Geochemical Characteristics and Factors of Transfer and Accumulation of Rare Earth Elements in Rock-Soil-Tea of the Mengku Tea Region in Yunnan Province, China
Abstract
:1. Introduction
2. Materials and Methods
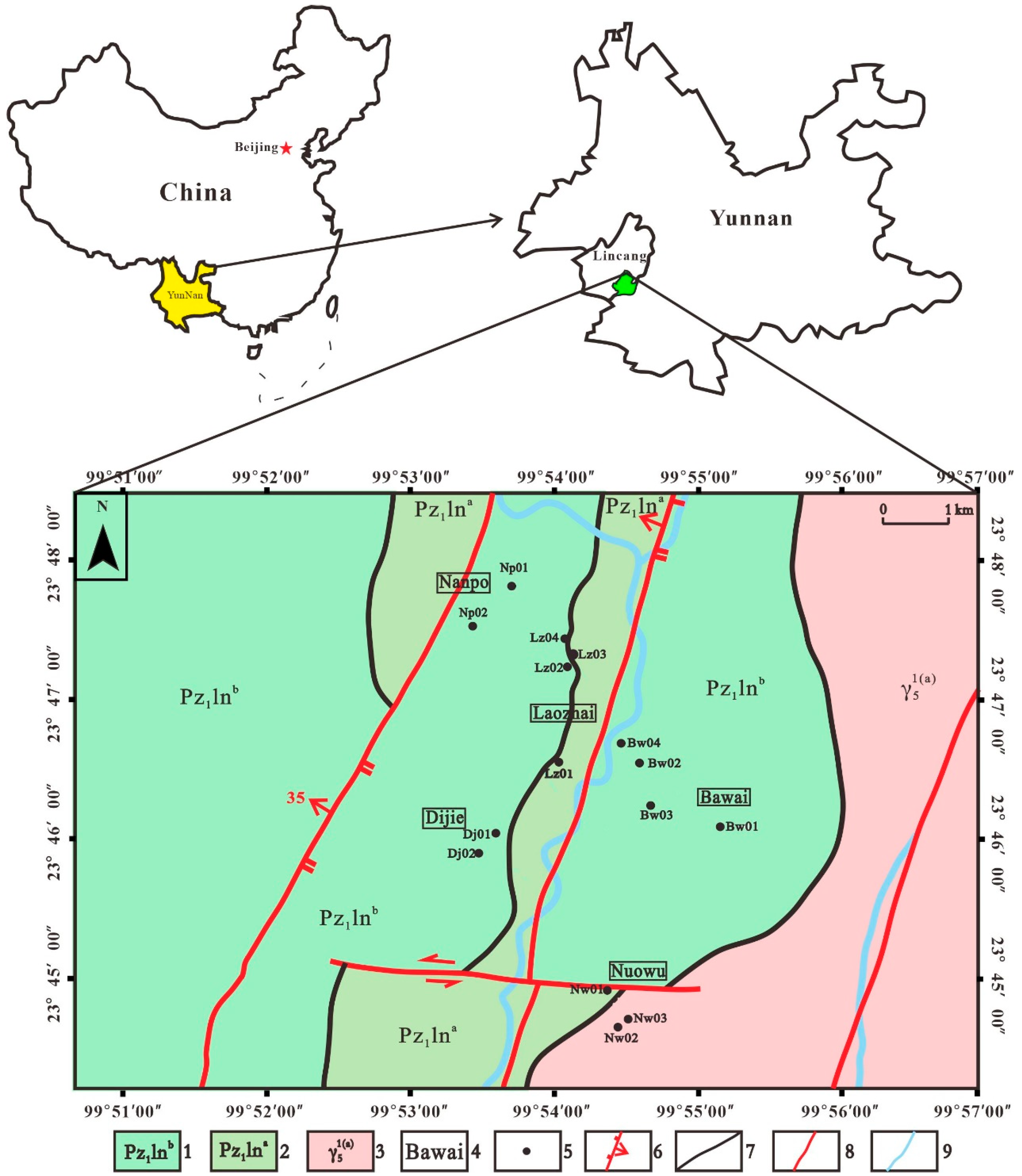
2.1. Study Area
2.2. Samples Collection and Pretreatment
2.3. Sample Test
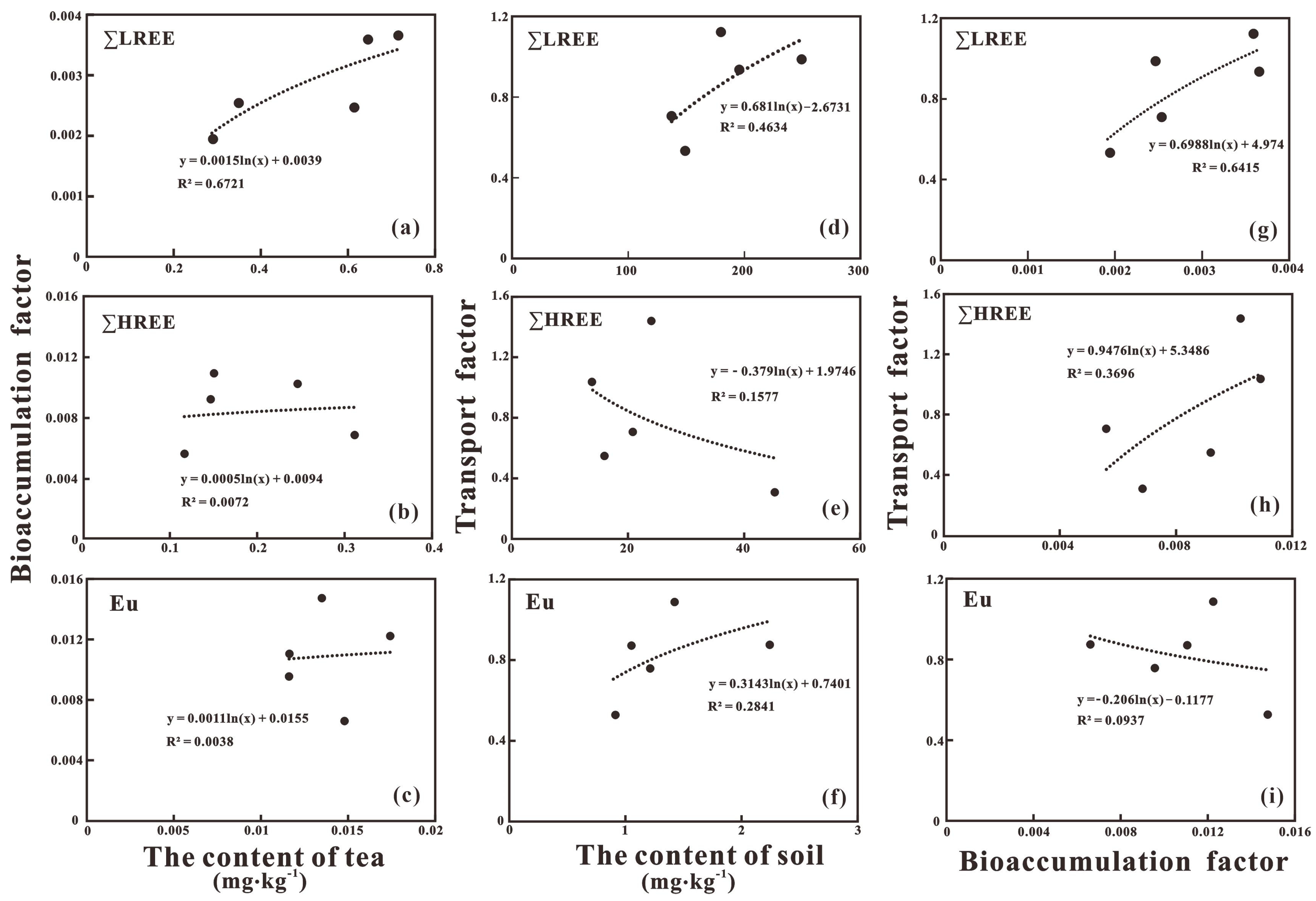
2.4. Bioaccumulation/Transport Factor
2.5. Health Risk Assessment Methods
2.6. Statistical Analysis
3. Results and Discussion
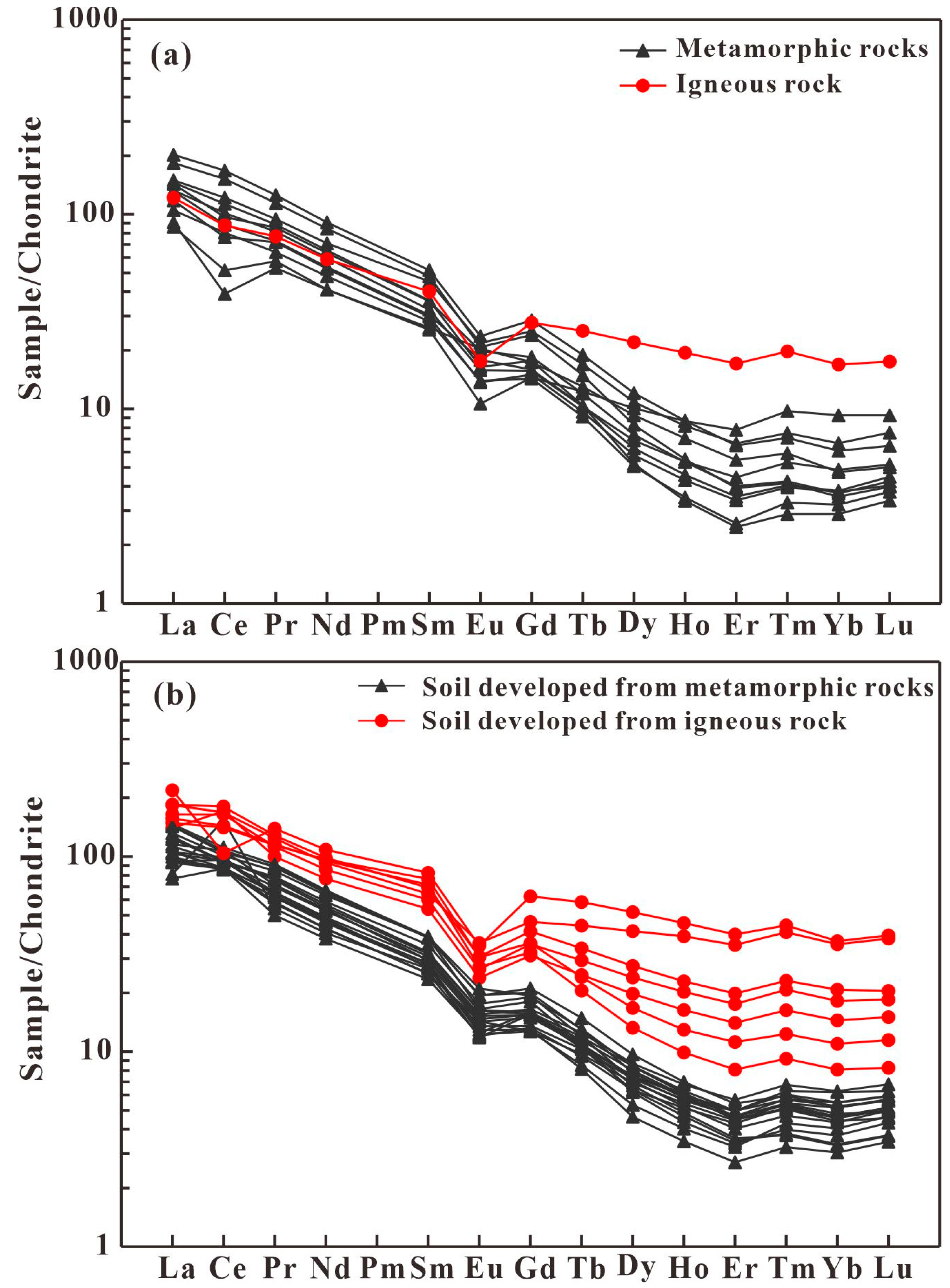
3.1. Geochemical Characteristics of REEs in Tea, Soil, and Rock
3.1.1. Levels of REEs in Tea, Soil, and Rock
| Rock | pH | OM | Soil Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bw01-D | 309 | 280 | 30 | 9.5 | 3.9 | 4.7 | 1.1 | 0.6 | — | — | — |
| Bw03 | 194 | 176 | 18 | 9.9 | 4.1 | 3.2 | 1.0 | 0.7 | — | — | — |
| Bw04 | 276 | 255 | 21 | 12.1 | 3.8 | 6.8 | 1.0 | 0.6 | — | — | — |
| Dj01-D | 208 | 195 | 13 | 14.6 | 4.0 | 6.1 | 1.0 | 0.7 | — | — | — |
| Dj02 | 153 | 141 | 13 | 11.0 | 3.7 | 4.4 | 1.0 | 0.7 | — | — | — |
| Lz01-D | 177 | 160 | 17 | 9.6 | 4.3 | 4.2 | 0.9 | 0.8 | — | — | — |
| Lz02-D | 160 | 145 | 15 | 9.7 | 4.0 | 4.0 | 0.8 | 0.6 | — | — | — |
| Lz03 | 122 | 97 | 24 | 4.0 | 3.5 | 3.6 | 0.6 | 1.0 | — | — | — |
| Lz04 | 198 | 179 | 19 | 9.5 | 4.0 | 5.0 | 0.9 | 0.8 | — | — | — |
| Np01-D | 238 | 209 | 29 | 7.2 | 3.3 | 3.8 | 1.0 | 0.6 | — | — | — |
| Nw01-D | 132 | 106 | 26 | 4.0 | 3.4 | 1.6 | 0.7 | 0.6 | — | — | — |
| Nw03 | 225 | 162 | 62 | 2.6 | 3.0 | 1.6 | 0.9 | 0.5 | — | — | — |
| Mean | 199 | 175 | 24 | 8.6 | 3.8 | 4.1 | 0.9 | 0.7 | — | — | — |
| China a | 235 | 185 | 49 | 3.8 | — | — | — | — | — | — | — |
| UCC b | 169 | 134 | 35 | 3.8 | — | — | — | — | — | — | — |
| Soil | |||||||||||
| Bw01-B | 170 | 149 | 21 | 7.2 | 3.4 | 3.0 | 1.2 | 0.6 | 5.69 | 8.6 | Alfisols |
| Bw01-C | 161 | 141 | 20 | 7.2 | 3.6 | 3.5 | 1.1 | 0.6 | 5.42 | 3.4 | Ultisols |
| Bw02-B | 157 | 140 | 17 | 8.3 | 3.8 | 2.8 | 1.2 | 0.7 | 6.06 | 5.9 | Alfisols |
| Bw02-C | 189 | 170 | 19 | 9.1 | 3.9 | 3.0 | 1.2 | 0.7 | 5.91 | 3.45 | Ultisols |
| Bw02-D | 180 | 158 | 22 | 7.2 | 3.7 | 2.6 | 1.1 | 0.7 | 5.76 | 2.6 | Ultisols |
| Dj01-B | 151 | 137 | 14 | 10.0 | 3.7 | 3.2 | 1.2 | 0.8 | 5.34 | 9.2 | Alfisols |
| Dj01-C | 208 | 189 | 19 | 10.1 | 3.7 | 4.5 | 0.9 | 0.8 | 5.70 | 5.0 | Ultisols |
| Lz01-A | 189 | 168 | 21 | 8.0 | 3.5 | 4.1 | 1.0 | 0.7 | 5.49 | 24.8 | Mollisols |
| Lz01-B | 204 | 180 | 24 | 7.5 | 3.4 | 4.1 | 1.0 | 0.7 | 5.49 | 30.1 | Alfisols |
| Lz01-C | 211 | 190 | 21 | 9.2 | 3.7 | 4.4 | 1.0 | 0.7 | 5.88 | 8.5 | Ultisols |
| Lz02-A | 145 | 128 | 17 | 7.6 | 3.3 | 2.7 | 1.4 | 0.7 | 5.12 | 49.1 | Mollisols |
| Lz02-B | 204 | 186 | 18 | 10.4 | 3.1 | 2.2 | 2.3 | 0.7 | 5.32 | 8.4 | Alfisols |
| Lz02-C | 175 | 155 | 20 | 7.9 | 3.7 | 2.7 | 1.2 | 0.7 | 5.84 | 2.6 | Ultisols |
| Np01-B | 212 | 196 | 16 | 12.3 | 3.8 | 4.9 | 1.0 | 0.6 | 5.07 | 2.6 | Alfisols |
| Np01-C | 159 | 146 | 13 | 11.5 | 3.9 | 4.3 | 1.1 | 0.7 | 5.50 | 1.6 | Ultisols |
| Np02-B | 171 | 153 | 18 | 8.5 | 3.5 | 2.8 | 1.2 | 0.7 | 4.98 | 18.0 | Alfisols |
| Np02-C | 181 | 165 | 16 | 10.5 | 3.9 | 4.6 | 1.0 | 0.7 | 5.12 | 10.9 | Ultisols |
| Np02-D | 183 | 166 | 17 | 9.9 | 3.9 | 4.8 | 1.0 | 0.7 | 5.23 | 3.5 | Ultisols |
| Nw01-A | 304 | 252 | 52 | 4.9 | 2.6 | 2.2 | 1.4 | 0.6 | 5.65 | 15.2 | Mollisols |
| Nw01-B | 365 | 293 | 72 | 4.1 | 2.7 | 2.0 | 1.2 | 0.6 | 5.24 | 8.6 | Alfisols |
