Altitudinal Distribution, Seasonal Dynamics and Borrelia burgdorferi Sensu Lato Infections in Hard Ticks (Acari: Ixodidae) in Different Forest Communities in Inland Croatia
Abstract
1. Introduction
2. Study Area
3. Materials and Methods
3.1. Tick Sampling and Identification
3.2. Borrelia burgdorferi s.l. Detection
3.3. Statystical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Tick Sampling Sites













Appendix B
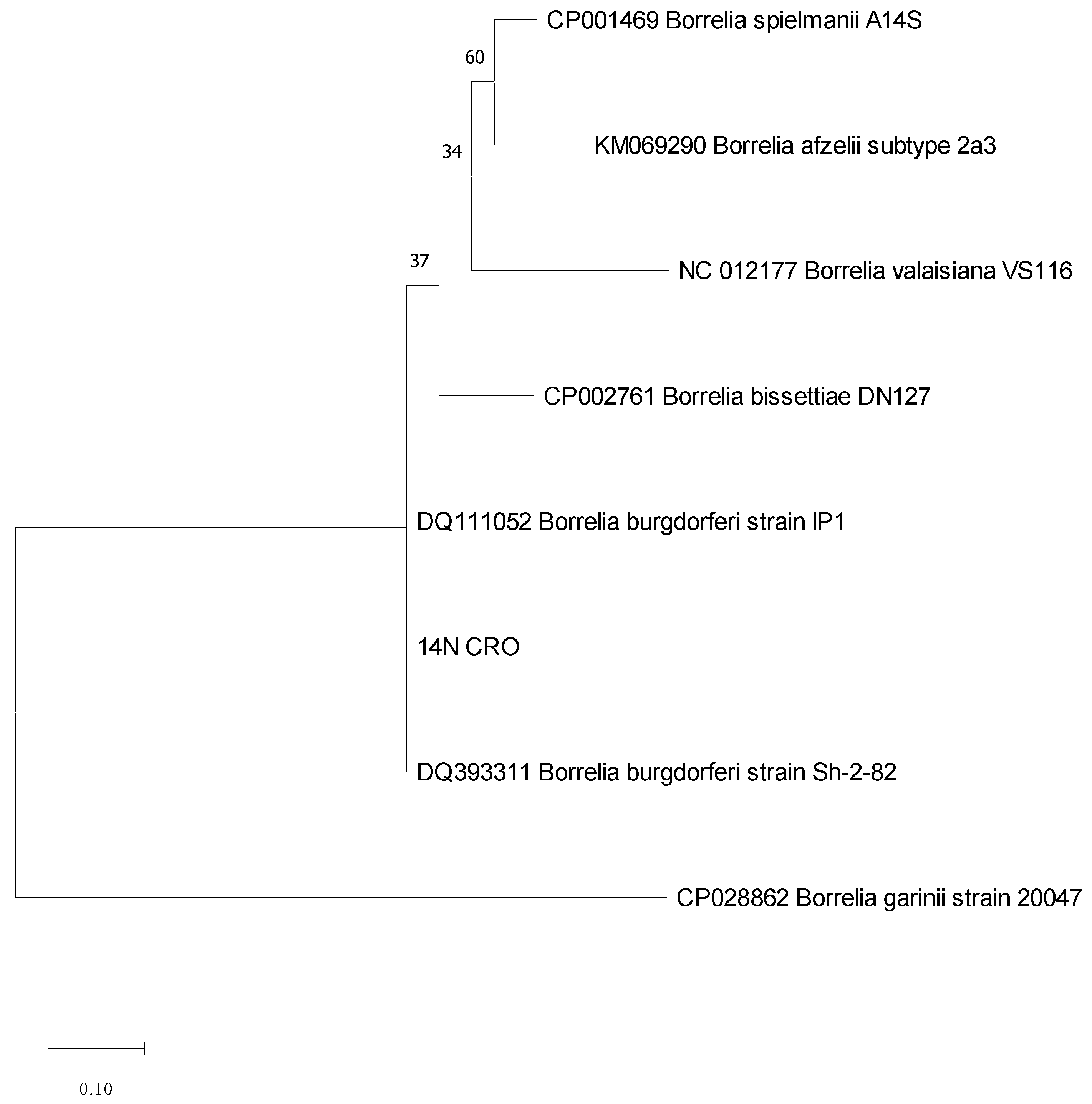
References
- Smith, T.; Kilbourne, F.L. Investigations into the nature, causation and prevention of Texas or southern cattle fever. Bull. Bur. Anim. Ind. 1893, 1, 177–304. [Google Scholar]
- Dutton, J.E.; Todd, J.L. The nature of tick fever in the eastern part of Congo Free State, with notes on the distribution and bionomics of the tick. Br. Med. J. 1905, 2, 1259–1260. [Google Scholar]
- Dennis, D.T.; Piesman, J.F. Overview of Tick-Borne Infections of Humans. In Tick-Borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 3–11. [Google Scholar]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Takkinen, J. Editorial team Collective. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Euro Surveill. 2006, 11, 2977. [Google Scholar] [CrossRef]
- Bacon, R.M.; Kugeler, K.J.; Mead, P.S. Surveillance for Lyme disease—United States, 1992–2006. MMWR Surveill. Summ. 2008, 57, SS-10. [Google Scholar]
- Mysterud, A.; Jore, S.; Østerås, O.; Viljugrein, H. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Sci. Rep. 2017, 7, 16316. [Google Scholar] [CrossRef]
- Bajer, A.; Beck, A.; Beck, R.; Behnke, J.M.; Dwuznik-Szarek, D.; Eichenberger, R.M.; Farkas, R.; Fuehrer, H.P.; Heddergott, M.; Jokelainen, P.; et al. Babesiosis in Southern, Central and Northeastern Europe: An Emerging and Re-Emerging Tick-Borne Disease of Humans and Animals. Microorganisms 2022, 10, 945. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljeva, I.; Jaenson, T.G.T.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Snegiriovaite, J.; Radzijevskaja, J.; Palauskas, A. A brief review: The prevalence of tick borne pathogens in urban and suburban areas. Biologia 2020, 66, 242–255. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Tabor, G.M. Global factors driving emerging infectious diseases: Impact on wildlife populations. Ann. N. Y. Acad. Sci. 2008, 1149, 1–3. [Google Scholar] [CrossRef]
- Spielman, A. The Emergence of Lyme Disease and human Babesiosis in a changing environment. Ann. N. Y. Acad. Sci. 1994, 740, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Maffey, G.L.; Ramsay, S.L.; Hester, A.J. The effect of deer management on the abundance of Ixodes ricinus in Scotland. Ecol. Appl. 2012, 22, 658–667. [Google Scholar] [CrossRef]
- Piesman, J.; Gern, L. Lyme borreliosis in Europe and North America. Parasitology 2004, 129, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L.; Alpers, K.; Bown, K.J.; Martin, S.J.; Birtles, R.J. Use of mass-participation outdoor events to assess human exposure to tickborne pathogens. Emerg. Infect. Dis. 2017, 23, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Mrljak, V.; Kuleš, J.; Mihaljević, Ž.; Torti, M.; Gotić, J.; Crnogaj, M.; Živičnjak, T.; Mayer, I.; Šmit, I.; Bhide, M.; et al. Prevalence and geographic distribution of vector-borne pathogens in apparently healthy dogs in Croatia. Vector Borne Zoonotic Dis. 2017, 17, 398–408. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Distribution, abundance, and habitat preferences of Ixodes ricinus (Acari: Ixodidae) in northern Spain. J. Med. Entomol. 2001, 38, 361–370. [Google Scholar] [CrossRef]
