Standardizing Pen Culture of Small Indigenous Fish Labeo bata in the Tropical Floodplain Wetland of the North Eastern Region, India: A Step towards Sustainable Fisheries Management
Abstract
1. Introduction
2. Materials and Methods
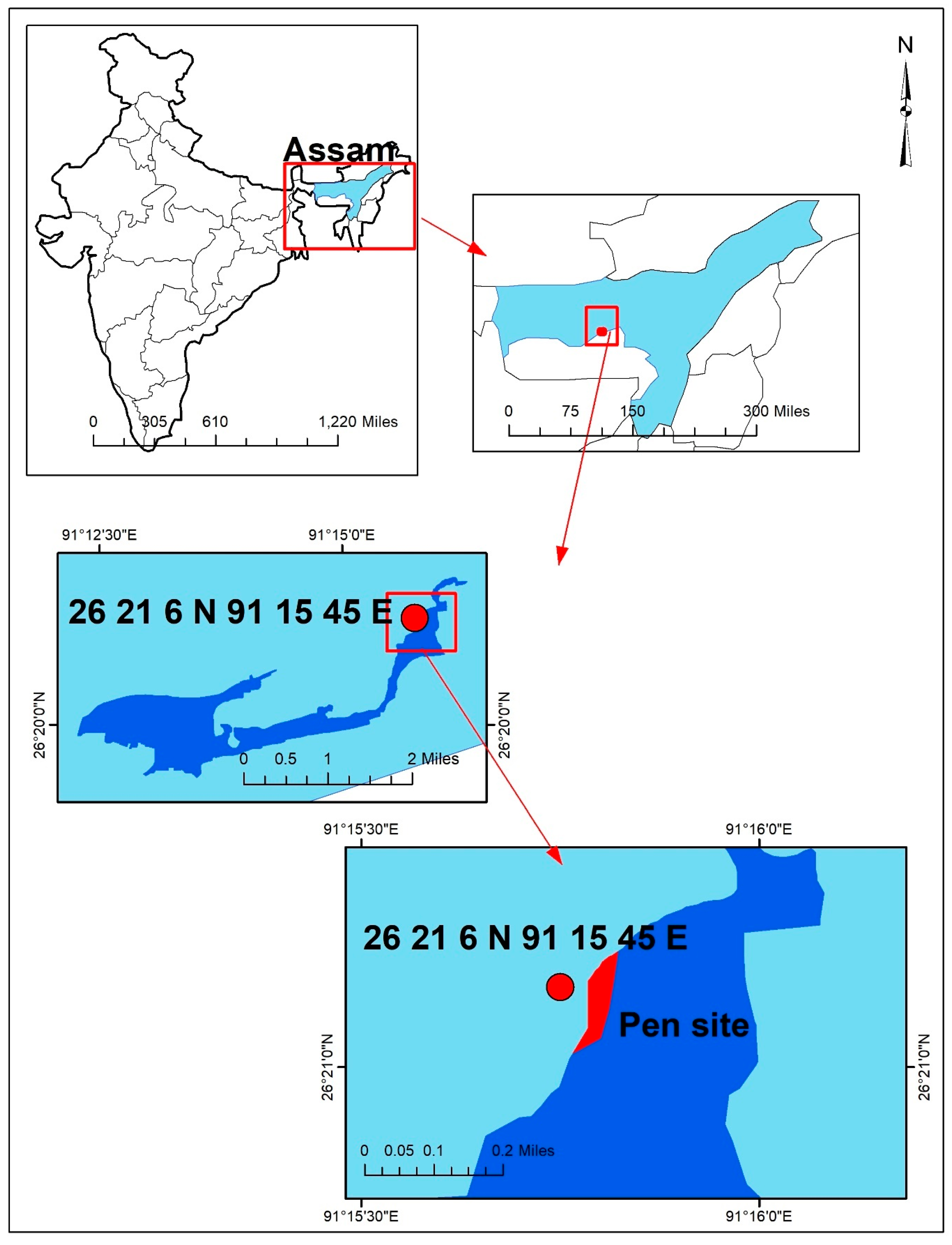
2.1. Study Area
2.2. Pen Culture
2.3. The Analysis of Water Quality Parameters
2.4. Growth Analysis
2.5. Economic Analysis
2.6. Fishers’ Income
2.7. Statistical Analysis
3. Results
3.1. Growth
3.2. Water Quality
3.3. Economics
3.4. Fishers’ Income
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutty, M.N.; Campbell, D. Pen Culture (Enclosure Culture) as an Aquaculture System; Lectures Presented at African Regional Aquaculture Centre (ARAC) for the Senior Aquaculturists Course; African Regional Aquaculture Centre: Port Harcourt, Nigeria, 1987. [Google Scholar]
- Sarkar, U.K.; Sandhya, K.M.; Mishal, P.; Karnatak, G.; Lianthuamluaia; Kumari, S.; Panikkar, P.; Palaniswamy, R.; Karthikeyan, M.; Mol, S.S.; et al. Status, prospects, threats, and the way forward for sustainable management and enhancement of the tropical Indian reservoir fisheries: An overview. Rev. Fish. Sci. Aquac. 2018, 26, 155–175. [Google Scholar] [CrossRef]
- Sarkar, U.K.; Mishal, P.; Borah, S.; Karnatak, G.; Chandra, G.; Kumari, S.; Meena, D.K.; Debnath, D.; Yengkokpam, S.; Das, P.; et al. Status, Potential, Prospects, and Issues of Floodplain Wetland Fisheries in India: Synthesis and Review for Sustainable Management. Rev. Fish. Sci. Aquac. 2021, 29, 1–32. [Google Scholar] [CrossRef]
- Biswas, G.; Sau, S.K.; Saurabh, S. Pen Culture and its uses in Indian Aquaculture. Fish. Chimes 2003, 23, 7–9. [Google Scholar]
- Sharma, A.P. Potentiality of enclosed fish farming in inland waters. In Fishery Management in M.P. Reservoirs Including Enclosure Culture; ICAR-CIFRI: Barrackpore, India, 2012; p. 116. [Google Scholar]
- Chandra, G.; Sharma, A.P.; Sahu, S.K. Impact of pen culture technology on fish productivity of floodplain wetlands in Asom. Indian J. Anim. Sci. 2013, 83, 209–215. [Google Scholar]
- Giresha, O.; Basavaraja, N.; Tyagi, A.C.; Shanmukha, S.N. Tungbhadra reservoir: Rearing of rohu spawn and common carp fingerlings in pens. Fish. Chimes 2003, 23, 21–24. [Google Scholar]
- Sugunan, V.V.; Bhattacharjya, B.K. Ecology and Fisheries of Beels in Assam; ICAR-Central Inland Fisheries Research Institute: Barrackpore, India, 2000; p. 65. [Google Scholar]
- Sugunan, V.V.; Sinha, M. Sustainable capture and culture-based fisheries in fresh waters of India. In Sustainable Indian Fisheries; National Academy of Agricultural Sciences: New Delhi, India, 2001; pp. 43–70. [Google Scholar]
- Nandi, B.; Priyadarshini, P.; Debnath, R.; Borah, S.; Yadav, A.K. Fisheries in Assam: Status and way forward. Biot. Res. Today 2022, 4, 768–770. [Google Scholar]