| Nw01-C | 327 | 263 | 21 | 4.1 | 2.7 | 2.0 | 1.2 | 0.6 | 5.45 | 8.4 | Ultisols |
| Nw02-A | 287 | 252 | 34 | 7.3 | 2.2 | 4.0 | 1.1 | 0.6 | 4.90 | 12.8 | Mollisols |
| Nw02-B | 295 | 249 | 45 | 5.5 | 1.9 | 3.3 | 1.1 | 0.6 | 5.04 | 12.2 | Alfisols |
| Nw02-C | 406 | 279 | 127 | 2.2 | 2.8 | 1.3 | 1.1 | 0.7 | 5.94 | 1.2 | Ultisols |
| Nw02-D | 400 | 253 | 147 | 1.7 | 2.6 | 1.7 | 0.6 | 0.5 | 5.93 | 1.3 | Ultisols |
| Mean | 225 | 190 | 35 | 7.7 | 3.3 | 3.2 | 1.1 | 0.7 | — | — | — |
| Yunnan soil c | 248 | 195 | 53 | 3.8 | — | — | — | — | — | — | — |
| China soil c | 187 | 148 | 39 | 3.8 | — | — | — | — | — | — | — |
3.1.2. Distribution Patterns of REEs in Rock, Soil, and Tea Samples
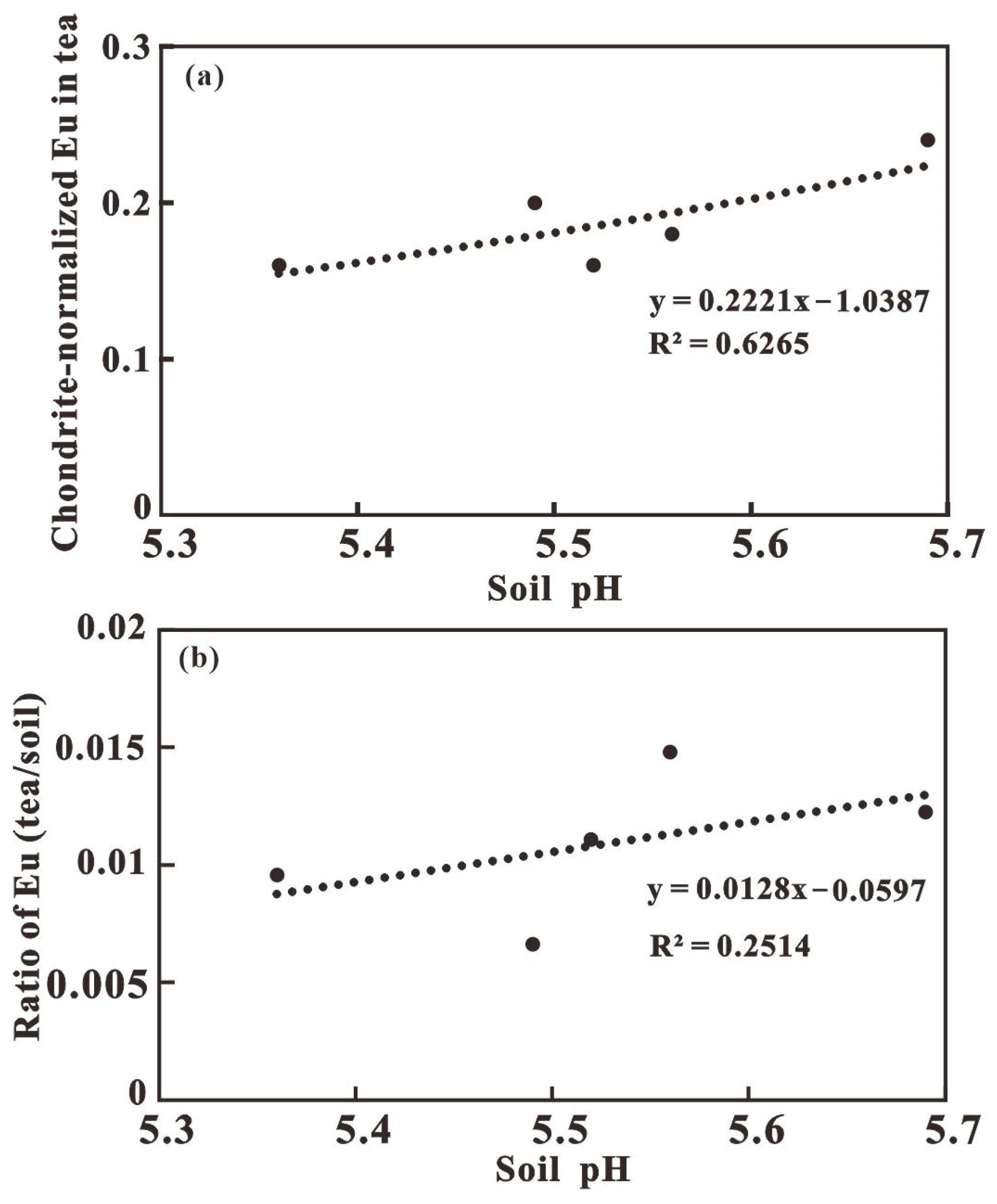
3.2. Transfer of REEs in Rock-Soil-Tea
3.3. Assessment of Potential Risk to Human Health through Tea Drinking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Gu, X.; Lian, M.; Wang, J.; Xin, M.; Wang, B.; Ouyang, W.; He, M.; Liu, X.; Lin, C. Occurrence, geochemical characteristics, enrichment, and ecological risks of rare earth elements in sediments of “the Yellow river-Estuary-bay” system. Environ. Pollut. 2023, 319, 121025. [Google Scholar] [CrossRef]
- Tao, Y.; Shen, L.; Feng, C.; Yang, R.; Qu, J.; Ju, H.; Zhang, Y. Distribution of rare earth elements (REEs) and their roles in plant growth: A review. Environ. Pollut. 2022, 298, 118540. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Zhou, D.; Gao, Z.; Jiang, X.X.; Luo, X.P. Effect of rice husk biochar on bioavailability of rare earth elements in polluted soil. China Environ. Sci. 2018, 38, 3823–3832. [Google Scholar]
- Wang, Y.; Chen, M.; Li, Y.; Liu, Z.; Li, C. Effects of Lanthanum on Morphological and Physiological Characteristics of Tobacco Root under Drought Stress. J. Chin. Soc. Rare Earths 2018, 36, 319–327. [Google Scholar]
- Yang, X.; Liu, Z.; Hu, F.; Lin, Y.; Shen, C. Soil Rare Earth Element and Nitride Pollution on Plant Growth and Physiology. J. Chin. Soc. Rare Earths 2019, 37, 1–11. [Google Scholar]
- Kotelnikova, A.D.; Rogova, O.B.; Stolbova, V.V. Lanthanides in the Soil: Routes of Entry, Content, Effect on Plants, and Genotoxicity (a Review). Eurasian Soil Sci. 2021, 54, 117–134. [Google Scholar] [CrossRef]
- Li, P.W.; Li, J.H.; Chen, S.X.; Meng, X.L. Determination of 16 heavy metal elements and 16 rare earth elements in Ya’an tibet tea by ICP-MS. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012181. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Stella, C.; Mantia, M.; Battuello, M.; Nurra, N.; Sartor, R.M.; Orusa, R.; Robetto, S.; Brusa, F.; et al. Rare earth elements in marine and terrestrial matrices of Northwestern Italy: Implications for food safety and human health. Sci. Total Environ. 2019, 660, 1383–1391. [Google Scholar] [CrossRef]
- Hong, T.; Xie, Y.Q.; Qin, X.M.; Kong, X.S.; Yu, Q.W. Geochemical characteristics and ecological interaction of earth elements in the soil-plant system in northwest Guizhou province. J. Ecol. Rural Environ. 2018, 34, 890–896. [Google Scholar]
- Huang, H.B.; Rui-Lian, Y.U.; Bian, K.; Gong-Ren, H.U.; Qiu, Q.J.; Yin-Jiao, W.U.; Wang, X.M. Geochemical Characteristics of Rare Earth Elements in the Soil-tea System in Tieguanyin Tea Gardens. J. Rare Earths 2018, 39, 141–147. [Google Scholar]
- Luo, X.Z.; Lin, S. Migration of rare earth elements in organic tea garden from soil through tea leaves to tea soup. J. Food Saf. Qual. 2017, 8, 265–269. [Google Scholar]