- Walker, A.R.; Alberdi, M.P.; Urquhart, K.A.; Rose, H. Risk factors in habitats of the tick Ixodes ricinus influencing human exposure to Ehrlichia phagocytophila bacteria. Med. Vet. Entomol. 2001, 15, 40–49. [Google Scholar] [CrossRef]
- Hansford, K.M.; Fonville, M.; Gillingham, E.L.; Copian, E.C.; Pietzch, M.E.; Krawczyk, A.I.; Vaux, A.G.C.; Cull, B.; Sprong, H.; Medlock, J.M. Ticks and Borrelia in urban and peri-urban green space habitats in a city in southern England. Ticks Tick Borne Dis. 2017, 8, 353–361. [Google Scholar] [CrossRef]
- Krčmar, S. Hard ticks (Acari, Ixodidae) of Croatia. ZooKeys 2012, 234, 19–57. [Google Scholar] [CrossRef]
- Gray, J.S. Biology of Ixodes species ticks in relation to tick-borne zoonoses. Wien. Klin. Wochenschr. 2002, 114, 473–478. [Google Scholar]
- García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Prevention and prophylaxis of tick-bites and tick-borne related diseases. Am. J. Infect. Dis. 2013, 9, 104–116. [Google Scholar] [CrossRef]
- Mulić, R.; Petković, B.; Klišmanić, Z.; Jerončić, I. Tick-borne diseases in the Republic of Croatia. Lječ. Vjesn. 2011, 133, 89–95. [Google Scholar]
- Beck, R.; Habrun, B.; Bosnić, S.; Benić, M.; Nemeth-Blažić, T.; Duvnjak, S. Identification of pathogens in Ixodes ricinus and Dermacentor reticulatus fron public gardens in Zagreb, Croatia. In Proceedings of the 12th International Conference on Lyme Borreliosis and Other Tick-Borne Diseases, Ljubljana, Slovenia, 26–29 September 2010. [Google Scholar]
- Golubić, D.; Rijpkema, S.; Tkalec-Makovec, N.; Ruzić, E. Epidemiologic, ecologic and clinical characteristics of Lyme borreliosis in northwest Croatia. Acta Med. Croat. 1998, 52, 7–13. [Google Scholar]
- Barišin, A.; Nemeth Blažić, T.; Jeličić, P.; Gjenero Margan, I.; Capak, K.; Petrović, G. Prikaz istraživanja krpelja na području Grada Zagreba u 2008. godini. Zbornik radova DDD-ZUPP 2011, 1, 203–211. [Google Scholar]
- Rijepkema, S.; Golubić, D.; Molkenboer, M.; Verbeek, D.E.; Kruif, N.; Schellekens, J.F.P. Identification of four genomic groups of B. burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region on northern Croatia. Exp. Appl. Acarol. 1996, 20, 25–30. [Google Scholar]
- Steere, A.C.; Malawista, S.E.; Snydman, D.R.; Shope, R.E.; Andiman, W.A.; Ross, M.R.; Steele, F.M. Lyme arthritis: An epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977, 20, 7–17. [Google Scholar] [CrossRef]
- Parola, F.; Raoult, D. Tick and tickborne bacterial diseases in humans: An emerging infectious threat. Ticks Tick Borne Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef]
- Quine, C.P.; Barnett, J.; Dobson, A.D.M.; Marcu, A.; Marzano, M.; Moseley, D.; O’ Brien, L.; Randolph, S.E.; Taylor, J.L.; Uzell, D. Frameworks for risk communication and disease management: The case of Lyme disease and countryside users. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Hauffe, H.C.; Carpi, G.; Vourc’h, G.I.; Neteler, M.; Rosà, R. Lyme borreliosis in Europe. Euro Surveill. 2011, 16, 19906. [Google Scholar] [CrossRef] [PubMed]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef]
