Optimized Artificial Intelligent Model to Boost the Efficiency of Saline Wastewater Treatment Based on Hunger Games Search Algorithm and ANFIS
Abstract
1. Introduction
- Creating an precise ANFIS model of the electrochemical oxidation process.
- For the first time, a hunger games search algorithm is used to define the best values of reaction time, pH, salt concentration, and DC applied voltage
- Boosting the COD and TOC removal efficiencies simultaneously
- Demonstration of the proposed methodology
2. Materials, Methods, and Dataset
3. Methodology
3.1. ANFIS-Modelling
| (Output Layer) | |
| Evaluating | (Defuzzification Layer) |
| (N Layer) | |
| (π Layer) | |
| are the MF values of the two inputs | (Fuzzification Layer) |
3.2. Hunger Games Search
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, E.T.; Olabi, A.G.; Elsaid, K.; Al Radi, M.; Alqadi, R.; Ali Abdelkareem, M. Recent progress in renewable energy based-desalination in the Middle East and North Africa MENA region. J. Adv. Res. 2022. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, B.; Jiang, L.; Zhang, X.-X.; Ren, H.-Q.; Li, M. Comparative analysis of toxicity reduction of wastewater in twelve industrial park wastewater treatment plants based on battery of toxicity assays. Sci. Rep. 2019, 9, 3751. [Google Scholar] [CrossRef] [PubMed]
- Heydari Orojlou, S.; Rastegarzadeh, S.; Zargar, B. Experimental and modeling analyses of COD removal from industrial wastewater using the TiO2–chitosan nanocomposites. Sci. Rep. 2022, 12, 11088. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.S.; Kalash, K.R.; Ahmed, A.N.; Albayati, T.M. Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci. Rep. 2022, 12, 16782. [Google Scholar] [CrossRef] [PubMed]
- Charazińska, S.; Burszta-Adamiak, E.; Lochyński, P. The efficiency of removing heavy metal ions from industrial electropolishing wastewater using natural materials. Sci. Rep. 2022, 12, 17766. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, X.; Zhao, H.; Zhang, W.; Liu, G.; Zhang, X. Isolation of two salt-tolerant strains from activated sludge and its COD degradation characteristics from saline organic wastewater. Sci. Rep. 2020, 10, 18421. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Zhang, H.-Y.; Ding, J.-R.; Peng, Y.-Z. The effects of salinity on nitrification using halophilic nitrifiers in a Sequencing Batch Reactor treating hypersaline wastewater. Sci. Rep. 2016, 6, 24825. [Google Scholar] [CrossRef]