- Pourret, O.; Lange; Martinez, R.E.; Wiche, O.; Faucon, M.P. Relationships Between Soil Chemical Properties and Rare Earth Element Concentrations in the Aboveground Biomass of a Tropical Herbaceous Plant; Hal Open Science: Lyon, France, 2019. [Google Scholar]
- Turniški, R.; Zupančič, N.; Grčman, H. Geochemical evidence of illuvial processes in clay-rich soils on limestones in a humid temperate climate. Geoderma 2023, 429, 116266. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Liu, L.; Heng, W.; Chen, C.; Long, J.; Wen, X. Geochemical characteristics of heavy metals of bedrock, soil, and tea in a metamorphic rock area of Guizhou Province, China. Environ. Sci. Pollut. Res. 2022, 30, 7402–7414. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Zhang, Y.; Analysis, R. Distribution Characteristics of Rare Earth Elements in Plants and Soils from the Bayan Obo Mining Area. Rock Miner. Anal. 2019, 40, 871–882. [Google Scholar]
- Tong, C.; He, S.; Ding, H. Research progress of element and isotope identification technology for the origin and quality of tea. Chin. J. Ecol. 2018, 37, 10. [Google Scholar]
- Zheng, M.; Tang, D.; Xu, A. Attribute-Driven or Green-Driven: The Impact of Subjective and Objective Knowledge on Sustainable Tea Consumption. Foods 2023, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Givelet, L.; Amlund, H.; Sloth, J.J.; Hansen, M. Risk assessment of rare earth elements, antimony, barium, boron, lithium, tellurium, thallium and vanadium in teas. EFSA J. 2022, 20, e200410. [Google Scholar] [CrossRef]
- Huang, X.G.; Dou, J.Z.; Wu, G.H.; He, J.; Chen, F. Source rocks control the geochemical diversity of granite: The Lincang pluton in the western Yunnan Tethyan belt, SW China. Lithos 2020, 382–383, 105950. [Google Scholar] [CrossRef]
- Sabir, M.; Baltrenaite-Gediene, E.; Ditta, A.; Ullah, H.; Kanwal, A.; Ullah, S.; Faraj, T.K. Bioaccumulation of Heavy Metals in a Soil-Plant System from an Open Dumpsite and the Associated Health Risks through Multiple Routes. Sustainability 2022, 14, 13223. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Risk assessment guidance for superfund: Volume II environmental evaluation manual, interim final. Saúde Pública 1989, 804, 636–640. [Google Scholar]
- US EPA National Center for Environmental Assessment. Exposure Factors Handbook 2011 Edition (Final Report); U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Peng, C.Y.; Zhu, X.H.; Hou, R.Y.; Ge, G.F.; Hua, R.M.; Wan, X.C.; Cai, H.M. Aluminum and Heavy Metal Accumulation in Tea Leaves: An Interplay of Environmental and Plant Factors and an Assessment of Exposure Risks to Consumers. J. Food Sci. 2018, 83, 1165. [Google Scholar] [CrossRef]
- Hartman, T.J.; Knight, C.A.; Teplansky, R.; Mitchell, D.C.; Hockenberry, J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar]
- Tao, C.; Song, Y.; Chen, Z.; Zhao, W.; Frost, R.L. Geological load and health risk of heavy metals uptake by tea from soil: What are the significant influencing factors? Catena 2021, 204, 105419. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Radionuclide Table: Radionuclide Carcinogenicity Slope Factors; United States Environmental Protection Agency (EPA): Washington, DC, USA, 2001.
- US EPA Office of Radiation and Indoor Air (ORIA), Radiation Protection Division. Radionuclide Carcinogenicity Slope Factors: Heast; US EPA Office of Radiation and Indoor Air (ORIA): Washington, DC, USA, 2001.