- Das, P.; Borah, S.; Yadav, A.K.; Bhattacharjya, B.K.; Das, B.K. Open water fisheries of Assam and strategies for its development. Fish. Chimes 2018, 38, 17–28. [Google Scholar]
- Yadav, A.K.; Das, K.K.; Borah, S.; Das, P.; Bhattacharjya, B.K.; Das, B.K. Impact of fish stock enhancement on fish yield of floodplain wetlands in different agro-climatic zones of Assam, India. Aquat. Ecosyst. Health Manag. 2021, 24, 54–63. [Google Scholar] [CrossRef]
- Bhattacharjya, B.K.; Borah, S.; Sharma, N.; Dewan, B.K.; Nath, K.D. Amblypharyngodon mola: A valuable candidate species for species enhancement in floodplain wetlands (beels) of Assam, India. Fish. Chimes 2018, 38, 51–53. [Google Scholar]
- Borah, S.; Sarkar, U.K.; Das, P.; Yadav, A.K.; Puthiyottil, M.; Pame, D.; Bhattacharjya, B.K.; Das, B.K. Managing floodplain wetlands through culture-based fisheries (CBF) for livelihood security and sustainable development: A study from a biodiversity hotspot region of India. Arab. J. Geosci. 2022, 15, 1245. [Google Scholar] [CrossRef]
- Borah, S.; Bhattacharjya, B.K.; Das, P.; Yadav, A.K.; Das, B.K.; Sarkar, U.K. Exploring the potential of culture-based fisheries for augmenting income of tribal beel fishers: A success story from Assam. Indian Farming 2022, 72, 23–26. [Google Scholar]
- Yengkokpam, S.; Das, B.K.; Debnath, D.; Das, P.; Yadav, A.K.; Sharma, N.; Borah, S.; Singh, N.S.; Sarma, K.K.; Ray, B.C.; et al. Effect of stocking density on growth and yield of Labeo bata fingerlings reared in cages. Aquac. Rep. 2020, 18, 100506. [Google Scholar] [CrossRef]
- Borah, S.; Das, P.; Yadav, A.K.; Bhattacharjya, B.K.; Das, B.K. Enhancing Income of Small Scale Tribal Fishers of Charan Beel, Assam through Culture- Based Fisheries and Pen Culture: A Participatory Approach. Biot. Res. Today 2022, 4, 392–394. [Google Scholar]
- Bhattacharjya, B.K.; Yadav, A.K.; Das, P.; Borah, S.; Debnath, D.; Yengkokpam, S.; Sharma, N.; Das, B.K. Decadal changes in fish yield rates in floodplain wetlands (beels) of Assam. CIFRI News 2017, 18, 7. [Google Scholar]
- Das, B.K.; Bhattacharjya, B.K.; Borah, S.; Das, P.; Debnath, D.; Yengkokpam, S.; Yadav, A.K.; Sharma, N.; Singh, N.S.; Pandit, A.; et al. Roadmap for Development of Open Water Fisheries in North-Eastern States of India; ICAR-Central Inland Fisheries Research Institute: Barrackpore, India, 2017; p. 120. [Google Scholar]
- Smith, H.; Basurto, X. Defining Small-Scale Fisheries and Examining the Role of Science in Shaping Perceptions of Who and What Counts: A Systematic Review. Front. Mar. Sci. 2019, 6, 236. [Google Scholar] [CrossRef]