- Chandnani, G.; Gandhi, P.; Kanpariya, D.; Parikh, D.; Shah, M. A comprehensive analysis of contaminated groundwater: Special emphasis on nature-ecosystem and socio-economic impacts. Groundw. Sustain. Dev. 2022, 19, 100813. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Hannington, T.; Awuchi, C.G.; Igwe, V.S.; Amagwula, I.O. Industrial waste management, treatment, and health issues: Wastewater, solid, and electronic wastes. Eur. Acad. Res. 2020, 8, 1081–1119. [Google Scholar]
- Aheto, J.H.; Huang, X.; Xiaoyu, T.; Bonah, E.; Ren, Y.; Alenyorege, E.A.; Chunxia, D. Investigation into crystal size effect on sodium chloride uptake and water activity of pork meat using hyperspectral imaging. J. Food Process. Preserv. 2019, 43, e14197. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Panda, R.C.; Kumar, V. Trends and advancements in sustainable leather processing: Future directions and challenges—A review. J. Environ. Chem. Eng. 2020, 8, 104379. [Google Scholar]
- Dowlath, M.J.H.; Karuppannan, S.K.; Rajan, P.; Khalith, S.M.; Rajadesingu, S.; Arunachalam, K.D. Application of advanced technologies in managing wastes produced by leather industries—An approach toward zero waste technology. In Concepts of Advanced Zero Waste Tools; Elsevier: Amsterdam, The Netherlands, 2021; pp. 143–179. [Google Scholar]
- Venkatachalam, M.; Rathinam, A.; Rao, J.; Krishnan, C. Bioconversion of animal hair waste using salt-and sulphide-tolerant Bacillus sp. KLP1 and depilation using keratinase. Int. J. Environ. Sci. Technol. 2022, 19, 6389–6398. [Google Scholar] [CrossRef]
