 67.96 | 85.79 | 0.010 | 0.998 | 5.38 | 0.42 | 0.974 |
| MOPH | 154.27 | 168.50 | 0.011 | 0.996 | 7.21 | 0.53 | 0.979 |
| WAC | 180.19 | 197.52 | 0.026 | 0.996 | 15.01 | 0.49 | 0.995 |
| ACZ1175 | 243.08 | 257.28 | 0.299 | 0.999 | 64.24 | 0.50 | 0.981 |
| Initial pH = 7 | |||||||
| GH | 55.02 | 69.42 | 0.005 | 0.984 | 1.97 | 0.56 | 0.956 |
| MGH | 157.98 | 172.77 | 0.014 | 0.985 | 10.56 | 0.48 | 0.943 |
| OPH | 65.54 | 81.68 | 0.009 | 0.994 | 4.47 | 0.43 | 0.964 |
| MOPH | 126.54 | 135.54 | 0.011 | 0.976 | 7.64 | 0.48 | 0.934 |
| WAC | 139.65 | 149.04 | 0.017 | 0.986 | 11.11 | 0.45 | 0.946 |
| ACZ1175 | 181.11 | 190.86 | 0.116 | 0.982 | 39.98 | 0.36 | 0.972 |
| Initial pH = 9.5 | |||||||
| GH | 40.65 | 51.21 | 0.002 | 0.968 | 0.33 | 0.73 | 0.951 |
| MGH | 115.51 | 126.23 | 0.010 | 0.995 | 5.31 | 0.51 | 0.979 |
| OPH | 51.97 | 55.66 | 0.005 | 0.995 | 1.27 | 0.55 | 0.978 |
| MOPH | 58.69 | 61.11 | 0.011 | 0.993 | 4.66 | 0.41 | 0.997 |
| WAC | 70.21 | 73.20 | 0.013 | 0.993 | 9.19 | 0.34 | 0.956 |
| ACZ1175 | 123.62 | 157.94 | 0.017 | 0.998 | 8.51 | 0.49 | 0.979 |
| Property Name | Chemical Formula | Molecular Weight | Molecular Structure | pKa1 | pKa2 | pKa3 |
|---|---|---|---|---|---|---|
| Tetracycline (TC) | C22H25ClN2O8 | 480.9 g/mol | 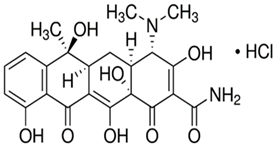 | 3.30 | 7.68 | 9.68 |
| (A) Cost Estimation of Modified Hydrochar | |||
|---|---|---|---|
| Particulars | Sub-Sections | Cost Break-Up | Total Cost (USD) |
| Processing of raw material | Collection of raw material | Orange peel was collected free of cost from the local market | 0.0 |
| Hand sorting and washing | DI water gained from laboratory set-up | 0.0 | |
| Drying cost | It was done under the sun | 0.0 | |
| Preparation of oxidized hydrochar | Hydrothermal carbonization cost | Hour × unit × cost per unit = 24 × 0.5 × 0.16 | 1.92 |
| Acid reflux with HNO3 | Unit consumed × cost per unit = 13.9 mL × 78.7/500 mL | 2.18 | |
| Cost of drying | Hour × unit × cost per unit = 6 × 0.3 × 0.16 | 0.28 | |
| Net cost | 4.38 | ||
| 10% to overhead charge | 0.43 | ||
| Total cost | 4.71 | ||
| (B) Cost Estimation of Hydrochar-Derived Activated Carbon | |||
| Processing of raw material | Collection of raw material | The teak (T. grandis) sawdust was obtained from a furniture factory | 0.0 |
| Washing cost | DI water gained from laboratory set-up | 0.0 | |
| Drying cost | It was done under the sun | 0.0 | |
| Preparation of AC | Hydrothermal carbonization cost | Hour × unit × cost per unit = 24 × 0.5 × 0.16 | 1.92 |
| Impregnation with ZnCl2 | Unit consumed × cost per unit = 1.75 g × 94/500 g | 0.32 | |
| Pyrolysis at 800 °C | Hour × unit × cost per unit = 4 × 1 × 0.16 | 0.64 | |
| Washing cost | DI water gained from laboratory set-up | 0.0 | |
| Cost of drying | Hour × unit × cost per unit = 6 × 0.3 × 0.16 | 0.28 | |
| Net cost | 3.16 | ||
| 10% to overhead charge | 0.31 | ||
| Total cost | 3.47 | ||
| Raw Materials | Pre–Post Treatment | Post-Treatment | SBET Surface Area (m2/g) | Cost/kg (USD) | References |
|---|---|---|---|---|---|
| Sugarcane bagasse | Pyrolysis | H3PO4 | 557 | 3.81 | [47] |
| Super-heated steam | 3.49 | ||||
| Orange peel | HTC | HNO3 | 20 | 4.71 | This study |
| Teak sawdust | HTC | ZnCl2 | 1757 | 3.47 | This study |
| Teff straw | Pyrolysis | H3PO4 | 627 | 3.73 | [48] |
| HTC | H3PO4 | 43 | 2.93 | ||
| Rice husk | Pyrolysis | Zeolite Z-RHA | 76 | 5.42 | [45] |
| Parthenium hysterophorus | Pyrolysis | NaOH | 308 | 2.88 | [49] |
| Ion-exchange resins | - | - | - | 150 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoc, D.M.; Hieu, N.C.; Trung, N.H.; Chien, H.H.; Thi, N.Q.; Hai, N.D.; Chao, H.-P. Tetracycline Removal from Water by Adsorption on Hydrochar and Hydrochar-Derived Activated Carbon: Performance, Mechanism, and Cost Calculation. Sustainability 2023, 15, 4412. https://doi.org/10.3390/su15054412
Ngoc DM, Hieu NC, Trung NH, Chien HH, Thi NQ, Hai ND, Chao H-P. Tetracycline Removal from Water by Adsorption on Hydrochar and Hydrochar-Derived Activated Carbon: Performance, Mechanism, and Cost Calculation. Sustainability. 2023; 15(5):4412. https://doi.org/10.3390/su15054412
Chicago/Turabian StyleNgoc, Duong Minh, Nguyen Chi Hieu, Nguyen Huy Trung, Hoang Huu Chien, Nguyen Quang Thi, Nguyen Duy Hai, and Huan-Ping Chao. 2023. "Tetracycline Removal from Water by Adsorption on Hydrochar and Hydrochar-Derived Activated Carbon: Performance, Mechanism, and Cost Calculation" Sustainability 15, no. 5: 4412. https://doi.org/10.3390/su15054412