- Bennet, L.; Halling, A.; Berglund, J. Increased incidence of Lyme borreliosis in southern Sweden following mild winters and during warm, humid summers. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 426–432. [Google Scholar] [CrossRef]
- Croatian Institute of Public Health. Statistical Yearbooks (1996–2021). Available online: https://www.hzjz.hr/hrvatski-zdravstveno-statisticki-ljetopis/hrvatski-zdravstveno-statisticki-ljetopis-za-2020-tablicni-podaci/ (accessed on 2 January 2023).
- Lipozenčić, J.; Šitum, M. Epidemiology of Lyme disease. Acta Derm. APA 2001, 10, 1–9. [Google Scholar]
- Estrada-Peña, A.; Cutler, S.; Potkonjak, A.; Vassier-Tussaut, M.; Van Bortel, W.; Zeller, H.; Fernández-Ruiz, N.; Mihalca, A.D. An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int. J. Health Geogr. 2018, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Kahl, O.; Gray, J.S. The biology of Ixodes ricinus with emphasis on its ecology. Ticks Tick Borne Dis. 2023, 14, 102114. [Google Scholar] [CrossRef] [PubMed]
- Mikačić, D. The ticks of the littoral zone in Yugoslavia. I. The morphology of the species from the genus Hyalomma. Vet. Arhiv. 1961, 31, 305–310. [Google Scholar]
- Mikačić, D. The ticks of the littoral zone of Yugoslavia. II. The genus Haemaphysalis with reference to the distinction between H. punctata and H. cholodkovskyi. Vet. Arhiv. 1963, 33, 133–136. [Google Scholar]
- Mikačić, D. Ticks in the littoral belt of Yugoslavia III. Distribution and dynamics of species in the course of the year. Vet. Arhiv. 1965, 35, 155–170. [Google Scholar]
- Tovornik, D.; Brelih, S. Ixodid ticks, the parasites of lizards (Lacertidae) in the karst and other districts of Yugoslavia. Scopolia 1980, 3, 1–21. [Google Scholar]
- Tovornik, D.; Vesenjak-Hirjan, J. A revision of ticks belonging to the Rhipicephalus sanguineus complex (Latreille), collected in the Yugoslav Coastal Region. Biol. Vestn. 1988, 36, 77–84. [Google Scholar]
- Vucelja, M.; Bjedov, L.; Boljfetić, M.; Klobučar, A.; Krčmar, S.; Borak, S.; Modrić, M.; Juričić, K.; Peleš, V.; Margaletić, J.; et al. Monitoring of Hard Ticks at Urban Recreational Sites in the City of Zagreb from 2016 to 2018. Infektol. Glasn. 2019, 39, 33–39. [Google Scholar] [CrossRef]
- Mikačić, D. Dinamika pojavljivanja krpelja (Ixodidae) u Sjevernoj Hrvatskoj. Vet. Arhiv. 1969, 39, 183–186. [Google Scholar]
- Vucelja, M. Rodent Control (Rodentia, Murinae, Arvicolinae) in Pedunculate Oak Forests (Quercus robur L.)—Integrated Approach and Zoonotic Aspect, 1st ed.; University of Zagreb, Faculty of forestry: Zagreb, Croatia, 2013; pp. 25–210. [Google Scholar]
- Borčić, B.; Aleraj, B.; Žutić, M.; Mikačić, D. The role of ticks (Ixodidae) in the maintenance of the tularemia natural focus in central Posavinia. Vet. Arhiv. 1978, 48, 277–283. [Google Scholar]
- Krčmar, S. Diversity, ecology, and seasonality of hard ticks (Acari: Ixodidae) in eastern Croatia. J. Vector Ecol. 2019, 44, 18–29. [Google Scholar] [CrossRef]
- Krčmar, S.; Klobučar, A.; Vucelja, M.; Boljfetić, M.; Kučinić, M.; Madić, J.; Cvek, M.; Bruvo Mađarić, B. DNA barcoding of hard ticks (Ixodidae), notes on distribution of vector species and new faunal record for Croatia. Ticks Tick Borne Dis. 2022, 13, 101920. [Google Scholar] [CrossRef]
- EEA. European Environment Agency Biogeographic Regions Dataset. 2016. Permalink: 2a7b433b388e432d9c3e8cf019af1db0. Available online: https://www.eea.europa.eu/data-and-maps/data/biogeographical-regions-europe-3 (accessed on 8 January 2023).