- Das, B.; Deka, S. A cost-effective and environmentally sustainable process for phycoremediation of oil field formation water for its safe disposal and reuse. Sci. Rep. 2019, 9, 15232. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.S.; Abu-Dieyeh, M.H.; Al Naemi, F.A.; Ahmed, T.; Al-Ghouti, M.A. Environmental impact of utilization of “produced water” from oil and gas operations in turfgrass systems. Sci. Rep. 2020, 10, 15051. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xu, X.; Lv, Z.; Mao, H.; Wu, J. Environmental impact assessment of wastewater discharge with multi-pollutants from iron and steel industry. J. Environ. Manag. 2019, 245, 210–215. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Wilberforce, T.; Sayed, E.T.; Abdelkareem, M.A.; Elsaid, K.; Olabi, A.G. Value added products from wastewater using bioelectrochemical systems: Current trends and perspectives. J. Water Process Eng. 2021, 39, 101737. [Google Scholar] [CrossRef]
- Sayed, E.T.; Alawadhi, H.; Elsaid, K.; Olabi, A.G.; Adel Almakrani, M.; Bin Tamim, S.T.; Alafranji, G.H.M.; Abdelkareem, M.A. A Carbon-Cloth Anode Electroplated with Iron Nanostructure for Microbial Fuel Cell Operated with Real Wastewater. Sustainability 2020, 12, 6538. [Google Scholar] [CrossRef]
- Sayed, E.T.; Alawadhi, H.; Olabi, A.G.; Jamal, A.; Almahdi, M.S.; Khalid, J.; Abdelkareem, M.A. Electrophoretic deposition of graphene oxide on carbon brush as bioanode for microbial fuel cell operated with real wastewater. Int. J. Hydrog. Energy 2021, 46, 5975–5983. [Google Scholar] [CrossRef]
- Rezk, H.; Olabi, A.G.; Abdelkareem, M.A.; Maghrabie, H.M.; Sayed, E.T. Fuzzy Modelling and Optimization of Yeast-MFC for Simultaneous Wastewater Treatment and Electrical Energy Production. Sustainability 2023, 15, 1878. [Google Scholar] [CrossRef]
- Sayed, E.T.; Olabi, A.G.; Shehata, N.; Al Radi, M.; Majdy Muhaisen, O.; Rodriguez, C.; Ali Atieh, M.; Abdelkareem, M.A. Application of bio-based electrodes in emerging capacitive deionization technology for desalination and wastewater treatment. Ain Shams Eng. J. 2022, 102030. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Obaid, M.; Sayed, E.T.; Abdelkareem, M.A.; Park, M.; Liu, Y.; Kim, H.-Y.; Barakat, N.A.M. Graphite Sheets as High-Performance Low-Cost Anodes for Microbial Fuel Cells Using Real Food Wastewater. Chem. Eng. Technol. 2017, 40, 2243–2250. [Google Scholar] [CrossRef]