- Funge-Smith, S. Review of the state of the world fishery resources: Inland fisheries, In FAO Fisheries and Aquaculture Circular; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Das, B.K.; Roy, A.; Som, S.; Chandra, G.; Kumari, S.; Sarkar, U.K.; Bhattacharjya, B.K.; Das, A.K.; Pandit, A. Impact of COVID-19 lockdown on small-scale fishers (SSF) engaged in floodplain wetland fisheries: Evidences from three states in India. Environ. Sci. Pollut. Res. 2021, 29, 8452–8463. [Google Scholar] [CrossRef]
- Karnatak, G.; Das, B.K.; Mishal, P.; Tayung, T.; Kumari, S.; Sarkar, U.K.; Das, A.K.; Ali, Y. Impact of stocking density on growth, feed utilization and survival of cage reared minor carp, Labeo bata (Hamilton, 1822) in Maithon reservoir, India. Aquaculture 2021, 532, 736078. [Google Scholar] [CrossRef]
- Gomes, L.C.; Baldisserotto, B.; Senhorini, J.A. Effect of stocking density on water quality, survival and growth of larvae of the matrinxa, Brycon cephalus (Characidae), in ponds. Aquaculture 2000, 183, 73–81. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Effects of stocking density and feeding levels on growth and feed efficiency of Nile tilapia (Oreochromis niloticus L.) fry. Aquac. Res. 2002, 33, 621–626. [Google Scholar] [CrossRef]
- Ellis, T.; North, B.; Scaott, A.P.; Bromage, N.R.; Porter, M.; Gadd, D. The relationship between stocking density and welfare in farmed rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, S.; Halder, G.C.; Tanaka, M. Cage culture of sutchi catfish, Pangasius sutchi (Fowler 1937): Effects of stocking density on growth, survival, yield and farm profitability. Aquac. Res. 2006, 37, 33–39. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Behera, B.K.; Das, P.; Singh, N.S.; Sahu, A.K. Observation on the induced breeding of Labeo bata (Hamilton) with Ovaprim and Ovatide as inducing agents with a note to its development. J. Aquac. 2007, 15, 11–17. [Google Scholar]
- Bhuyan, P.C.; Goswami, C.; Kakati, B.K. Study of fish consumption patterns in Assam for development of market driven strategies. Res. J. Chem. Env. Sci. 2017, 5, 42–52. [Google Scholar]
- Debnath, D.; Das, B.K.; Yengkokpam, S.; Das, P.; Yadav, A.K.; Sharma, N.; Borah, S.; Ray, B.C.; Kakati, A.; Sarkar, U.K.; et al. Evaluating growth, production and economics of a new candidate species Labeo bata in cages: A regional model for table fish production in floodplain wetlands of North East India. Aquaculture 2021, 546, 737344. [Google Scholar] [CrossRef]
- Datta, A.K.; Bhowmik, M.L.; Mandal, S.C.; Tripathi, S.D. Rearing of Labeo bata in sewage-fed fish culture pond. J. Indian Fish. Assoc. 1996, 26, 105–109. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species, Version 2020-2; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2020. Available online: https://marine.gov.scot/sma/content/iucn-red-list-threatened-species-version-2020-2 (accessed on 28 August 2021).