- Filipčić, A. Klimatska regionalizacija Hrvatske po Köppenu za standardno razdoblje 1961–1990. u odnosu na razdoblje 1931–1960. Acta Geograph. Croat. 1998, 34, 1–15. [Google Scholar]
- Zaninović, K.; Gajić-Čapka, M.; Perčec Tadić, M.; Vučetić, M.; Milković, J.; Bajić, A.; Cindrić, K.; Cvitan, L.; Katušin, Z.; Kaučić, D.; et al. Klimatski atlas Hrvatske/Climate atlas of Croatia 1961–1990, 1971–2000, 1st ed.; Državni Hidrometeorološki Zavod Zagreb: Zagreb, Hrvatska, 2008; pp. 1–200. [Google Scholar]
- Šegota, T.; Filipčić, A. Köppenova podjela klima i hrvatsko nazivlje. Geoadria 2003, 8, 17–31. [Google Scholar] [CrossRef]
- Vukelić, J.; Rauš, Đ. Forest phytocenology and forest communities in Croatia (Šumarska fitocenologija i šumske zajednice u Hrvatskoj), 1st ed.; University of Zagreb, Faculty of forestry: Zagreb, Croatia, 1988; pp. 173–177. [Google Scholar]
- Vukelić, J. Croatian Forest Vegetation (Šumska Vegetacija Hrvatske), 1st ed.; University of Zagreb, Faculty of forestry: Zagreb, Croatia, 2012; pp. 81–101. [Google Scholar]
- Martinović, J. Management of Forest Soils in Croatia, 1st ed.; Gračan, J., Ed.; Croatian Forests Limited Liability Company, Ministry of Science and technology: Zagreb, Croatia, 2003; pp. 118–119. [Google Scholar]
- Cohnstaedt, L.W.; Rochon, K.; Duehl, A.J.; Anderson, J.F.; Barrera, R.; Yao Su, N.; Gerry, A.C.; Obenauer, P.J.; Campbell, J.F.; Lysyk, T.J.; et al. Arthropod Surveillance Programs: Basic Components, Strategies, and Analysis. Ann. Entomol. Soc. Am. 2012, 105, 135–149. [Google Scholar] [CrossRef]
- Falco, R.; Fish, D. The comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp. Appl. Acarol. 1992, 14, 165–173. [Google Scholar] [CrossRef]
- Carroll, J.F.; Schmidtmann, E.T. Tick sweep: Modification of the tick drag—Flag method for sampling nymphs of the deer tick (Acari: Ixodidae). J. Med. Entomol. 1992, 29, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region—A Guide to Identification of Species, 1st ed.; University of Zaragoza: Zaragoza, Spain, 2004; pp. 1–128. [Google Scholar]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa. A Guide to Species Identification, 1st ed.; Springer International Publishing AG: Cham, Szwitzerland, 2017; pp. 1–404. [Google Scholar]
- Guy, E.C.; Stanek, G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J. Clin. Pathol. 1991, 44, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.; Andersson, Y.; Suk, J.E.; Sudre, B.; Semenza, J.C. Public health. Monitoring EU emerging infectious disease risk due to climate change. Science 2012, 336, 418–419. [Google Scholar] [CrossRef]
