Preparation of Red Iron by Magnetization Roasting-Hydrothermal Method Using Ultra-Low-Grade Limonite
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Methods
2.2.1. Grinding Fineness
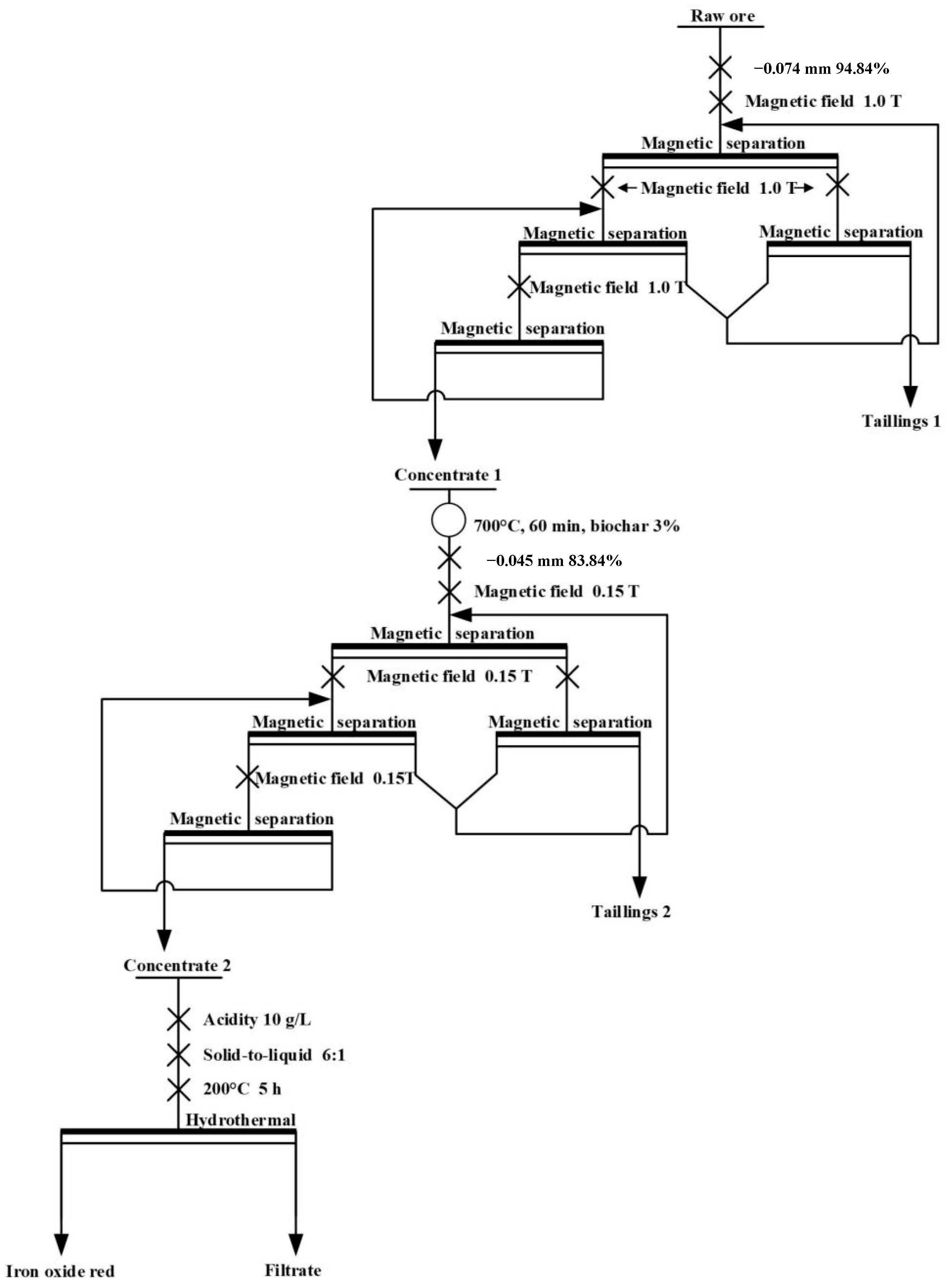
2.2.2. High-Intensity Magnetic Separation
2.2.3. Magnetization Roasting
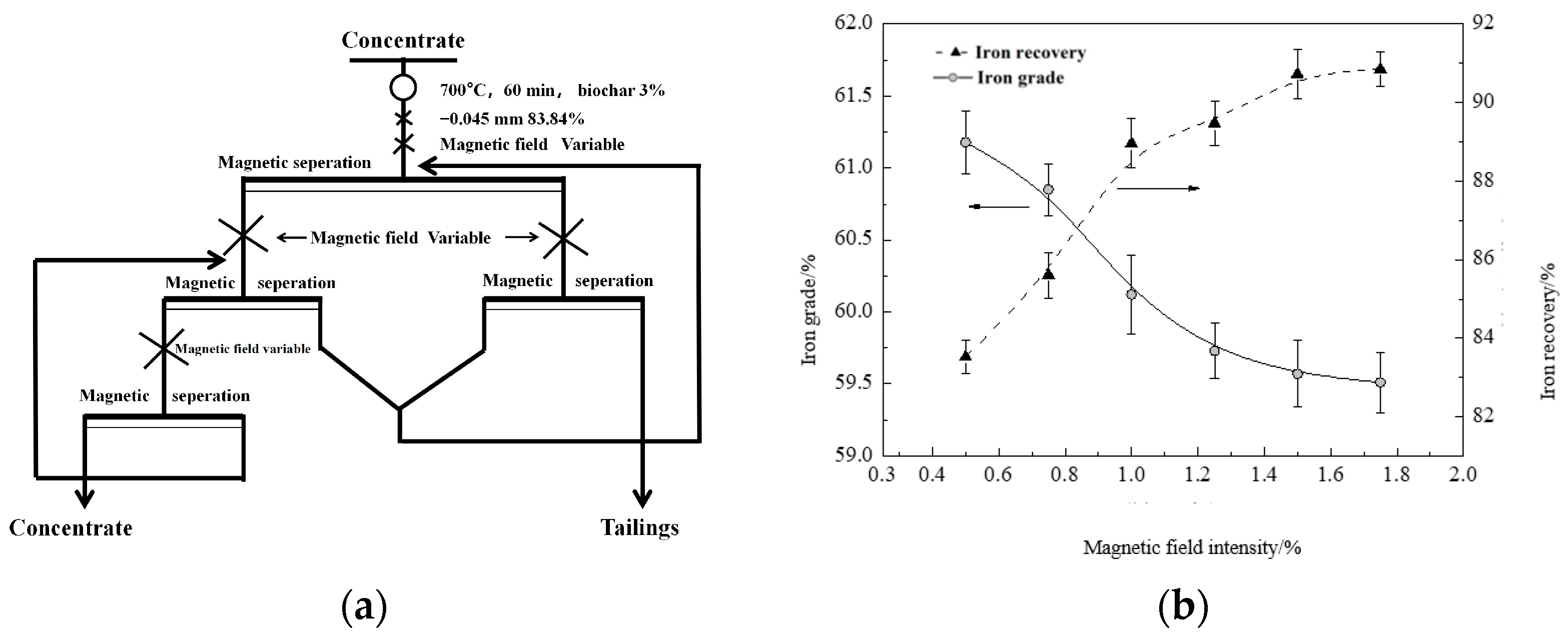
2.2.4. Weak Magnetic Separation
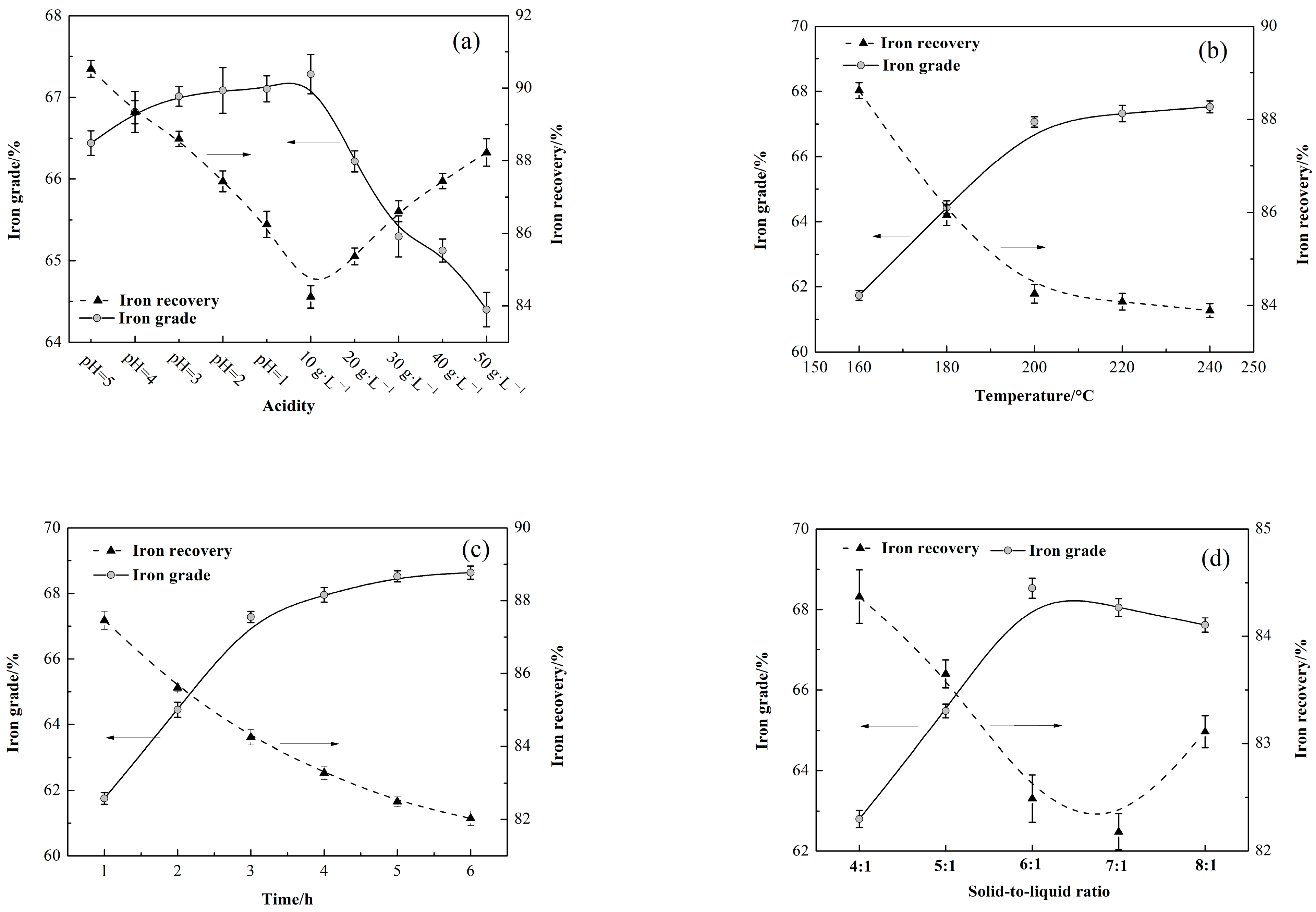
2.2.5. Red Iron Preparation
2.3. Characterizations
3. Results and Discussions
3.1. Physicochemical Properties of Raw Material
3.1.1. Raw Ore
3.1.2. Biochar
3.2. Grinding Fineness
3.3. High-Intensity Magnetic Separation
3.4. Magnetization Roasting-Weak Magnetic Separation
3.4.1. Orthogonal Test
3.4.2. Optimization Verification
3.5. Preparation of Red Iron
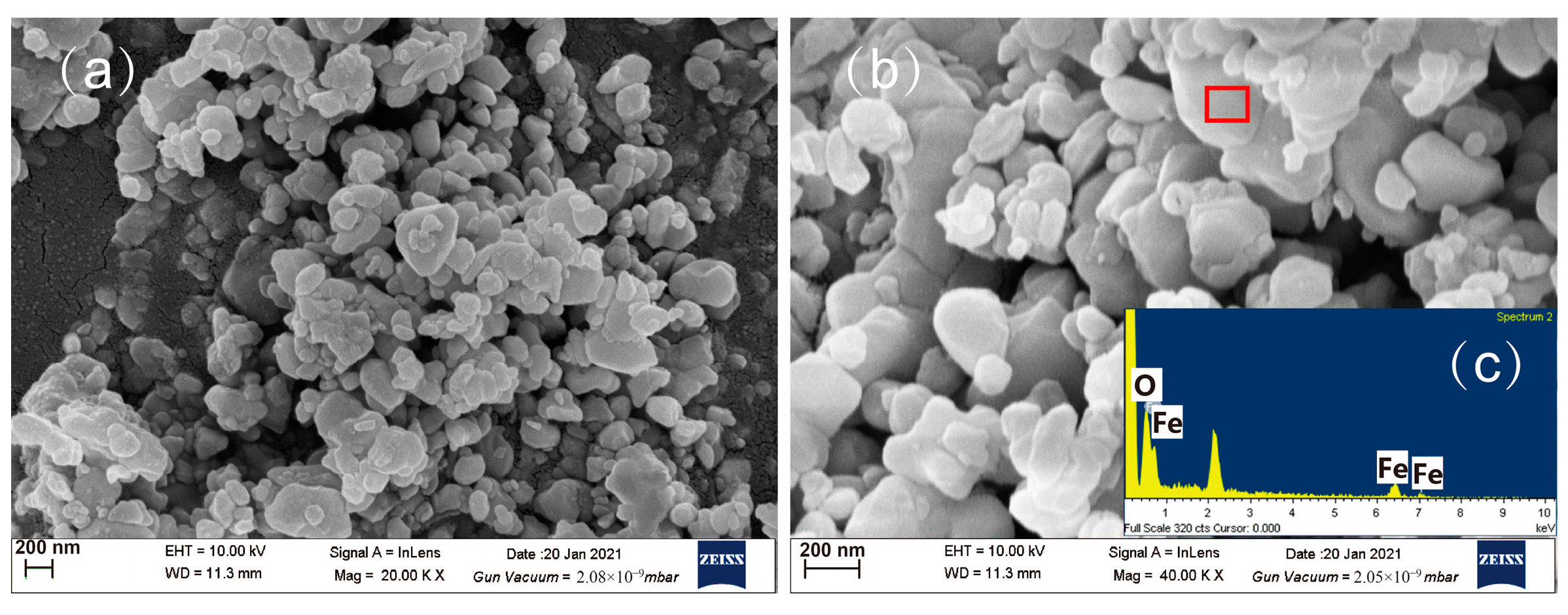
3.6. Reaction Mechanism
3.7. Quality Inspection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, M. Composition of the Earth’s Crust. Encyclopedia of Geology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 178–186. [Google Scholar]