- Li, T. Element Abundances of China's Continental Crust and Its Sedimentary Layer and Upper Continental Crust. J. Geochem. 1995, 14, 7. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust; University of Maryland: College Park, MD, USA; China University of Geosciences: Xi’an, China, 2013; Volume 4. [Google Scholar]
- Morgenstern, R.; Turnbull, R.E.; Ashwell, P.A.; Horton, T.W.; Oze, C. Petrological and geochemical characteristics of REE mineralization in the A-type French Creek Granite, New Zealand. Miner. Depos. 2018, 54, 935–958. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Liu, J.; Deng, K.; Liu, Y.; Wei, T.; Ran, W. Geochemical characteristics and significance of trace elements in Caijiaba bauxite deposit, Guizhou Province. Chin. J. Geol. 2022, 57, 879–896. [Google Scholar]
- Li, C.; Zhang, C.; Qin, H.; Jiang, J. Geochemical characteristics of REE and trace elements compositions of Poyan antimony deposit in Guangxi. Miner. Resour. Geol. 2018, 32, 1035–1042. [Google Scholar]
- Chen, Z.Y.; Chen, Y.L.; Shang-Yi, G.U.; Zheng, C.Y.; Ji, L.I.; Long, W.U. Geochemical characteristics and significance of REE and trace elements compositions of Kengtou gold deposit in southwestern margin of Jiangnan orogenic belt. Miner. Explor. 2019, 10, 567–574. [Google Scholar]
- Yue, C.; Yu, F.; Zhang, X. Geochemical Characteristics of Igneous Rock around the Baihebu Reservoir in Yanqing, Beijing. Acta Geol. Sichuan 2022, 42, 282–287+295. [Google Scholar]
- Lu, L.; Liu, Y.; Liu, H.; Zhao, Z.; Xu, X. Research from Hefei University of Technology Has Provided New Data on Mineral Research (Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China). Minerals 2020, 10, 1116. [Google Scholar] [CrossRef]
- Zhao, F.; Li, G.; Zhang, P.; Wang, C.; Tang, X. Petrogenesis and tectonic implications of the Lincang batholith in the Sanjiang, Southwest China: Constraints by geochemistry, zircon U-Pb chronology and Hf isotope. Minerals 2018, 8, 516. [Google Scholar]
- China National Environmental Monitoring Centre. China Environmental Background Values of Soil; China Environmental Press: Beijing, China, 1990. [Google Scholar]
- Boynton, W.V. Cosmochemistry of the Rare Earth Elements: Meteorite Studies. Dev. Geochem. 1984, 3, 114. [Google Scholar]
- Wang, H.; Chen, X.; Ye, J.; Jia, X.; Zhang, Q.; He, H. Analysis of the absorption and accumulation characteristics of rare earth elements in Chinese tea. J. Sci. Food Agric. 2020, 100, 3360–3369. [Google Scholar] [CrossRef]
- Yuan, L.; Guo, X.; Wei, Y.; Tianhua, T.U.; Liao, Q.; Zhang, L.; Dong, Q.; Xiang, J. Compositions and health risk assessment of rare-earth elements in soil, animal and plant products around rare earth mining area in Southern Jiangxi Province. Environ. Chem. 2019, 38, 1850–1863. [Google Scholar]
- Miao, L.; Ma, Y.; Xu, R.; Yan, W. Environmental biogeochemical characteristics of rare earth elements in soil and soil-grown plants of the Hetai goldfield, Guangdong Province, China. Environ. Earth Sci. 2010, 63, 501–511. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Z.; Chen, Z.; Feng, L. Distribution and Enrichment of Rare Earth Elements of Pinus massoniana in Ionic Type Rare Earth Mining Area, Southern China. J. Chiness Soc. Rare Earths 2020, 38, 564–572. [Google Scholar]