- Kovačević, J.; Bučanović, T.; Krčmar, S. Hard tick fauna (Acari: Ixodidae) in different types of habitats in the city of Osijek (Eastern Croatia). Nat. Croat. 2020, 29, 63–72. [Google Scholar] [CrossRef]
- Cull, B.; Vaux, A.G.C.; Ottowell, L.J.; Gillingham, E.L.; Medlock, J.M. Tick infestation of small mammals in an English woodland. J. Vector Ecol. 2017, 42, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.; Radulović, Ž.; Tomanović, S.; Tomanović, Ž. Seasonal distribution of Borreliae in Ixodes ricinus ticks in the Belgrade region, Serbia. Arch. Biol. Sci. Belgrade 2006, 58, 183–186. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Venzal, J.M.; Sánchez Acedo, C. The tick Ixodes ricinus: Distribution and climate preferences in the western Palaearctic. Med. Vet. Entomol. 2006, 20, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Accrosi, A.; Schiavetti, I.; Listorti, V.; Dellepiane, M.; Masotti, C.; Ercolini, C.; Guardone, L.; Razzuoli, E. Hard ticks (Ixodidae) from Wildlife in Liguria, Northwest Italy: Tick Species Diversity and Tick-Host Associations. Insects 2022, 13, 199. [Google Scholar] [CrossRef]
- Bertola, M.; Momtarsi, F.; Obber, F.; Da Rold, G.; Carlin, S.; Toniolo, F.; Porcellato, E.; Falcaro, C.; Mondardini, V.; Ormelli, S.; et al. Occurrence and identification of Ixodes ricinus borne pathogens in Northeastern Italy. Pathogens 2021, 10, 1181. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfeiffer, M.; Chitimia-Dobler, I.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef]
- Bilbija, B.; Spitzweg, C.; Papoušek, I.; Fritz, U.; Földvári, G.; Mullett, M.; Ihlow, F.; Sprong, H.; Civáňová Krížová, K.; Anisimov, N.; et al. Dermacentor reticulatus—A tick on its way from glacial refugia to a panmictic Eurasian population. Int. J. Parasitol. 2023, 53, 91–101. [Google Scholar] [CrossRef]
- Široký, P.; Kubelová, M.; Bednář, M.; Modrý, D.; Hubálek, Z.; Tkadlec, E. The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic—How much is range of this vector expanding? Vet. Parasitol. 2011, 183, 130–135. [Google Scholar] [CrossRef]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 2016, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M. Influence of the microclimate on the vertical distribution of the tick Ixodes ricinus (L.) in Central Europe. Acarologia 1993, 34, 105–113. [Google Scholar]