- Phung, L.D.; Pham, D.V.; Sasaki, Y.; Masuda, S.; Takakai, F.; Kaku, N.; Watanabe, T. Continuous sub-irrigation with treated municipal wastewater for protein-rich rice production with reduced emissions of CH4 and N2O. Sci. Rep. 2020, 10, 5485. [Google Scholar] [CrossRef] [PubMed]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Zhu, T.-T.; Su, Z.-X.; Lai, W.-X.; Zhang, Y.-B.; Liu, Y.-W. Insights into the fate and removal of antibiotics and antibiotic resistance genes using biological wastewater treatment technology. Sci. Total Environ. 2021, 776, 145906. [Google Scholar] [CrossRef]
- Obaideen, K.; Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Olabi, A.G. Biogas role in achievement of the sustainable development goals: Evaluation, Challenges, and Guidelines. J. Taiwan Inst. Chem. Eng. 2022, 131, 104207. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Huang, W.; Yang, Y.; Ji, M.; Nghiem, L.D.; Trinh, Q.T.; Tran, N.H. Insights into biofilm carriers for biological wastewater treatment processes: Current state-of-the-art, challenges, and opportunities. Bioresour. Technol. 2019, 288, 121619. [Google Scholar] [CrossRef]
- Aziz, A.; Basheer, F.; Sengar, A.; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current advances in membrane technologies for saline wastewater treatment: A comprehensive review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Goh, P.; Wong, K.; Ismail, A. Membrane technology: A versatile tool for saline wastewater treatment and resource recovery. Desalination 2022, 521, 115377. [Google Scholar] [CrossRef]
- Alam, R.; Khan, S.U.; Usman, M.; Asif, M.; Farooqi, I.H. A critical review on treatment of saline wastewater with emphasis on electrochemical based approaches. Process Saf. Environ. Prot. 2022, 158, 625–643. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Tang, J.; Wang, X.; Wu, Z. A pilot-scale forward osmosis membrane system for concentrating low-strength municipal wastewater: Performance and implications. Sci. Rep. 2016, 6, 21653. [Google Scholar] [CrossRef]
- Hellal, M.S.; Hemdan, B.A.; Youssef, M.; El-Taweel, G.E.; Abou Taleb, E.M. Novel electro-oxidation unit for electro-disinfection of E. coli and some waterborne pathogens during wastewater treatment: Batch and continuous experiments. Sci. Rep. 2022, 12, 16417. [Google Scholar] [CrossRef]