- Gao, S.; He, P.; Lin, T.; Liu, H.; Guo, B.; Lin, H.; Hu, Y.; Chen, Q.; Xiang, P.; Zou, L. Consecutive soybean (Glycine max) planting and covering improve acidified tea garden soil. PLoS ONE 2021, 16, e0254502. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; Yong, H. Tea saponins: Effective natural surfactants beneficial for soil remediation, from preparation to application. RSC Adv. 2018, 8, 24312–24321. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, G.; Liu, M.; Wang, L. Geochemical Characteristics of Rare Earth Elements in Soils from Puding Karst Critical Zone Observatory, Southwest China. Sustainability 2019, 11, 4963. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, W.; Xue, Y.U.; Mengxi, Q.I.; Sun, C. The Space Distribution Characteristics Study of Rare Earth Elements in the Surface Soil of Urban Areas in Beijing. Ecol. Environ. Sci. 2017, 26, 1736–1746. [Google Scholar]
- Liu, Y.; Luo, K.; Yuan, Y. Content and Spatial Distribution Characteristics of Rare Earth of Surface Soil in Jiangjin District, Chongqing City. JOurnal Chin. Soc. Rare Earths 2020, 38, 10. [Google Scholar]
- Xie, Z.; Zhou, Z.; Quan, L.; Chu, G.; Wang, C.; Zhang, W.; Xu, Y. Content characteristics of rare earth elements in tea in Shiqian tea producing region, Guizhou province. Oper. Technol. 2020, 229, 72–73. [Google Scholar]
- Luo, C. Study on the Content of Rare Earth Elements in Tea in Mianyang City, Sichuan Province. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2019. [Google Scholar]
- Yu, Y.-F.; Huang, L. Rapid Mass Spectrometric Analysis and Quality Control of 16 Rare Earth Elements in Broadleaf holly Leaf. Anhui Agric.Sci. 2019, 47, 4. [Google Scholar]
- Liu, X.-R.; Liu, W.-S.; Tang, Y.-T.; Wang, S.-Z.; Cao, Y.-J.; Chen, Z.-W.; Xie, C.-D.; Liu, C.; Guo, M.-N.; Qiu, R.-L. Effects of in situ leaching on the origin and migration of rare earth elements in aqueous systems of South China: Insights based on REE patterns, and Ce and Eu anomalies. J. Hazard. Mater. 2022, 435, 128959. [Google Scholar] [CrossRef]
- Lee, H.M.; Lee, S.G.; Kim, H.; Lee, J.I.; Mi, J.L. REE Tetrad Effect and Sr-Nd Isotope Systematics of A-Type Pirrit Hills Granite from West Antarctica. Minerals 2021, 11, 792. [Google Scholar] [CrossRef]
- Pourret, O.; Van der Ent, A.; Hursthouse, A.; Irawan, D.E.; Liu, H.Y.; Wiche, O. The 'europium anomaly' in plants: Facts and fiction. Plant Soil 2022, 476, 721–728. [Google Scholar] [CrossRef]
- Qiu, Q. Characteristics of Metals and Isotopic Tracing Research in Soil-Tea System from Typical Tea Gardens in Fujian Province; Huaqiao University: Quanzhou, China, 2018. [Google Scholar]
- Xie, Z.; Li, G.; Wang, X.; Wu, C.; Quan, L.; Wang, R.; Qiang, X. Monitoring and Pollution Assessment of Heavy Metals in Soil and Tea in Shiqiantai Tea Producing Area of Guizhou Province. J. Agric. Catastropholgy 2020, 10, 3. [Google Scholar]
- Zhao, S. Key Points of Kuding Tea Cultivation Technology. Agric. Technol. Ext. China 2002, 2, 44. [Google Scholar]
- Yang, J.; Hu, J.; Zhang, T.; Peng, B.; Deng, Z. A Preliminary Study of Physical and Chemical Properties of Davidia involucrata Soil in Beichuan Piankou Nature Reserve. J. Sichuan For. Sci. Technol. 2013, 3, 40–44. [Google Scholar]
- Luo, X.; Wu, J.; Jin, Y.; Wang, Y.; Gu, B. Soil Nutrient and Vegetation Restoration on Earthquake-stricken Slopes in Beichuan County, Sichuan Province. Bull. Soil Water Conserv. 2017, 37, 7. [Google Scholar]
- Shtangeeva, I.; Ayrault, S. Effects of Eu and Ca on yield and mineral nutrition of wheat (Triticum aestivum) seedlings. Environ. Exp. Bot. 2007, 59, 49–58. [Google Scholar] [CrossRef]