- Materna, J.; Daniel, M.; Danielova, V. Altitudinal distribution limit of the tick Ixodes ricinus shifted considerably towards higher altitudes in central Europe: Results of thre years monitoring in the Krkonoše Mts. (Czech Republic). Cent. Eur. J. Publ. Health 2005, 13, 24–28. [Google Scholar]
- Pelsmaeker, N.D.; Korslund, L.; Steifetten, Ø. High-elevational occurrence of two tick species, Ixodes ricinus and I. trianguliceps, at their northern distribution range. Parasit. Vectors 2021, 14, 161. [Google Scholar] [CrossRef]
- Hornok, S.; Farkas, R. Influence of biotope on the distribution and peak activity of questing ixodid ticks in Hungary. Med. Vet. Entomol. 2009, 23, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.; Szymaňska, H.; Dmitryjuk, M.; Dzika, E. Abundance of Ixodes ricinus ticks (Acari: Ixodidae) and the diversity of Borrelia species in Northeastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 7378. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F.; Santos-Silva, M.M. Ixodes ricinus (Linnaeus, 1758) (Figs.67–69). In Ticks of Europe and North Africa: A Guide to Species Identification, 1st ed.; Estrada-Peña, A., Mihalca, A.D., Petney, T.N., Eds.; Springer International Publishing AG: Cham, Szwitzerland, 2017; pp. 189–195. [Google Scholar]
- Paul, R.E.L.; Cote, M.; Le Naour, E.; Bonnet, S.I. Environmental factors influencing tick densities over seven years in a French suburban forest. Parasit. Vectors 2016, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; De La Fuente, J. Species interactions in occurrence data for a community of tick transmitted pathogens. Sci. Data 2016, 3, 160056. [Google Scholar] [CrossRef]
- Schwarz, A.; Maier, W.A.; Kistemann, T.; Kampen, H. Analysis of the distribution of the tick Ixodes ricinus L. (Acari: Ixodidae) in a nature reserve of western Germany using geographic information systems. Int. J. Hyg. Environ. Health 2009, 212, 87–96. [Google Scholar] [CrossRef]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of Climate Change on Ticks and Tick-Borne Diseases in Europe. Inter. Perspect. Infect. Dis. 2009, 212, 87–96. [Google Scholar] [CrossRef]
- Ehrmann, S.; Liira, J.; Gärtner, S.; Hansen, K.; Brunet, J.; Cousins, S.A.O.; Deconchat, M.; Decocq, G.; De Frenne, P.; De Smedt, P.; et al. Environmental drivers of Ixodes ricinus abundance in forest fragments of rural European landscapes. BMC Ecol. 2017, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Petney, T.N.; Pfäffie, M.P.; Skuballa, J.D. An annotated checklist of the ticks (Acari: Ixodidae) of Germany. Syst. Appl. Acarol. 2012, 17, 115–170. [Google Scholar] [CrossRef]