- Darvishmotevalli, M.; Zarei, A.; Moradnia, M.; Noorisepehr, M.; Mohammadi, H. Optimization of saline wastewater treatment using electrochemical oxidation process: Prediction by RSM method. MethodsX 2019, 6, 1101–1113. [Google Scholar] [CrossRef]
- Nassef, A.M.; Fathy, A.; Sayed, E.T.; Abdelkareem, M.A.; Rezk, H.; Tanveer, W.H.; Olabi, A.G. Maximizing SOFC performance through optimal parameters identification by modern optimization algorithms. Renew. Energy 2019, 138, 458–464. [Google Scholar] [CrossRef]
- Olabi, A.G.; Haridy, S.; Sayed, E.T.; Radi, M.A.; Alami, A.H.; Zwayyed, F.; Salameh, T.; Abdelkareem, M.A. Implementation of Artificial Intelligence in Modeling and Control of Heat Pipes: A Review. Energies 2023, 16, 760. [Google Scholar] [CrossRef]
- Sayed, E.T.; Rezk, H.; Abdelkareem, M.A.; Olabi, A.G. Artificial neural network based modelling and optimization of microalgae microbial fuel cell. Int. J. Hydrog. Energy 2023. [Google Scholar] [CrossRef]
- Nassef, A.M.; Sayed, E.T.; Rezk, H.; Abdelkareem, M.A.; Rodriguez, C.; Olabi, A.G. Fuzzy-modeling with Particle Swarm Optimization for enhancing the production of biodiesel from Microalga. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2094–2103. [Google Scholar] [CrossRef]
- Meena, M.; Shubham, S.; Paritosh, K.; Pareek, N.; Vivekanand, V. Production of biofuels from biomass: Predicting the energy employing artificial intelligence modelling. Bioresour. Technol. 2021, 340, 125642. [Google Scholar] [CrossRef] [PubMed]
- Rezk, H.; Nassef, A.M.; Inayat, A.; Sayed, E.T.; Shahbaz, M.; Olabi, A.G. Improving the environmental impact of palm kernel shell through maximizing its production of hydrogen and syngas using advanced artificial intelligence. Sci. Total Environ. 2019, 658, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.F.; Rodrigues, A.E.; Loureiro, J.M.; Ribeiro, A.M.; Nogueira, I.B.R. Artificial Intelligence-oriented economic non-linear model predictive control applied to a pressure swing adsorption unit: Syngas purification as a case study. Sep. Purif. Technol. 2021, 276, 119333. [Google Scholar] [CrossRef]