- Rahman, M.M.; Hossain, M.Y.; Ahamed, F.; Fatematuzzhura; Subba, B.R.; Abdallah, E.M.; Ohtomi, J. Biodiversity in the Padma (distributary of the Ganges River), Northwestern Bangladesh: Recommendations for conservation. World J. Zool. 2012, 7, 328–337. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; p. 1545. [Google Scholar]
- Karnatak, G.; Das, B.K.; Sarkar, U.K.; Borah, S.; Roy, A.; Parida, P.; Lianthuamluaia, L.; Das, A.K.; Behera, B.K.; Pandit, A.; et al. Integration of pen aquaculture into ecosystem-based enhancement of small-scale fisheries in a macrophyte dominated floodplain wetland of India. Environ. Sci. Pollut. Res. 2022, 29, 75431–75440. [Google Scholar] [CrossRef]
- Fraenkel, J.; Wallen, N.; Hyun, H. How to Design and Evaluate Research in Education, 8th ed.; McGraw-Hill: New York, NY, USA, 2012; p. 642. [Google Scholar]
- Das, B.K.; Jha, D.N.; Sahu, S.K.; Yadav, A.K.; Raman, R.K.; Kartikeyan, M. Concept Building in Fisheries Data Analysis; Springer: Singapore, 2022; p. 257. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 9 November 2022).
- Gorai, B.K.; Jha, B.C.; Nath, D.; Choudhury, M. Culture of carps in pens in a wetland of Assam–A case study. J. Inland Fish. Soc. India 2006, 38, 53–58. [Google Scholar]
- Chandra, G. Impact of adoption of pen culture technology on well-being of fishers of Haribhanga Wetland in Assam. Indian Res. J. Ext. Edu. 2010, 10, 61–65. [Google Scholar]
- Bhattacharjya, B.K.; Sharma, A.P.; Debnath, D.; Das, P.; Yadav, A.K.; Yengkokpam, S.; Sarma, K.K.; Ray, B.C.; Kakati, A. Pen culture in floodplain wetlands of Assam. CIFRI News 2015, 20, 8. [Google Scholar]
- Das, A.; Bhattacharjya, B.K.; Goswami, S.N.; Sawant, P.B.; Debnath, D.; Yengkokpam, S.; Das, A.; Kakati, A.; Sarma, K.K.; Chadha, N.K.; et al. Assessment of economic feasibility of pen aquaculture technology in floodplain wetlands (beels) of Assam, India. Indian J. Fish. 2017, 64, 1–7. [Google Scholar] [CrossRef]
- Alam, A.; Joshi, K.D.; Das, S.C.S.; Jha, D.N.; Srivastava, K.; Kumar, V.; Bhattacharjya, B.K. Enhancing fish productivity through pen culture: A case study in Sareni wetland of Uttar Pradesh. Indian J. Fish. 2017, 64, 8–13. [Google Scholar] [CrossRef]
- Yengkokpam, S.; Debnath, D.; Bhattacharjya, B.K.; Yadav, A.K.; Das, P.; Sarma, K.K.; Das, B.K. Polyculture of Osteobrama belangeri with major carps in pen enclosures in Takmu lake of Manipur. J. Inland Fish. Soc. India 2019, 51, 125–132. [Google Scholar] [CrossRef]
- Gorai, B.K.; Sugunan, V.V.; Jha, B.C. Raising of stocking materials of Indian major carps in pen enclosures in selected flood-plain wetlands of Assam, India. Asian Fish. Sci. 2006, 19, 185–197. [Google Scholar]
- Paul, B.N.; Datta, A.K.; Giri, S.S.; Mohanty, S.N. Dietary protein requirement of Labeo bata fry. Anim. Nutr. Feed Techn. 2009, 9, 179–184. [Google Scholar]