- Olsthoorn, F. The Effect of Woodland Expansion on Tick Populations and Lyme Disease Risk. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 2021. [Google Scholar]
- Milutinović, M.; Radulović, Ž.; Jovičić, V.; Oreščanin, Z. Population dynamics and Borrelia burgdorferi infection rate of Ixodes ricinus ticks in the Belgrade area. Acta Vet. 2004, 54, 219–225. [Google Scholar]
- Rauter, C.; Hartung, T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Appl. Environ. Microbiol. 2005, 71, 7203–7216. [Google Scholar] [CrossRef] [PubMed]
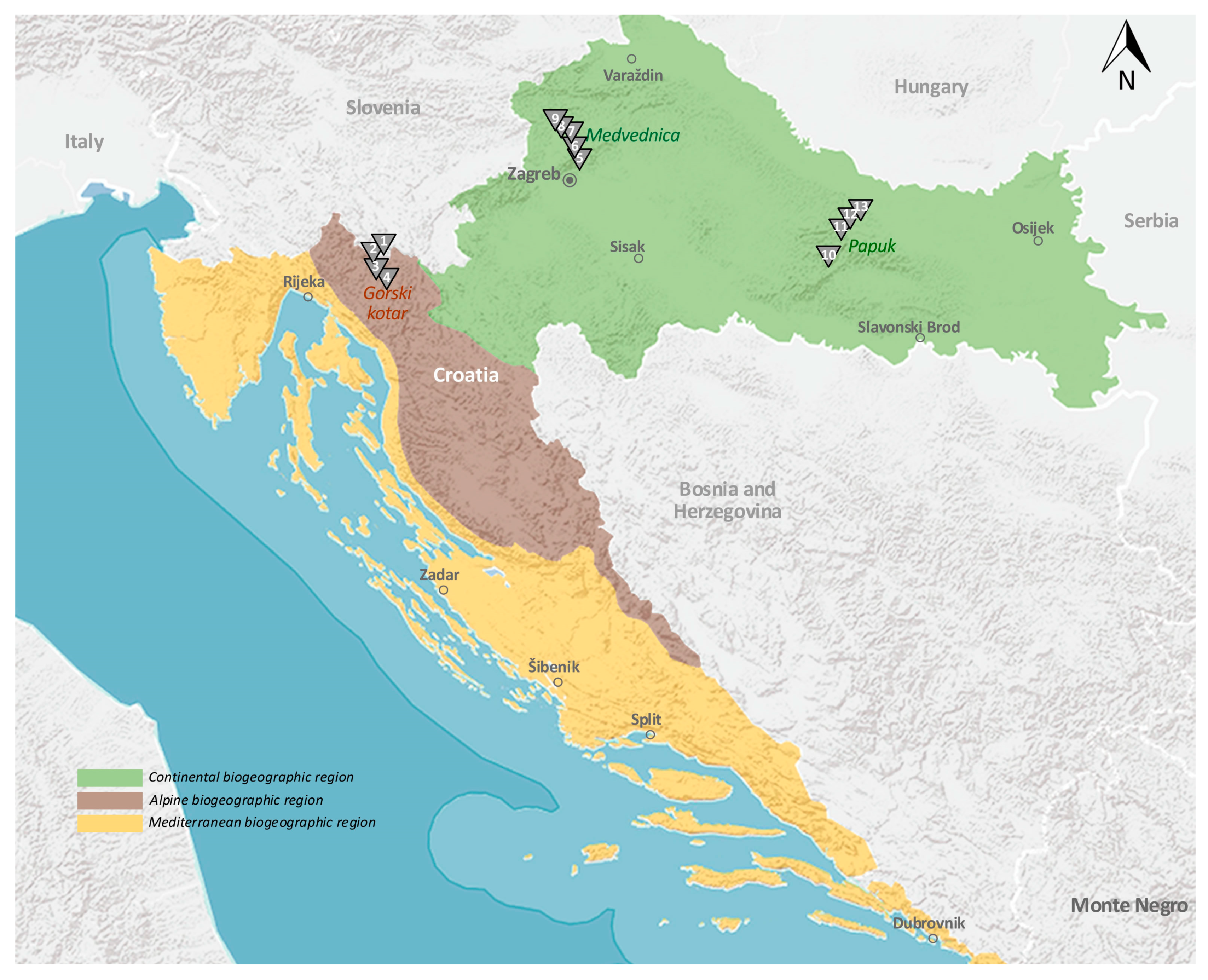


| Mountain Site | Tick Sampling Site | Altitude, Latitude (Degree/min/s) | Altitude (m asl.) | Forest Soil | Forest Community |
|---|---|---|---|---|---|
| GOK | 1 Donje Tihovo | N45 25.927 E14 50.814; | 400 | Pseudogley on sloping terrain | EBDF |
| 2 Marija Trošt | N45 24.798 E14 49.351; | 600 | Dystric cambisol | EFDF | |
| 3 Lučice | N45 22.359 E14 48.402; | 800 | Dystric cambisol | DBF | |
| 4 Stari Laz | N45 20.740 E14 51.486; | 1000 | Dystric cambisol | MPF | |
| MED | 5 Dotrščina | N45 51.174 E16 01.172; | 200 | Dystric cambisol | POEH |
| 6 Medvednica a | N45 52.748 E15 58.568: | 400 | Dystric cambisol | EBW | |
| 7 Medvednica b | N45 53.201 E15 58.330; | 600 | Dystric cambisol | EBW | |
| 8 Medvednica c | N45 53.394 E15 57.634; | 800 | Dystric cambisol | EBW | |
| 9 Medvednica d | N45 53.916 E15 56.753; | 1000 | Dolomitic cambisol | PBF | |
| PAP | 10 Markovac | N45 25.583 E17 32.227; | 200 | Luvisol | SOFD |
| 11 Slatinski Drenovac a | N45 31.553 E17 41.306; | 400 | Dystric cambisol | EBW | |
| 12 Slatinski Drenovac b | N45 30.850 E17 39.990 | 600 | Dystric cambisol | EBW | |
| 13 Radovanci | N45 30.673 E17 39.430; | 800 | Dystric cambisol | MEBBLA |
| GOK/Air Temperature (°C) (min–max) | GOK/Air Humidity (%) (min–max) | |||||
|---|---|---|---|---|---|---|
| Year Season | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 |
| S | 10.7 (0.2–14.9) | 10.2 (−4.5–22.2) | 9.9 (−3.9–25.3) | 82 (57–97) | 78.5 (45–97) | 85.8 (71–98) |
| A | 8.5 (−4.1–18.6) | 7.4 (−4.8–18.0) | 6.7 (−3.7–18.8) | 89.3 (78–98) | 89.8 (75–98) | 90 (76–98) |
| MED/air temperature (°C) (min–max) | MED/air humidity (%) (min–max) | |||||