- Ma, K.; Deng, J.; Wang, G.; Zhou, Q.; Xu, J. Utilization and impacts of hydrogen in the ironmaking processes: A review from lab-scale basics to industrial practices. Int. J. Hydrog. Energy 2021, 46, 26646–26664. [Google Scholar] [CrossRef]
- Song, Y.; Wang, N.; Yu, A. Temporal and spatial evolution of global iron ore supply-demand and trade structure. Resour. Policy 2019, 64, 101506. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, X.; Wang, M. Forecasting iron ore import and consumption of China using grey model optimized by particle swarm optimization algorithm. Resour. Policy 2013, 38, 613–620. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Han, Y.; Li, Y.; Gao, P. Iron recovery from refractory limonite ore using suspension magnetization roasting: A pilot-scale study. J. Clean. Prod. 2020, 261, 121221. [Google Scholar] [CrossRef]
- Liu, C.; Deng, J.; Ni, C.; Wang, D.; Xue, K.; Xu, L.; Zhang, X. Reverse froth flotation separation of limonite and quartz with cationic gemini surfactant. Miner. Eng. 2022, 177, 107391. [Google Scholar] [CrossRef]
- O’Connor, F.; Cheung, W.H.; Valix, M. Reduction roasting of limonite ores: Effect of dehydroxylation. Int. J. Min. Sci. Technol. 2006, 80, 88–99. [Google Scholar] [CrossRef]
- Wu, F.; Cao, Z.; Wang, S.; Zhong, H. Novel and green metallurgical technique of comprehensive utilization of refractory limonite ores. J. Clean. Prod. 2018, 171, 831–843. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, Y.; Guo, W.; Li, N.; Wang, Z.; Luo, H.; Hu, Q.; Li, B.; Wu, W.; Shang, S. Phase transition and magnetic properties of low-grade limonite during reductive roasting. Vacuum 2019, 167, 163–174. [Google Scholar] [CrossRef]
- Song, S.; Lu, S.; Lopez-Valdivieso, A. Magnetic separation of hematite and limonite fines as hydrophobic flocs from iron ores. Miner. Eng. 2002, 15, 415–422. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, D.; Pan, J.; Luo, Y.; Liu, X. Upgrading of High-Aluminum Hematite-Limonite Ore by High Temperature Reduction-Wet Magnetic Separation Process. Metals 2016, 6, 57. [Google Scholar] [CrossRef]