- Anon. Handbook of Fisheries and Aquaculture, 1st ed.; Indian Council of Agricultural Research: New Delhi, India, 2006; p. 755. [Google Scholar]
- Bhattacharjya, B.K.; Yengkokpam, S.; Gogoi, P.; Sarma, K.K.; Debnath, D. Rearing of carried over carp seed in pen enclosure in a closed floodplain wetland of Assam. J. Inland Fish. Soc. India 2015, 47, 43–48. [Google Scholar]
- Leatherland, J.F.; Cho, C.O. Effect of rearing density on thyroid and internal gland activity and plasma and hepatic metabolite levels in rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1985, 27, 583–592. [Google Scholar] [CrossRef]
- Pfuderer, P.; Williams, P.; Francis, A.A. Partial purification of the crowding factor from Carassius auratus and Cyprinus carpio. J. Exp. Zool. 1973, 187, 375–382. [Google Scholar] [CrossRef]
- Jiwyam, W. The effect of stocking density on yield, growth and survival of Asian river catfish (Pangasius bocourti Sauvage, 1880) cultured in cages. Aquac. Int. 2011, 19, 987–997. [Google Scholar] [CrossRef]
- Cruz, E.M.; Ridha, M. Preliminary study on the production of the tilapia, Oreochromis spilurus (Günther), cultured in seawater cages. Aquac. Res. 1989, 20, 381–388. [Google Scholar] [CrossRef]
- Hengsawat, K.; Ward, F.; Jaruratjamorn, P. The effect of stocking density on yield, growth and mortality of African catfish (Clarias gariepinus Burchell 1822) cultured in cages. Aquaculture 1997, 152, 67–76. [Google Scholar] [CrossRef]
- Garcia, F.; Romera, D.M.; Gozi, K.S.; Onaka, E.M.; Fonseca, F.S.; Schalch, S.H.; Candeira, P.G.; Guerra, L.O.; Carmo, F.J.; Carneiro, D.J.; et al. Stocking density of Nile tilapia in cages placed in a hydroelectric reservoir. Aquaculture 2013, 410–411, 51–56. [Google Scholar] [CrossRef]
- Debnath, D.; Bhattacharjya, B.K.; Yengkokpam, S.; Yadav, A.K.; Sarma, K.K.; Gogoi, P.; Kakati, A. Standardization of Stocking Density of Cirrhinus mrigala Fry in Floating Net Cages in Charan Beel, Assam. In Book of Abstracts, Proceedings of the Wetland 2012 (National Symposium), Silchar, India, 21–23 November 2012; Assam University: Silchar, India, 2012; pp. 11–12. [Google Scholar]
- Kijima, Y.; Otsuka, K.; Sserunkuuma, D. An Inquiry into Constraints on a Green Revolution in Sub-Saharan Africa: The Case of NERICA Rice in Uganda. World Dev. 2011, 39, 77–86. [Google Scholar] [CrossRef]
- Haque, A.B.M.M.; Dey, M.M. Impacts of community-based fish culture in seasonal floodplains on income, food security and employment in Bangladesh. Food Secur. 2017, 9, 25–38. [Google Scholar] [CrossRef]
- Das, P.; Borah, S.; Yadav, A.K.; Bhattacharjya, B.K.; Das, B.K. Culture-Based Fisheries and Pen Culture Technologies Enhanced Income of Tribal Fishers in Bamuni Beel, Assam. Biot. Res. Today 2022, 4, 255–258. [Google Scholar]
- Boyd, C.E. Water Quality Management for Pond Fish Culture; Elsevier: Amsterdam, The Netherlands, 1982; p. 318. [Google Scholar]