- Brioschi, L.; Steinmann, M.; Lucot, E.; Pierret, M.C.; Stille, P.; Prunier, J.; Badot, P.M. Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant Soil 2013, 366, 143–163. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, L.; Chen, Y.; Jiang, C.; Chen, X.; Chen, Y. Geochemical characteristics of rare earth elements in surface sediments from Huainan coal mining subsidence area, Anhui Province. Coal Geol. Explor. 2022, 50, 7. [Google Scholar]
- Zheng, Y.; Gu, L.; Tang, X.; Wu, C.; Li, H.; Liu, S. Element mobilization and mass-change quantification of highly metamorphosed footwall alteration zones in Hongtoushan voicanogenic massive sulfide deposit, Liaoning Province. Miner. Depos. 2010, 5, 25. [Google Scholar]
- Yuan, M.; Guo, M.N.; Liu, W.S.; Liu, C.; Ent, A.V.D.; Morel, J.L.; Huot, H.; Zhao, W.Y.; Wei, X.G.; Qiu, R.L. The accumulation and fractionation of Rare Earth Elements in hydroponically grown Phytolacca americana L. Plant Soil. 2017, 421, 67–82. [Google Scholar] [CrossRef]
- Yuan, M.; Chang, L.; Liu, W.; Guo, M.; Morel, J.L.; Huot, H.; Yu, H.; Tang, Y.; Qiu, R. Accumulation and fractionation of rare earth elements (REEs) in the naturally grown Phytolacca americana L. in southern China. Int. J. Phytoremediation 2018, 20, 415–423. [Google Scholar] [CrossRef]
- Han, F.; Shan, X.Q.; Zhang, J.; Xie, Y.N.; Pei, Z.G.; Zhang, S.Z.; Zhu, Y.G.; Wen, B. Organic acids promote the uptake of lanthanum by barley roots. New Phytol. 2005, 165, 481–492. [Google Scholar] [CrossRef]
- Latteo, V.; Cibella, F.; Censi, P.; Saiano, E.; Falcone, E. Rare earths and trace elements contents in leaves: A new indicator of the composition of atmospheric dust. Chemosphere: Environ. Toxicol. Risk Assess. 2017, 169, 342–350. [Google Scholar]
- Zeng, F.L.; Tian, H.E.; Wang, Z.P.; An, Y.; Gao, F.Y.; Zhang, L.J.; Li, F.M.; Shan, L. Effect of rare earth element europium on amaranthin synthesis in Amarathus caudatus seedlings. Biol. Trace Elem. Res. 2003, 93, 271–282. [Google Scholar] [CrossRef]
- Censi, P.; Saiano, F.; Pisciotta, A.; Tuzzolino, N. Geochemical behaviour of rare earths in Vitis vinifera grafted onto different rootstocks and growing on several soils. Sci. Total Environ. 2014, 473-474, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.E.; Pourret, O.; Faucon, M.P.; Dian, C. Effect of rare earth elements on rice plant growth. Chem. Geol. 2018, 489, 28–37. [Google Scholar] [CrossRef]
- Shaban, G.; Idrus, A.; Fadlin, F.; Priadi, B.; Basuki, N.I. Saprolitization’s Characteristics of Rare Earth Elements in Volcanic Regolith on Drill Core #65 in Western Sulawesi, Indonesia. Asian J. Appl. Sci. 2019, 7, 435–450. [Google Scholar]
- Zhu, W.; Xu, S.; Shao, P.; Zhang, H.; Feng, j.; Wu, D.; Yang, W. Investigation on intake allowance of rare earth-A study on bio-effect of rare earth in South Jiangxi. China Environ. Sci. 1997, 17, 4. [Google Scholar]
- Zhou, Y. Characteristics of Rare Earth Elements in Tea from Chinese Market and Their Exposure Risk Assessment. Master’s Thesis, Huazhong University of Science and Technology, Huazhong, China, 2020. [Google Scholar]
- Loell, M.; Albrecht, C.; Felix-Henningsen, P. Rare earth elements and relation between their potential bioavailability and soil properties, Nidda catchment (Central Germany). Plant Soil 2011, 349, 303–317. [Google Scholar] [CrossRef]
- Thorsten, R.; Daus, B.; Saatz, J.; Vetterlein, D.; Richnow, H. Location and speciation of gadolinium and yttrium in roots of Zea mays by LA-ICP-MS and ToF-SIMS. Environ. Pollut. 2016, 216, 245–252. [Google Scholar]