- Çetintaş, S.; Yildiz, U.; Bingöl, D. A novel reagent-assisted mechanochemical method for nickel recovery from lateritic ore. J. Clean. Prod. 2018, 199, 616–632. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.; Han, Y.; Li, Y. Individual enrichment of manganese and iron from complex refractory ferromanganese ore by suspension magnetization roasting and magnetic separation. Powder Technol. 2020, 373, 689–701. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Gao, P.; Han, Y.; Li, Y. Mechanism for suspension magnetization roasting of iron ore using straw-type biomass reductant. Int. J. Min. Sci. Technol. 2021, 31, 1075–1083. [Google Scholar] [CrossRef]
- Li, Y.; Sun, T.; Zou, A.; Xu, C. Effect of coal levels during direct reduction roasting of high phosphorus oolitic hematite ore in a tunnel kiln. Int. J. Min. Sci. Technol. 2012, 22, 323–328. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Liu, F.; Yang, F.; Zhou, S.; Zheng, K.; Chu, C.; Liu, L.; Ju, M. Effective removal of Cr(vi) from aqueous solution by biochar supported manganese sulfide. RSC Adv. 2019, 9, 31333–31342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Ge, Q.; Guo, G. Iron oxide red wastewater treatment and recycling of iron-containing sludge. J. Clean. Prod. 2015, 87, 558–566. [Google Scholar] [CrossRef]
- Mi, R.; Pan, G.; Li, Y. Distinguishing between new and old mortars in recycled aggregate concrete under carbonation using iron oxide red. Constr. Build. Mater. 2019, 222, 601–609. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Liu, L.; Ju, M. Study on the long-term effects of DOM on the adsorption of BPS by biochar. Chemosphere 2020, 242, 125165. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Li, C.; Tang, X.; Sun, Y. Removal of tetracycline via the synergistic effect of biochar adsorption and enhanced activation of persulfate. Chem. Eng. J. 2020, 382, 122916. [Google Scholar] [CrossRef]
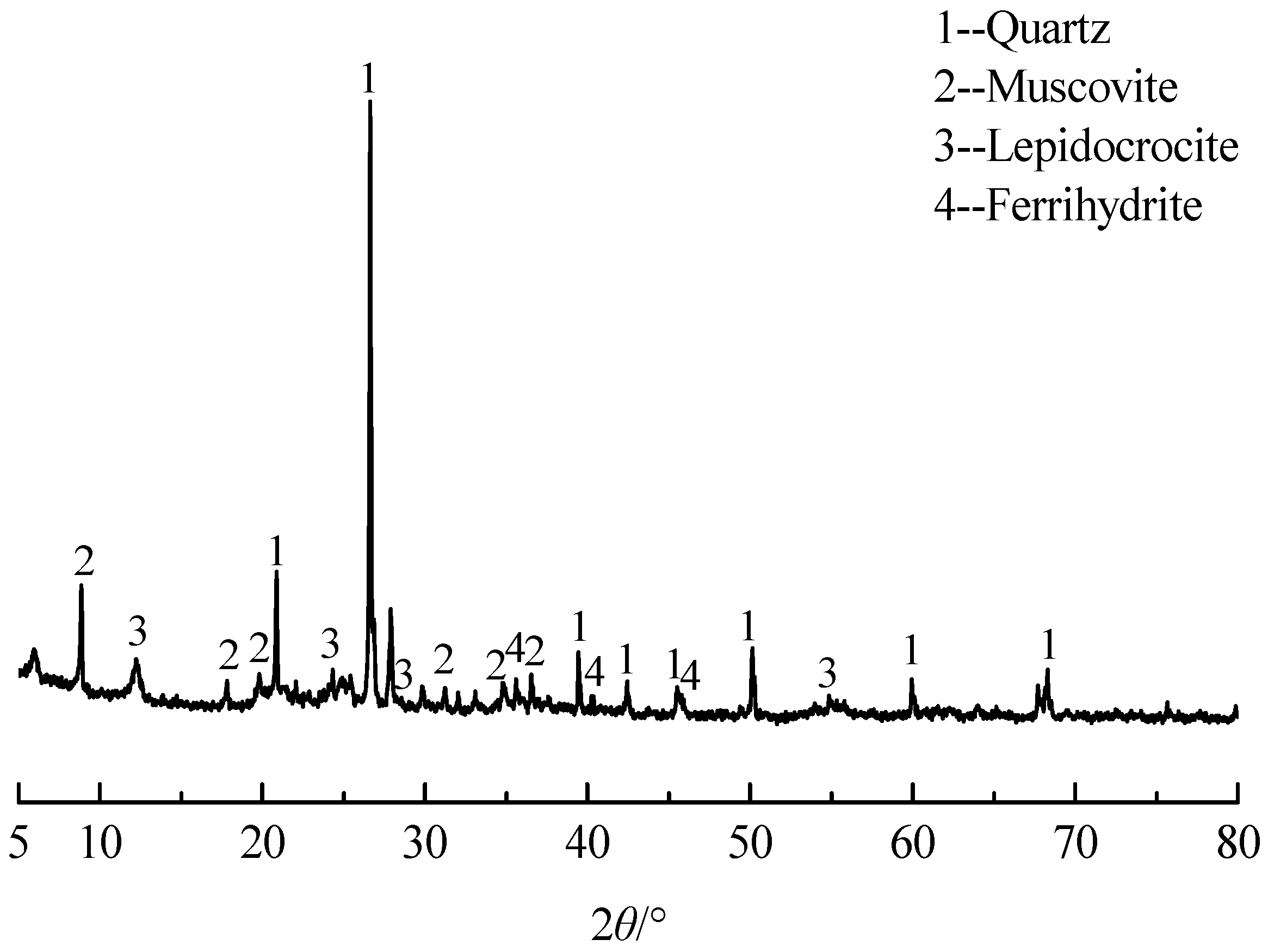
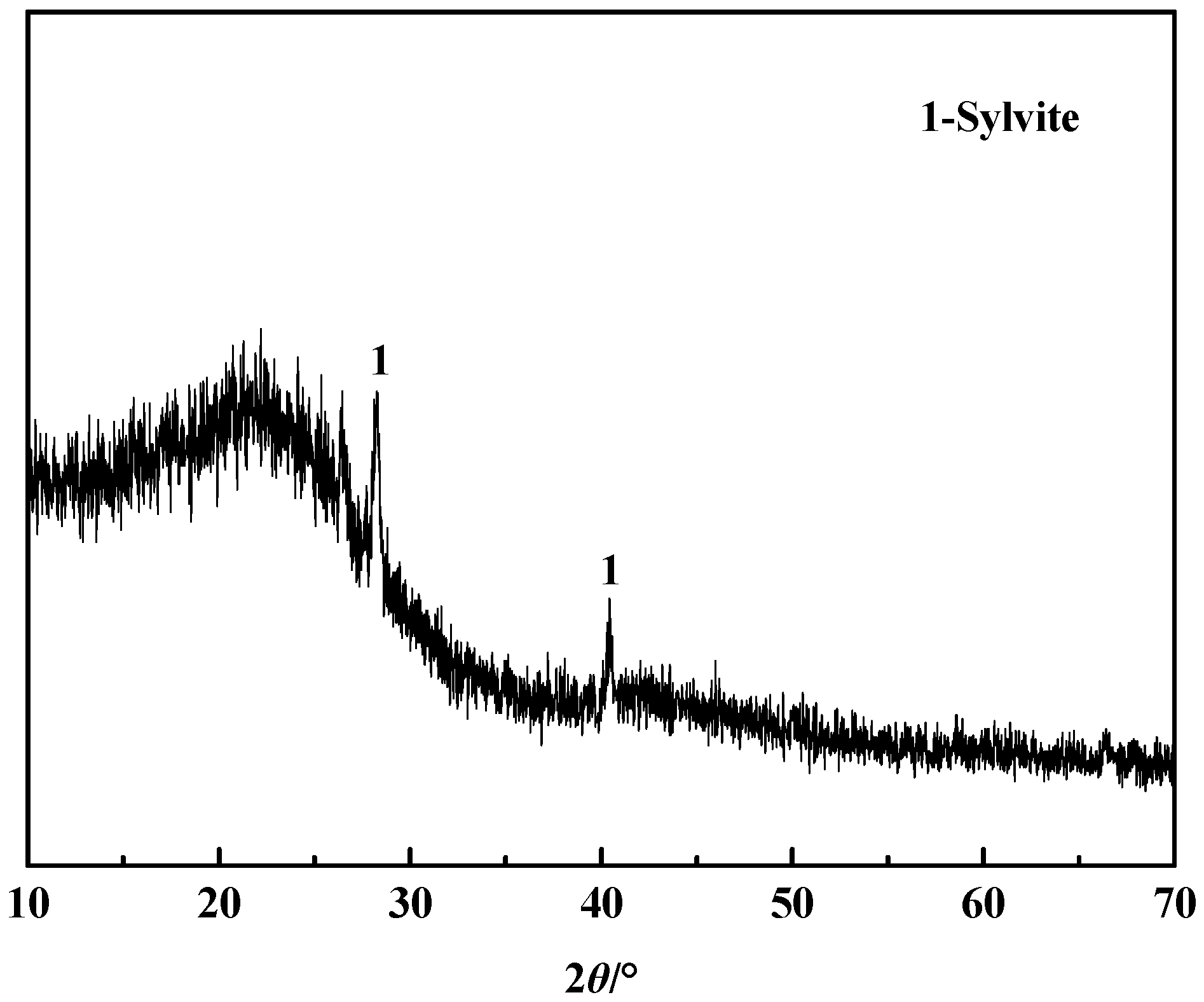

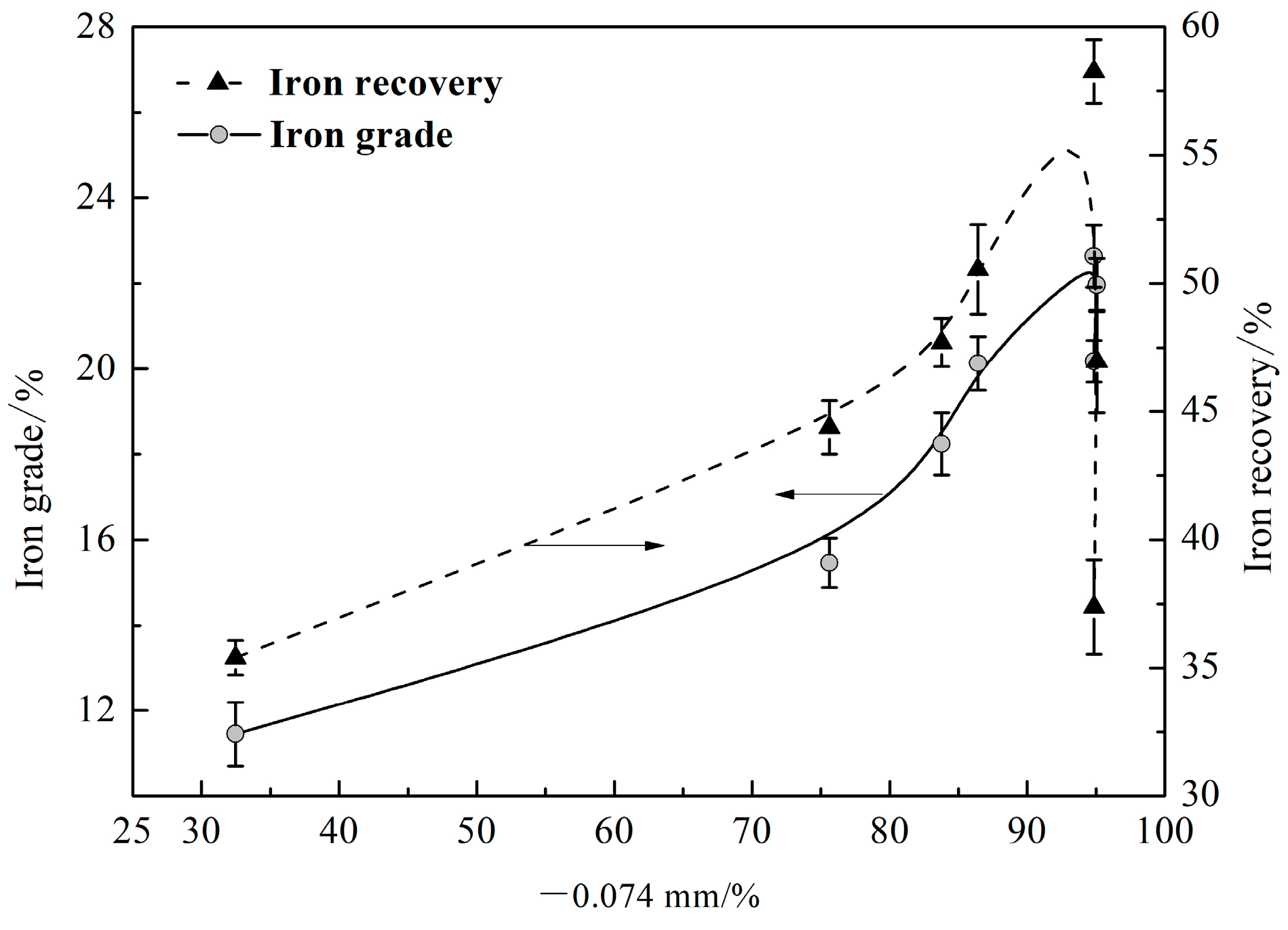