- Karnatak, G.; Sarkar, U.K.; Naskar, M.; Roy, K.; Gupta, S.; Nandy, S.K.; Srivastava, P.K.; Das Sarkar, S.; Sudheesan, D.; Bose, A.K.; et al. Understanding the role of climatic and environmental variables in gonadal maturation and spawning periodicity of spotted snakehead, Channa punctata (Bloch, 1793) in a tropical floodplain wetland, India. Environ. Biol. Fishes 2018, 101, 595–607. [Google Scholar] [CrossRef]
- Karnatak, G.; Das, B.K.; Puthiyottil, M.; Tayung, T.; Kumari, S.; Lianthuamluaia, L.; Sarkar, U.K.; Behera, B.K.; Tiwari, V.K.; Chadha, N.K.; et al. Environmental parameters and stocking density influence growth, feed utilization and economics of butter catfish, Ompok bimaculatus (Bloch, 1794) production in floating net cages in a large tropical reservoir, India. Environ. Sci. Pollut. Res. 2021, 28, 59720–59730. [Google Scholar] [CrossRef]
- Mizanur, R.M.; Yun, H.; Moniruzzaman, M.; Ferreira, F.; Kim, K.W.; Bai, S.C. Effects of feeding rate and water temperature on growth and body composition of juvenile Korean rockfish, Sebastes schlegeli (Hilgendorf 1880). Asian-Australas. J. Anim. Sci. 2014, 27, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Gardeur, J.-N.; Mathis, N.; Kobilinsky, A.; Brun-Bellut, J. Simultaneous effects of nutritional and environmental factors on growth and flesh quality of Perca fluviatilis using a fractional factorial design study. Aquaculture 2007, 273, 50–63. [Google Scholar] [CrossRef]
- Wurts, W.A.; Durborow, R.M. Interactions of pH, Carbon Dioxide, Alkalinity and Hardness in Fish Ponds; Southern Regional Aquaculture Center: Stoneville, MS, USA, 1992; pp. 1–4. [Google Scholar]




| Parameters | Stocking Densities | |||
|---|---|---|---|---|
| SD3 | SD5 | SD7 | SD9 | |
| Final length (cm) | 20.31 a ± 0.11 | 19.84 ab ± 0.13 | 19.26 bc ± 0.01 | 18.82 c ± 0.46 |
| Final weight (g) | 82.78 a ± 3.18 | 75.94 ab ± 0.89 | 71.94 b ± 0.89 | 61.81 c ± 3.35 |
| WG% | 3371.33 a ± 133.16 | 3084.78 ab ± 37.66 | 2917.03 b ± 37.66 | 2491.85 c ± 140.56 |
| SGR | 1.55 a ± 0.02 | 1.50 ab ± 0.01 | 1.47 b ± 0.01 | 1.41 c ± 0.02 |
| DWG | 0.80 a ± 0.03 | 0.74 ab ± 0.01 | 0.70 b ± 0.01 | 0.59 c ± 0.03 |
| FCR | 2.32 ± 0.19 | 2.34 ± 0.19 | 2.35 ± 0.19 | 2.37 ± 0.18 |
| PER | 1.52 ± 0.30 | 1.42 ± 0.27 | 1.35 ± 0.26 | 1.20 ± 0.22 |
| CV | 0.37 ± 0.06 | 0.42 ± 0.07 | 0.47 ± 0.08 | 0.34 ± 0.07 |
| Survival (%) | 95.60 a ± 0.81 | 94.30 ab ± 0.86 | 92.30 b ± 1.01 | 89.70 c ± 0.70 |
| Gross yield (kg pen−1) | 118.70 c ± 4.55 | 179.04 b ± 2.12 | 232.42 a ± 2.90 | 249.48 a ± 13.53 |
| Net yield (kg pen−1) | 115.28 c ± 4.55 | 173.42 b ± 2.12 | 224.71 a ± 2.90 | 239.85 a ± 13.53 |
| Parameter | Inside Pens | Reference Site |
|---|---|---|
| Water temperature °C) | 23.93 ± 0.28 | 23.85 ± 0.60 |
| pH | 7.18 ± 0.28 | 7.24 ± 0.27 |
| DO (mg L−1) | 7.62 ± 0.34 | 7.79 ± 0.52 |