- Weber, R.J. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues. US EPA Region 2012, 8, 189–200. [Google Scholar]
- Tyler, G.; Olsson, T. Plant uptake of major and minor mineral elements as influenced by soil acidity and liming. Plant Soil 2001, 230, 307–321. [Google Scholar] [CrossRef]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Khan, A.M.; Abu Bakar, N.K.; Abu Bakar, A.F.; Ashraf, M.A. Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: A review. Environ. Sci. Pollut. Res. 2017, 24, 22764–22789. [Google Scholar] [CrossRef] [PubMed]


| Elements | Bw | Dj | Lz | Np | Nw | Mean |
|---|---|---|---|---|---|---|
| Na | 45.340 | 50.590 | 80.490 | 64.460 | 49.160 | 58.008 |
| Mg | 2584 | 2074 | 2308 | 2387 | 3302 | 2531 |
| K | 15,420 | 11,050 | 13,830 | 12,880 | 16,040 | 13,844 |
| Ca | 4357 | 4536 | 4647 | 4265 | 4352 | 4429 |
| La | 0.059 | 0.081 | 0.231 | 0.157 | 0.207 | 0.147 |
| Ce | 0.137 | 0.162 | 0.171 | 0.343 | 0.188 | 0.200 |
| Pr | 0.017 | 0.017 | 0.041 | 0.036 | 0.037 | 0.030 |
| Nd | 0.050 | 0.064 | 0.156 | 0.139 | 0.137 | 0.109 |
| Sm | 0.015 | 0.013 | 0.030 | 0.029 | 0.031 | 0.024 |
| Eu | 0.014 | 0.012 | 0.017 | 0.012 | 0.015 | 0.014 |
| Gd | 0.014 | 0.015 | 0.033 | 0.023 | 0.031 | 0.023 |
| Tb | 0.005 | 0.003 | 0.005 | 0.003 | 0.005 | 0.004 |
| Dy | 0.013 | 0.015 | 0.025 | 0.017 | 0.031 | 0.020 |
| Ho | 0.005 | 0.003 | 0.005 | 0.003 | 0.006 | 0.004 |
| Er | 0.008 | 0.010 | 0.014 | 0.009 | 0.018 | 0.012 |
| Tm | 0.004 | 0.002 | 0.002 | 0.001 | 0.003 | 0.002 |
| Yb | 0.008 | 0.009 | 0.011 | 0.009 | 0.016 | 0.011 |
| Lu | 0.003 | 0.002 | 0.002 | 0.001 | 0.002 | 0.002 |
| Y | 0.057 | 0.092 | 0.149 | 0.080 | 0.198 | 0.115 |
| ∑REE | 0.407 | 0.499 | 0.891 | 0.862 | 0.924 | 0.717 |
| ∑LREEs | 0.290 | 0.349 | 0.646 | 0.715 | 0.615 | 0.523 |
| ∑HREEs | 0.117 | 0.150 | 0.245 | 0.147 | 0.310 | 0.194 |
| ∑LREEs/∑HREEs | 2.487 | 2.327 | 2.634 | 4.881 | 1.984 | 2.863 |
| Principal Component | ||||
|---|---|---|---|---|
| Elements | 1 | 2 | 3 | 4 |
| Ca | 0.113 | 0.070 | −0.167 | 0.977 |
| K | 0.174 | 0.299 | 0.908 | −0.236 |
| Mg | 0.071 | 0.677 | 0.588 | −0.437 |
| Na | 0.869 | −0.166 | −0.248 | 0.394 |
| La | 0.844 | 0.524 | −0.006 | 0.111 |
| Ce | 0.499 | −0.171 | −0.483 | −0.698 |
| Pr | 0.933 | 0.351 | −0.028 | −0.078 |
| Nd | 0.916 | 0.374 | −0.132 | −0.066 |
| Sm | 0.881 | 0.419 | 0.023 | −0.218 |
| Eu | 0.573 | 0.284 | 0.508 | 0.577 |
| Gd | 0.819 | 0.557 | 0.098 | 0.099 |
| Tb | 0.236 | 0.264 | 0.923 | 0.154 |
| Dy | 0.496 | 0.845 | 0.182 | 0.089 |
| Ho | 0.062 | 0.643 | 0.758 | 0.092 |
| Er | 0.293 | 0.930 | 0.195 | 0.103 |
| Tm | −0.381 | −0.099 | 0.919 | 0.036 |
| Yb | 0.240 | 0.959 | 0.152 | −0.002 |
| Lu | −0.334 | 0.047 | 0.941 | −0.005 |
| Y | 0.387 | 0.900 | 0.126 | 0.157 |
| Cumulative variance contribution rate | 32.13% | 61.16% | 87.71% | 100.00% |
| Elements | pH | K | Ca | Mg | Na | Organic Matter |
|---|---|---|---|---|---|---|
| La | −0.466 | −0.785 | 0.618 | 0.853 | 0.634 | 0.610 |
| Ce | −0.678 | 0.072 | −0.495 | −0.193 | −0.136 | −0.454 |
| Pr | −0.511 | −0.724 | 0.462 | 0.709 | 0.506 | 0.464 |
| Nd | −0.563 | −0.669 | 0.439 | 0.692 | 0.489 | 0.446 |
| Sm | −0.597 | −0.769 | 0.357 | 0.718 | 0.591 | 0.346 |
| Eu | 0.270 | −0.765 | 0.946 * | 0.787 | 0.472 | 0.919 * |
| Gd | −0.436 | −0.850 | 0.634 | 0.893 * | 0.694 | 0.615 |
| Tb | 0.314 | −0.809 | 0.560 | 0.621 | 0.568 | 0.486 |