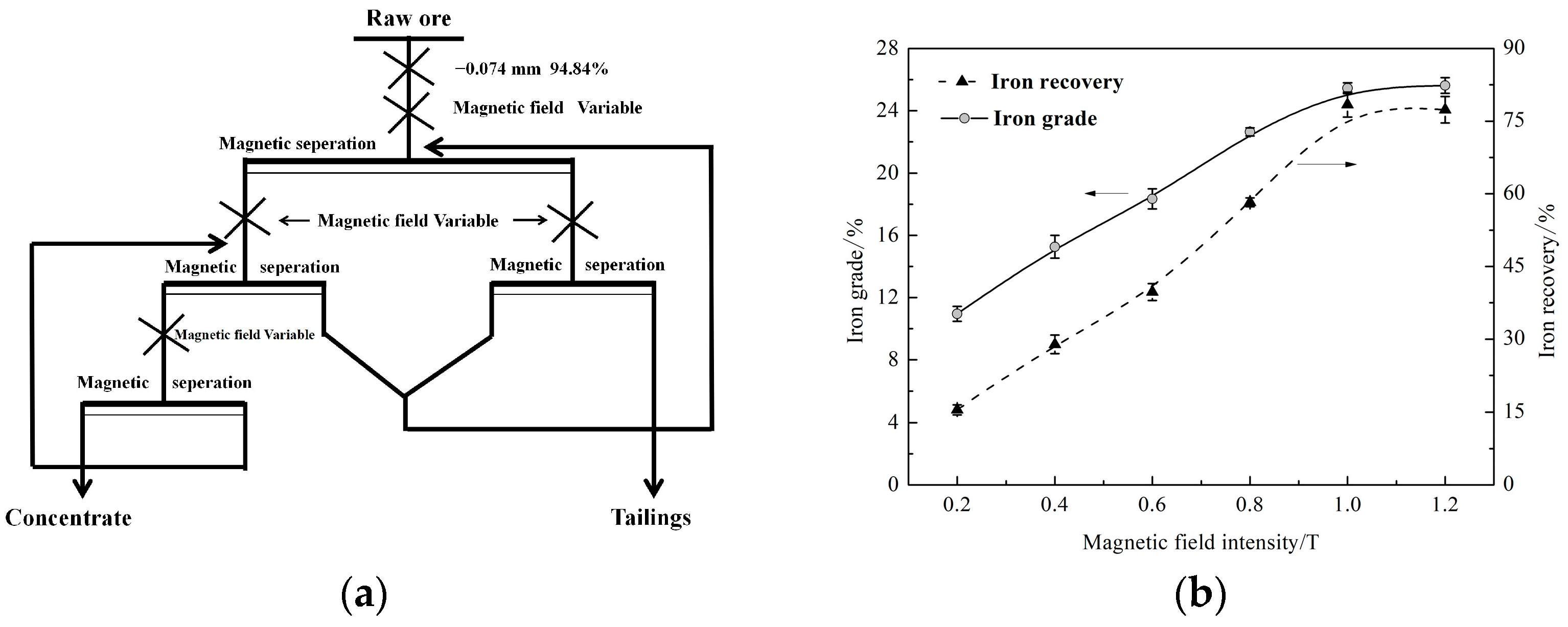



| Elements | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | TiO2 |
|---|---|---|---|---|---|---|
| Content | 53.54 | 24.48 | 9.48 | 3.01 | 1.77 | 1.43 |
| Sample | Elements (%) | Ash (%) | H/C | O/C | (O + N)/C | |||
|---|---|---|---|---|---|---|---|---|
| N | C | H | O | |||||
| B600 | 0.86 | 77.19 | 3.14 | 7.81 | 11.00 | 0.49 | 0.08 | 0.09 |
| Sample | Yield (%) | pH | SSA/(m2·g−1) | PV/(cm3·g−1) | CEC/(cmol·kg−1) | |||
| B600 | 32.41 | 10.61 | 136.13 | 0.1721 | 10.62 | |||
| Level | Roasting Temperature/°C | Roasting Time/Min | Addition Amount of Reducing Agent/wt% |
|---|---|---|---|
| 1 | 600 | 30 | 3 |
| 2 | 700 | 40 | 5 |
| 3 | 800 | 50 | 8 |
| 4 | 900 | 60 | 10 |
| Test No. | Level of Factors | Fe Recovery/% | Fe Grade/% | Efficiency/% | ||
|---|---|---|---|---|---|---|
| Roasting Temperature/°C | Roasting Time/Min | Addition amount of Reducing Agent/% | ||||
| 1 | 600 | 30 | 3 | 59.99 | 58.16 | 31.86 |
| 2 | 600 | 40 | 5 | 52.50 | 58.22 | 26.76 |