- Wen, H.-T.; Lu, J.-H.; Phuc, M.-X. Applying Artificial Intelligence to Predict the Composition of Syngas Using Rice Husks: A Comparison of Artificial Neural Networks and Gradient Boosting Regression. Energies 2021, 14, 2932. [Google Scholar] [CrossRef]
- Rezk, H.; Olabi, A.G.; Abdelkareem, M.A.; Alami, A.H.; Sayed, E.T. Optimal Parameter Determination of Membrane Bioreactor to Boost Biohydrogen Production-Based Integration of ANFIS Modeling and Honey Badger Algorithm. Sustainability 2023, 15, 1589. [Google Scholar] [CrossRef]
- Salameh, T.; Sayed, E.T.; Olabi, A.G.; Hdaib, I.I.; Allan, Y.; Alkasrawi, M.; Abdelkareem, M.A. Adaptive Network Fuzzy Inference System and Particle Swarm Optimization of Biohydrogen Production Process. Fermentation 2022, 8, 483. [Google Scholar] [CrossRef]
- Rezk, H.; Ferahtia, S.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A.G. Robust parameter identification strategy of solid oxide fuel cells using bald eagle search optimization algorithm. Int. J. Energy Res. 2022, 46, 10535–10552. [Google Scholar] [CrossRef]
- Chen, X.; Yi, Z.; Zhou, Y.; Guo, P.; Farkoush, S.G.; Niroumandi, H. Artificial neural network modeling and optimization of the Solid Oxide Fuel Cell parameters using grey wolf optimizer. Energy Rep. 2021, 7, 3449–3459. [Google Scholar] [CrossRef]
- Olabi, A.G.; Rezk, H.; Sayed, E.T.; Ghoniem, R.M.; Abdelkareem, M.A. Boosting carbon dioxide adsorption capacity applying Jellyfish optimization and ANFIS-based modelling. Ain Shams Eng. J. 2023, 14, 101931. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Lee, K.Y. The mutual benefits of renewables and carbon capture: Achieved by an artificial intelligent scheduling strategy. Energy Convers. Manag. 2021, 233, 113856. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, T.; Qiao, Z.; Sun, P.; Hao, J.; Yang, Y. Application of artificial intelligence to wastewater treatment: A bibliometric analysis and systematic review of technology, economy, management, and wastewater reuse. Process Saf. Environ. Prot. 2020, 133, 169–182. [Google Scholar] [CrossRef]
- Federation, W.E.; Association, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005; p. 21. [Google Scholar]