| Electrical conductivity (μS cm−1) | 176.50 ± 11.76 | 172.13 ± 14.31 |
| TDS (mg L−1) | 116.94 ± 8.67 | 108.38 ± 12.92 |
| CO2 (mg L−1) | 3.90 ± 0.42 | 3.25 ± 1.89 |
| Alkalinity (mg L−1) | 20.63 ± 2.20 | 20.25 ± 3.31 |
| Transparency (cm) | 47.88 ± 4.45 | 48.50 ± 6.96 |
| Stocking Densities (No. m−2) | |||||
|---|---|---|---|---|---|
| Sl. No. | Particulars | 3 | 5 | 7 | 9 |
| 1 | Seed cost (USD) | 20.21 | 33.68 | 47.16 | 60.63 |
| 2 | Feed cost (USD) | 127.97 | 193.71 | 252.08 | 272.06 |
| 3 | Labor cost (USD) | 13.47 | 13.47 | 13.47 | 13.47 |
| 4 | Total operational cost (1 + 2 + 3) (USD) | 161.65 | 240.87 | 312.72 | 346.17 |
| 5 | Total capital cost (USD) | 128.47 | 128.47 | 128.47 | 128.47 |
| 6 | Total cost (4 + 5) (USD) | 290.12 | 369.34 | 441.18 | 474.64 |
| 7 | Gross fish yield (kg) | 118.70 | 179.04 | 232.42 | 249.48 |
| 8 | Gross revenue (USD) | 319.87 | 482.45 | 626.29 | 672.27 |
| 9 | Net revenue (8–6) (USD) | 29.75 | 113.12 | 185.11 | 197.63 |
| 10 | BCR | 1.10 | 1.31 | 1.42 | 1.41 |
| 11 | YPC (kg USD−1) | 0.41 | 0.48 | 0.53 | 0.52 |
| 12 | CPY (USD kg−1) | 2.45 | 2.06 | 1.90 | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borah, S.; Das, B.K.; Bhattacharjya, B.K.; Karnatak, G.; Yadav, A.K.; Pandit, A.; Parida, P.K.; Roy, A.; Sahoo, A.K.; Behera, B.K.; et al. Standardizing Pen Culture of Small Indigenous Fish Labeo bata in the Tropical Floodplain Wetland of the North Eastern Region, India: A Step towards Sustainable Fisheries Management. Sustainability 2023, 15, 4423. https://doi.org/10.3390/su15054423
Borah S, Das BK, Bhattacharjya BK, Karnatak G, Yadav AK, Pandit A, Parida PK, Roy A, Sahoo AK, Behera BK, et al. Standardizing Pen Culture of Small Indigenous Fish Labeo bata in the Tropical Floodplain Wetland of the North Eastern Region, India: A Step towards Sustainable Fisheries Management. Sustainability. 2023; 15(5):4423. https://doi.org/10.3390/su15054423
Chicago/Turabian StyleBorah, Simanku, Basanta Kumar Das, Birendra Kumar Bhattacharjya, Gunjan Karnatak, Anil Kumar Yadav, Arun Pandit, Pranaya Kumar Parida, Aparna Roy, Amiya Kumar Sahoo, Bijay Kumar Behera, and et al. 2023. "Standardizing Pen Culture of Small Indigenous Fish Labeo bata in the Tropical Floodplain Wetland of the North Eastern Region, India: A Step towards Sustainable Fisheries Management" Sustainability 15, no. 5: 4423. https://doi.org/10.3390/su15054423
APA StyleBorah, S., Das, B. K., Bhattacharjya, B. K., Karnatak, G., Yadav, A. K., Pandit, A., Parida, P. K., Roy, A., Sahoo, A. K., Behera, B. K., Das, A. K., Rabha, N., & Priyadarshini, P. (2023). Standardizing Pen Culture of Small Indigenous Fish Labeo bata in the Tropical Floodplain Wetland of the North Eastern Region, India: A Step towards Sustainable Fisheries Management. Sustainability, 15(5), 4423. https://doi.org/10.3390/su15054423