| 3 | 600 | 50 | 8 | 72.69 | 56.06 | 38.61 |
| 4 | 600 | 60 | 10 | 77.40 | 55.58 | 41.70 |
| 5 | 700 | 30 | 5 | 72.08 | 56.34 | 38.55 |
| 6 | 700 | 40 | 3 | 76.93 | 51.31 | 34.53 |
| 7 | 700 | 50 | 10 | 59.73 | 58.18 | 31.71 |
| 8 | 700 | 60 | 8 | 78.43 | 51.87 | 36.53 |
| 9 | 800 | 30 | 8 | 86.90 | 41.03 | 23.75 |
| 10 | 800 | 40 | 10 | 77.99 | 49.41 | 32.25 |
| 11 | 800 | 50 | 3 | 71.37 | 56.74 | 38.57 |
| 12 | 800 | 60 | 5 | 85.08 | 46.07 | 31.55 |
| 13 | 900 | 30 | 10 | 55.90 | 60.38 | 31.47 |
| 14 | 900 | 40 | 8 | 53.72 | 65.89 | 35.83 |
| 15 | 900 | 50 | 5 | 54.69 | 60.81 | 31.06 |
| 16 | 900 | 60 | 3 | 55.68 | 65.51 | 37.06 |
| Factor | Average Beneficiation Efficiency at Each Level | Range | Comparatively Excellent Level | |||
|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | |||
| Temperature | 34.71 | 35.33 | 31.53 | 33.86 | 3.80 | 2 |
| Time | 31.41 | 32.32 | 34.99 | 36.71 | 5.30 | 4 |
| Biochar | 35.51 | 31.96 | 33.68 | 34.28 | 3.55 | 1 |
| Product Name | Yield/% | Fe Grade/% | Fe Recovery Rate/% |
|---|---|---|---|