| Dy | −0.488 | −0.886 * | 0.566 | 0.982 ** | 0.923 * | 0.516 |
| Ho | 0.020 | −0.846 | 0.475 | 0.774 | 0.836 | 0.384 |
| Er | −0.470 | −0.830 | 0.505 | 0.957 * | 0.964 ** | 0.444 |
| Tm | 0.616 | −0.244 | 0.082 | 0.002 | 0.124 | 0.012 |
| Yb | −0.557 | −0.796 | 0.391 | 0.917 * | 0.978 ** | 0.328 |
| Lu | 0.504 | −0.372 | 0.115 | 0.138 | 0.281 | 0.034 |
| Y | −0.478 | −0.824 | 0.566 | 0.973 ** | 0.926 * | 0.515 |
| ADI | HQ | Risk | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| La | 1.08 × 10−5 | 4.26 × 10−5 | 2.71 ×10−5 | 5.41 × 10−4 | 2.13 × 10−3 | 1.36 × 10−3 | 1.19 × 10−16 | 4.69 ×10−16 | 2.98 × 10−16 |
| Ce | 2.52 × 10−5 | 6.33 × 10−5 | 3.69 ×10−5 | — | — | — | 8.88 × 10−16 | 2.23 × 10−15 | 1.30 × 10−15 |
| Pr | 3.12 × 10−6 | 7.62 × 10−6 | 5.48 ×10−6 | 6.24 × 10−6 | 1.52 × 10−5 | 1.10 × 10−5 | 2.47 × 10−17 | 6.04 × 10−17 | 4.34 × 10−17 |
| Nd | 9.19 × 10−6 | 2.87 × 10−5 | 2.01 ×10−5 | 1.84 × 10−5 | 5.75 × 10−5 | 4.03 × 10−5 | 5.00 × 10−18 | 1.56 × 10−17 | 1.10 × 10−17 |
| Sm | 2.49 × 10−6 | 5.69 × 10−6 | 4.36 × 10−6 | 4.98 × 10−6 | 1.14 × 10−5 | 8.73 × 10−6 | 9.32 × 10−17 | 2.13 × 10−16 | 1.63 × 10−16 |
| Eu | 2.14 × 10−6 | 3.21 × 10−6 | 2.54 × 10−6 | 1.07 × 10−3 | 1.61 × 10−3 | 1.27 × 10−3 | 2.21 × 10−17 | 3.31 × 10−17 | 2.62 × 10−17 |
| Gd | 2.57 × 10−6 | 6.07 × 10−6 | 4.29 × 10−6 | — | — | — | 3.90 × 10−18 | 9.23 × 10−18 | 6.53 × 10−18 |
| Tb | 4.98 × 10−7 | 9.60 × 10−7 | 7.75 × 10−7 | — | — | — | 2.43 × 10−18 | 4.68 × 10−18 | 3.78 × 10−18 |
| Dy | 2.36 × 10−6 | 5.69 × 10−6 | 3.70 × 10−6 | — | — | — | 9.78 × 10−19 | 2.35 × 10−18 | 1.53 × 10−18 |
| Ho | 5.72 × 10−7 | 1.13 × 10−6 | 8.16 × 10−7 | — | — | — | 5.27 × 10−18 | 1.04 × 10−17 | 7.52 × 10−18 |
| Er | 1.55 × 10−6 | 3.32 × 10−6 | 2.19 × 10−6 | — | — | — | 3.92 × 10−18 | 8.41 × 10−18 | 5.53 × 10−18 |
| Tm | 2.58 × 10−7 | 6.65 × 10−7 | 4.14 × 10−7 | — | — | — | 1.81 × 10−19 | 4.65 × 10−19 | 2.89 × 10−19 |
| Yb | 1.40 × 10−6 | 2.88 × 10−6 | 1.93 × 10−6 | — | — | — | 5.61 × 10−18 | 1.15 × 10−17 | 7.71 × 10−18 |
| Lu | 2.58 × 10−7 | 5.72 × 10−7 | 3.88 × 10−7 | 2.87 × 10−4 | 6.36 × 10−4 | 4.31 × 10−4 | 9.12 × 10−19 | 2.02 × 10−18 | 1.37 × 10−18 |
| Y | 1.06 × 10−5 | 3.65 × 10−5 | 2.13 × 10−5 | 2.65 × 10−3 | 9.12 × 10−3 | 5.31 × 10−3 | 1.92 × 10−16 | 6.61 × 10−16 | 3.85 × 10−16 |
| Total∑ | 7.31 × 10−5 | 2.09 × 10−4 | 1.32 × 10−4 | 4.58 × 10−3 | 1.36 × 10−2 | 8.43 × 10−3 | 1.37 × 10−15 | 3.73 × 10−15 | 2.26 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Chang, H.; Zhou, X.; Zhu, J.; Chen, Z.; Yang, T.; Long, K.; Song, Y. Geochemical Characteristics and Factors of Transfer and Accumulation of Rare Earth Elements in Rock-Soil-Tea of the Mengku Tea Region in Yunnan Province, China. Sustainability 2023, 15, 4836. https://doi.org/10.3390/su15064836
Xie M, Chang H, Zhou X, Zhu J, Chen Z, Yang T, Long K, Song Y. Geochemical Characteristics and Factors of Transfer and Accumulation of Rare Earth Elements in Rock-Soil-Tea of the Mengku Tea Region in Yunnan Province, China. Sustainability. 2023; 15(6):4836. https://doi.org/10.3390/su15064836
Chicago/Turabian StyleXie, Mengli, He Chang, Xiaohua Zhou, Jieyong Zhu, Zhong Chen, Tianfu Yang, Kun Long, and Yinxian Song. 2023. "Geochemical Characteristics and Factors of Transfer and Accumulation of Rare Earth Elements in Rock-Soil-Tea of the Mengku Tea Region in Yunnan Province, China" Sustainability 15, no. 6: 4836. https://doi.org/10.3390/su15064836