- Rezk, H.; Mohammed, R.H.; Rashad, E.; Nassef, A.M. ANFIS-based accurate modeling of silica gel adsorption cooling cycle. Sustain. Energy Technol. Assess. 2022, 50, 101793. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Heidari, A.A.; Gandomi, A.H. Hunger games search: Visions, conception, implementation, deep analysis, perspectives, and towards performance shifts. Expert Syst. Appl. 2021, 177, 114864. [Google Scholar] [CrossRef]
- Un, U.T.; Altay, U.; Koparal, A.S.; Ogutveren, U.B. Complete treatment of olive mill wastewaters by electrooxidation. Chem. Eng. J. 2008, 139, 445–452. [Google Scholar] [CrossRef]
- Bhatti, M.S.; Reddy, A.S.; Thukral, A.K. Electrocoagulation removal of Cr(VI) from simulated wastewater using response surface methodology. J. Hazard. Mater. 2009, 172, 839–846. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Olive mill wastewater treatment by anodic oxidation with parallel plate electrodes. Water Res. 2006, 40, 1179–1184. [Google Scholar] [CrossRef]



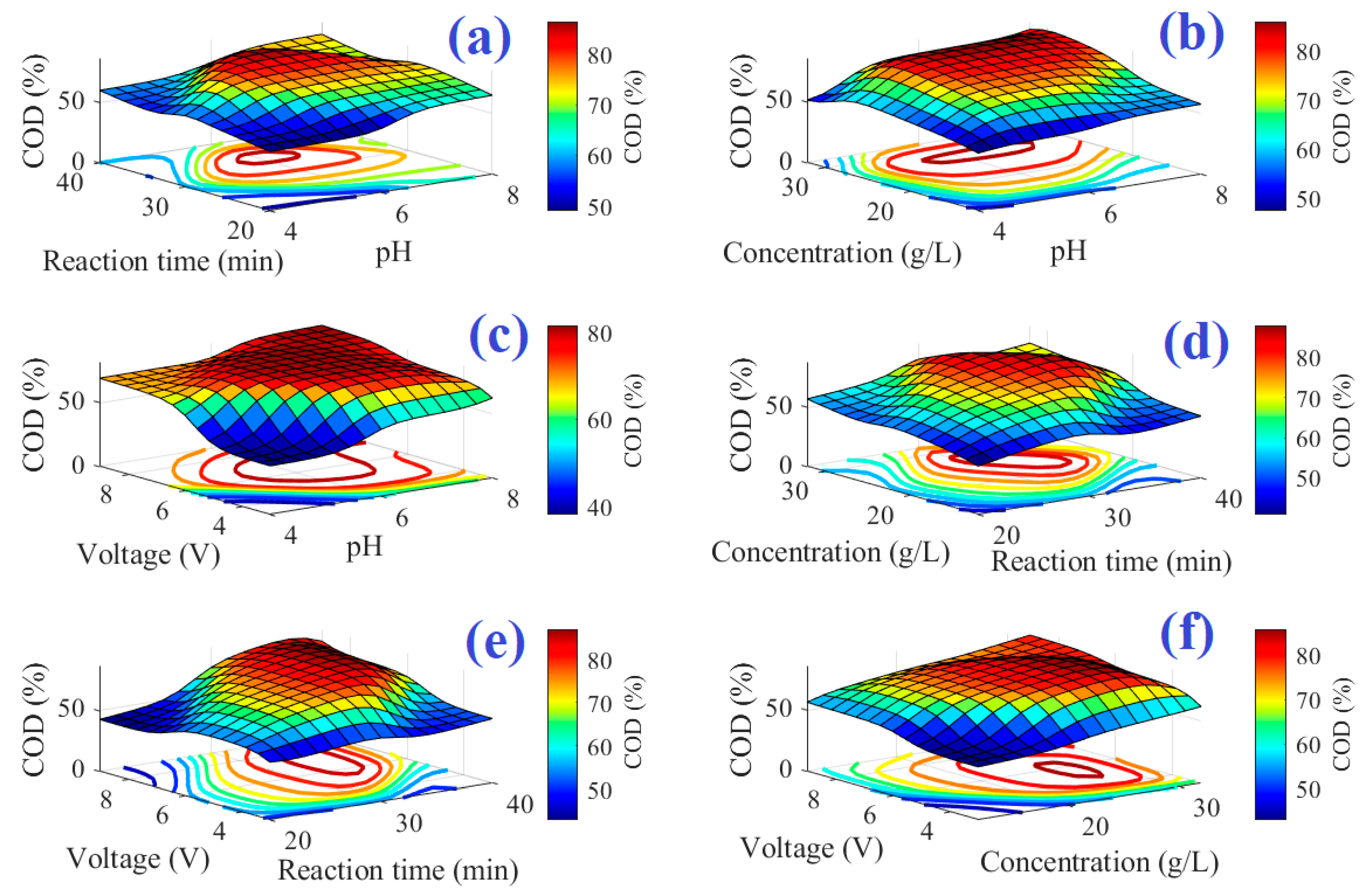
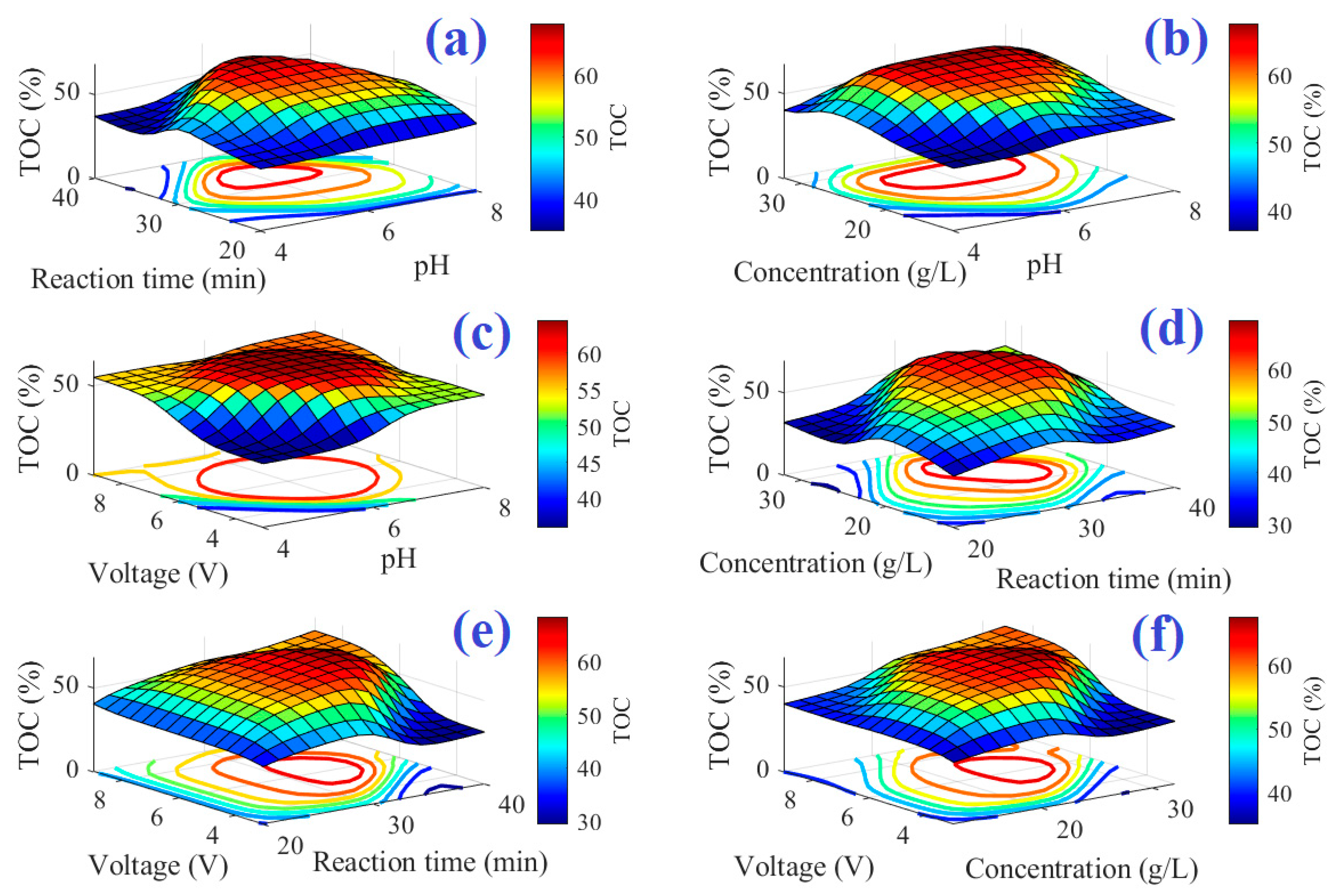
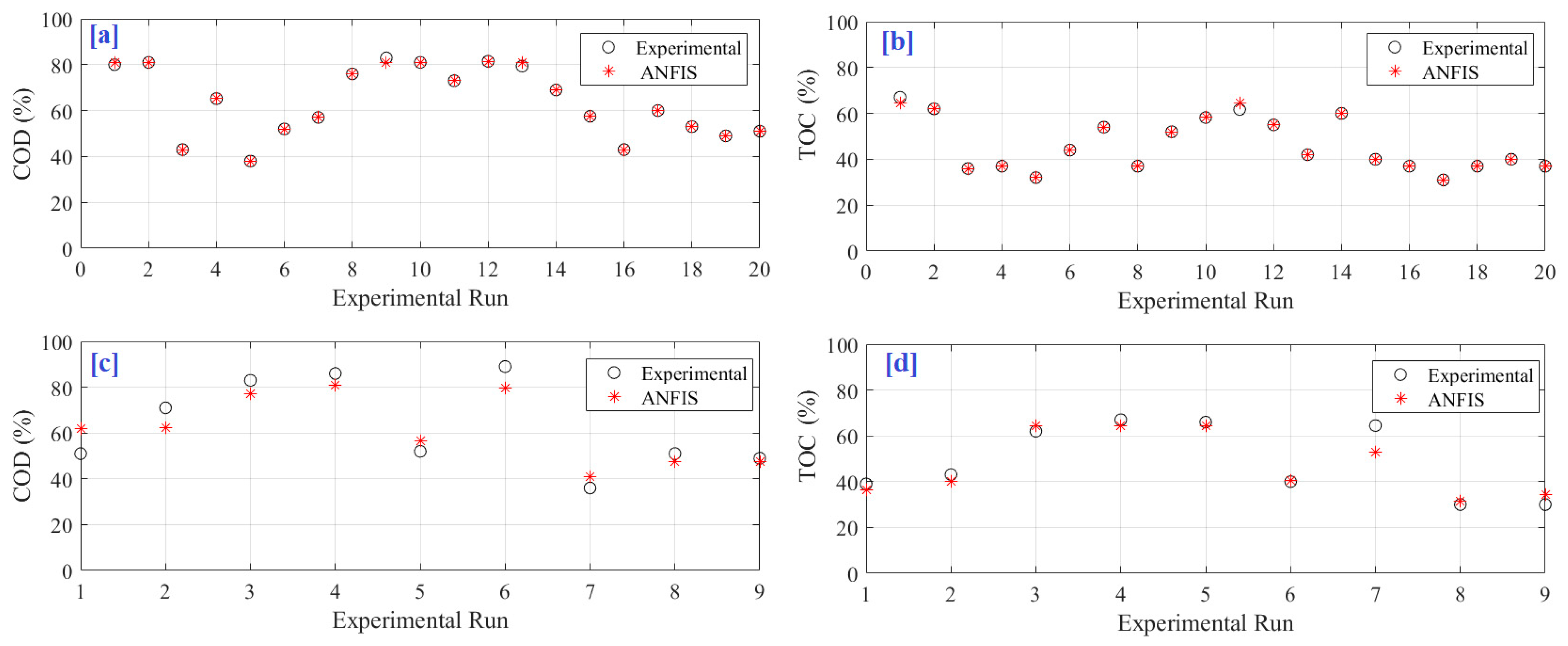


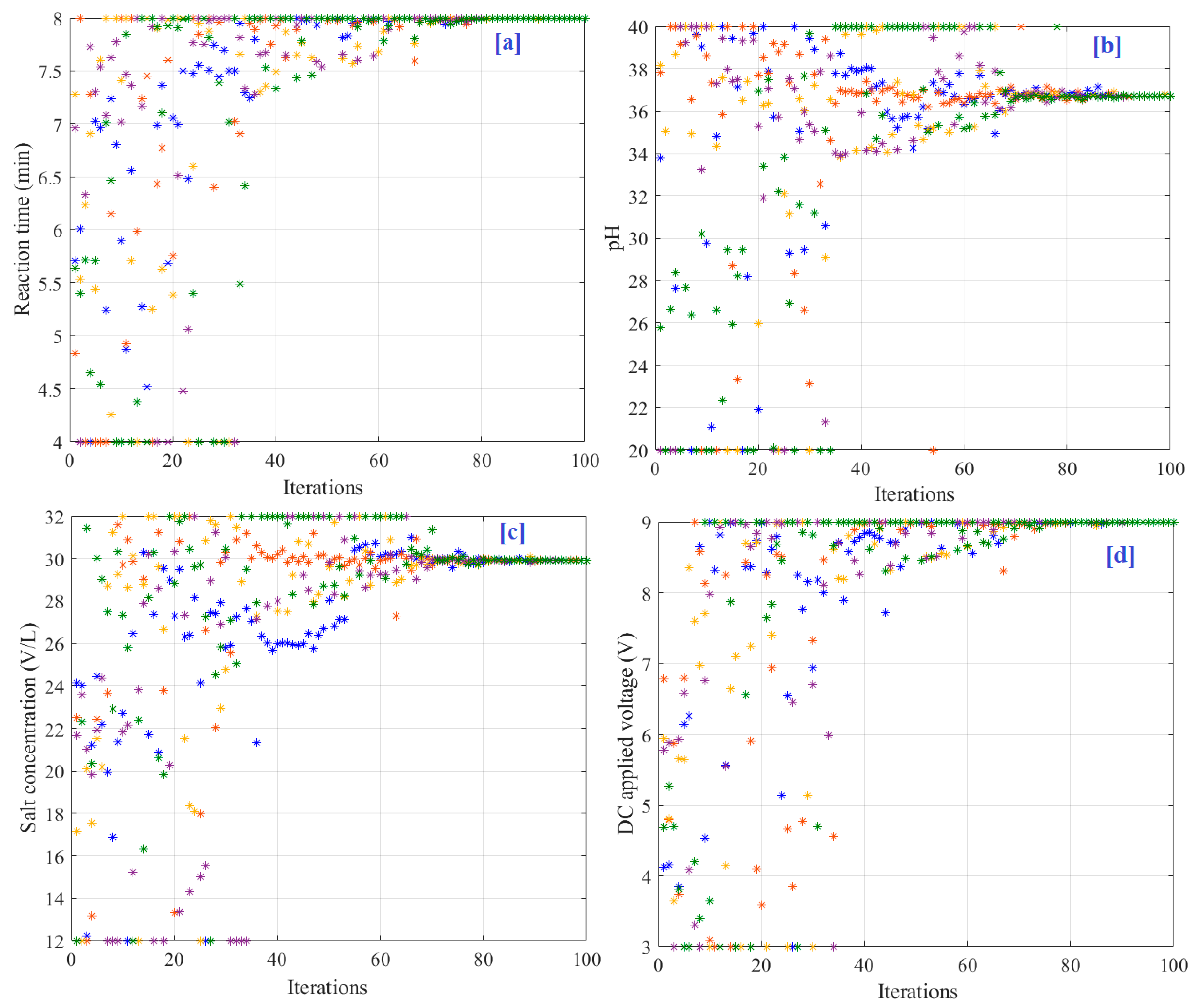
| Independent Variables | Values |
|---|---|
| Reaction time, min | 20, 30, 40 |
| pH | 4, 6, 8 |
| Applied voltage, V | 3, 6, 9 |
| Salt concentration, mg/L | 12,000, 22,000, 32,000 |
| pH | Time (min) | Salinity (gr/L) | Voltage (Volt) | COD Removal (%) | TOC Removal (%) |
|---|---|---|---|---|---|
| 4 | 20 | 22 | 6 | 49 | 37 |
| 4 | 30 | 22 | 3 | 38 | 36 |
| 4 | 30 | 12 | 6 | 49 | 37 |
| 4 | 30 | 22 | 9 | 69 | 55 |
| 4 | 30 | 32 | 9 | 51 | 40 |
| 4 | 40 | 22 | 6 | 60 | 37 |
| 6 | 20 | 12 | 6 | 36 | 30 |
| 6 | 20 | 22 | 3 | 51 | 30 |
| 6 | 20 | 22 | 9 | 43 | 40 |
| 6 | 20 | 32 | 6 | 57 | 32 |
| 6 | 30 | 12 | 3 | 43 | 39 |
| 6 | 30 | 12 | 9 | 52 | 40 |
| 6 | 30 | 22 | 6 | 80 | 67 |
| 6 | 30 | 22 | 6 | 79.4 | 61.7 |
| 6 | 30 | 22 | 6 | 86 | 66 |
| 6 | 30 | 22 | 6 | 83 | 62 |
| 6 | 30 | 22 | 6 | 81 | 67 |
| 6 | 30 | 32 | 3 | 51 | 37 |
| 6 | 30 | 32 | 9 | 81 | 62 |
| 6 | 40 | 12 | 6 | 52 | 37 |
| 6 | 40 | 22 | 3 | 53 | 31 |
| 6 | 40 | 22 | 9 | 83 | 60 |
| 6 | 40 | 32 | 6 | 73 | 54 |