| Rough concentrate | 40.63 | 55.87 | 89.19 |
| Tailings | 59.37 | 4.36 | 10.81 |
| Ore feeding | 100.00 | 25.45 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Li, F.; Jiang, P.; Zhang, S. Preparation of Red Iron by Magnetization Roasting-Hydrothermal Method Using Ultra-Low-Grade Limonite. Sustainability 2023, 15, 4708. https://doi.org/10.3390/su15064708
Xu G, Li F, Jiang P, Zhang S. Preparation of Red Iron by Magnetization Roasting-Hydrothermal Method Using Ultra-Low-Grade Limonite. Sustainability. 2023; 15(6):4708. https://doi.org/10.3390/su15064708
Chicago/Turabian StyleXu, Geng, Fei Li, Peipei Jiang, and Shiqiu Zhang. 2023. "Preparation of Red Iron by Magnetization Roasting-Hydrothermal Method Using Ultra-Low-Grade Limonite" Sustainability 15, no. 6: 4708. https://doi.org/10.3390/su15064708
APA StyleXu, G., Li, F., Jiang, P., & Zhang, S. (2023). Preparation of Red Iron by Magnetization Roasting-Hydrothermal Method Using Ultra-Low-Grade Limonite. Sustainability, 15(6), 4708. https://doi.org/10.3390/su15064708