| 8 | 30 | 12 | 6 | 57.5 | 42 |
| 8 | 30 | 22 | 3 | 71 | 52 |
| 8 | 30 | 22 | 9 | 81.5 | 58.2 |
| 8 | 30 | 32 | 6 | 89 | 64.5 |
| 8 | 40 | 22 | 6 | 76 | 44 |
| RMSE | R-Squared | ||||
|---|---|---|---|---|---|
| Train | Test | All | Train | Test | All |
| COD model | |||||
| 0.611 | 6.74 | 3.79 | 0.999 | 0.91 | 0.95 |
| TOC model | |||||
| 0.838 | 4.547 | 2.63 | 0.994 | 0.92 | 0.96 |
| Method | Reaction Time | pH | Salt Concentration (g/L) | DC Applied Voltage | COD (%) | TOC (%) |
|---|---|---|---|---|---|---|
| Exp. [36] | 30 | 8 | 32 | 6 | 89 | 64.5 |
| RSM [36] | 30.71 | 7.69 | 30.94 | 7.41 | 91.78 | 68.49 |
| HGSA | 36.69 | 8 | 29.9 | 9.0 | 97.63 | 69.42 |
| HGSA | SMA | SCA | HHO | |
|---|---|---|---|---|
| Maximum | 167.0600 | 167.0600 | 166.8933 | 167.0442 |
| Minimum | 166.9904 | 158.1047 | 155.2735 | 156.5687 |
| Mean | 167.0577 | 165.8614 | 163.0845 | 165.0988 |
| STD | 0.0127 | 3.0856 | 3.1572 | 3.2264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezk, H.; Olabi, A.G.; Sayed, E.T.; Alshathri, S.I.; Abdelkareem, M.A. Optimized Artificial Intelligent Model to Boost the Efficiency of Saline Wastewater Treatment Based on Hunger Games Search Algorithm and ANFIS. Sustainability 2023, 15, 4413. https://doi.org/10.3390/su15054413
Rezk H, Olabi AG, Sayed ET, Alshathri SI, Abdelkareem MA. Optimized Artificial Intelligent Model to Boost the Efficiency of Saline Wastewater Treatment Based on Hunger Games Search Algorithm and ANFIS. Sustainability. 2023; 15(5):4413. https://doi.org/10.3390/su15054413
Chicago/Turabian StyleRezk, Hegazy, Abdul Ghani Olabi, Enas Taha Sayed, Samah Ibrahim Alshathri, and Mohammad Ali Abdelkareem. 2023. "Optimized Artificial Intelligent Model to Boost the Efficiency of Saline Wastewater Treatment Based on Hunger Games Search Algorithm and ANFIS" Sustainability 15, no. 5: 4413. https://doi.org/10.3390/su15054413
APA StyleRezk, H., Olabi, A. G., Sayed, E. T., Alshathri, S. I., & Abdelkareem, M. A. (2023). Optimized Artificial Intelligent Model to Boost the Efficiency of Saline Wastewater Treatment Based on Hunger Games Search Algorithm and ANFIS. Sustainability, 15(5), 4413. https://doi.org/10.3390/su15054413