| S | 9.73 (−1.0–24.4) | 9.01 (−5.9–21.2) | 8.25 (−5.6–23.6) | 74.5 (36–100) | 67.5 (29–99) | 72.5 (31–100) |
| A | 7.53 (−6.6–21.3) | 6.63 (−6.4–18.4) | 5.9 (−5.4–19.0) | 82.5 (45–100) | 67.1 (48–100) | 84.5 (32–100) |
| PAP/air temperature (°C) (min–max) | PAP/air humidity (%) (min–max) | |||||
| S | 12.3 (0.2–24.6) | 11.3 (−3.0–22.7) | 10.8 (−2.0–26.4) | 76.3 (67–98) | 82.5 (59–96.6) | 81.4 (63–97.3) |
| A | 8.95 (−4–21.1) | 8.1 (−2.6–19.4) | 7.2 (−2.7–19.6) | 88.3 (77–98) | 88.8 (70.2–96.2) | 88.5 (73.6–97.1) |
| Species | Females | Males | Nymphs | Larvae | Total |
|---|---|---|---|---|---|
| Ixodes ricinus | 72 | 103 | 1179 | 1583 | 2937 |
| Ixodes frontalis | 0 | 0 | 1 | 0 | 1 |
| Haemaphysalis concinna | 0 | 0 | 3 | 1 | 4 |
| Ʃ | 72 | 103 | 1183 | 1584 | 2942 |
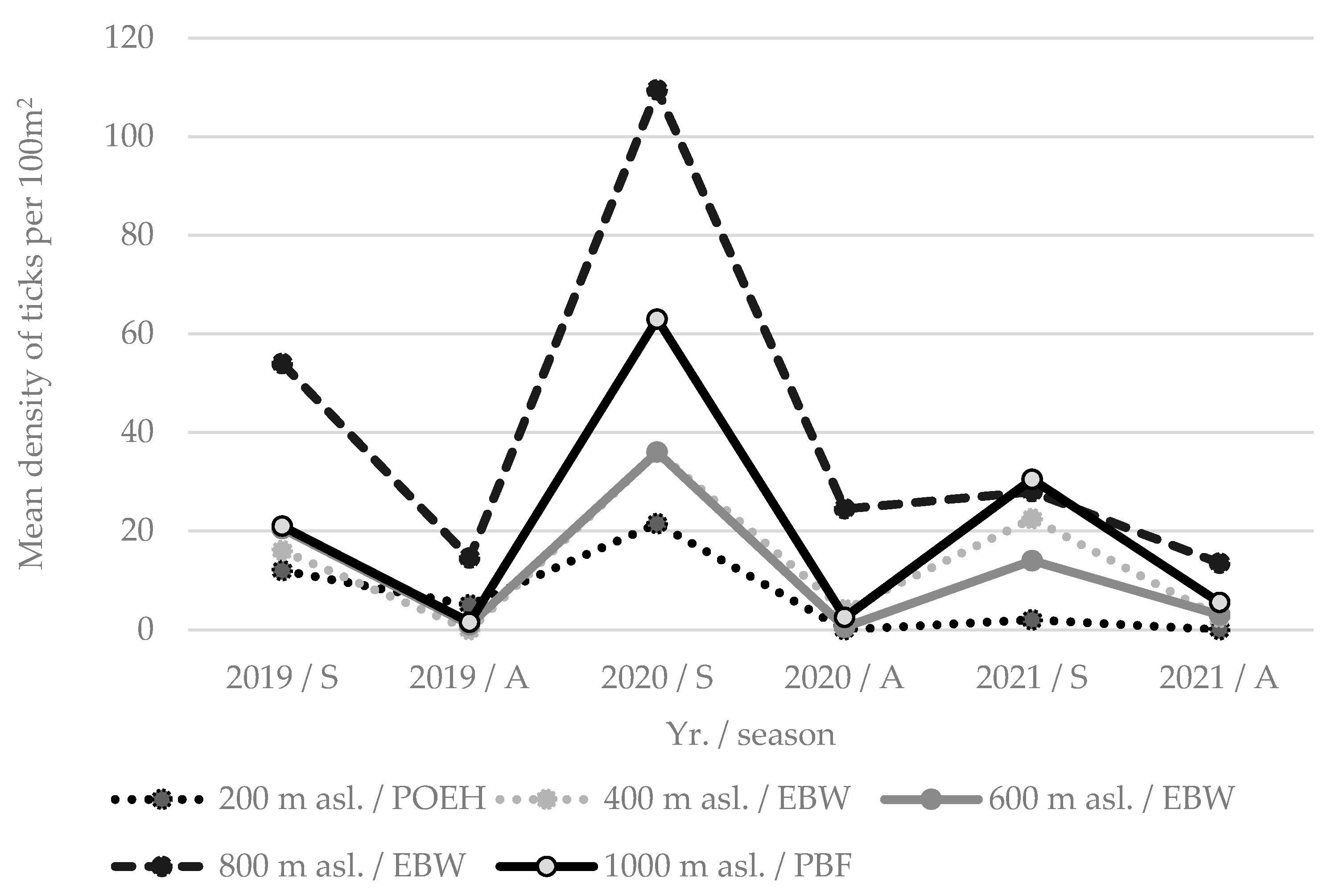
| Mountain Site | Altitude (m asl.) | Forest Community | Ir ♀ | Ir ♂ | Ir n | Ir l | If n | Hc n | Hc l | Ʃ | Mean Density per 100 m2 (min–max) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GOK | 400 | EBDF | 0 | 2 | 116 | 250 | 0 | 0 | 0 | 368 | 30.7 (1–131.5) |
| 600 | EFDF | 5 | 4 | 84 | 42 | 0 | 0 | 0 | 135 | 11.3 (0.5–32.5) | |
| 800 | DBF | 6 | 4 | 66 | 383 | 0 | 0 | 0 | 459 | 38.3 (0–207.5) | |
| 1000 | MPF | 2 | 5 | 51 | 410 | 0 | 0 | 0 | 468 | 39 (0–177.5) | |
| Ʃ | 13 | 15 | 317 | 1085 | 0 | 0 | 0 | 1430 | 29.8 | ||
| MED | 200 | POEH | 3 | 5 | 34 | 38 | 1 | 0 | 0 | 81 | 6.75 (0–21.5) |
| 400 | EBW | 25 | 29 | 94 | 14 | 0 | 0 | 0 | 162 | 13.5 (0–22.5) | |
| 600 | EBW | 4 | 14 | 107 | 25 | 0 | 0 | 0 | 150 | 12.5 (0.5–20.5) | |
| 800 | EBW | 8 | 18 | 237 | 225 | 0 | 0 | 0 | 488 | 40.7 (13.5–109.5) | |
| 1000 | PBF | 15 | 16 | 208 | 9 | 0 | 0 | 0 | 248 | 20.7 (1.5–63) | |
| Ʃ | 55 | 82 | 680 | 311 | 1 | 0 | 0 | 1129 | 18.8 | ||
| PAP | 200 | SOFD | 3 | 3 | 89 | 78 | 0 | 3 | 0 | 176 | 14.7 (0–42.5) |
| 400 | EBW | 1 | 2 | 29 | 10 | 0 | 0 | 0 | 42 | 3.5 (0–11.5) | |
| 600 | EBW | 0 | 0 | 23 | 69 | 0 | 0 | 1 | 93 | 7.8 (0–38) | |
| 800 | MEBBLA | 0 | 1 | 41 | 30 | 0 | 0 | 0 | 72 | 6 (0–23.5) | |
| Ʃ | 4 | 6 | 182 | 187 | 0 | 3 | 1 | 383 | 8.0 | ||
| Ʃ Ʃ | 72 | 103 | 1179 | 1583 | 1 | 3 | 1 | 2942 | 18.9 |
| Mountain Site | Altitude (m asl.) | Forest Community | Sp. | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|---|---|---|
| S | A | S | A | S | A | ||||
| GOK | 400 | EBDF | Ir | 0 ♀, 0 ♂, 26 n, 22 l | 0 ♀, 0 ♂, 2 n, 0 l | 0 ♀, 0 ♂, 41 n, 222 l | 0 ♀, 1 ♂, 26 n, 0 l | 0 ♀, 1 ♂, 4 n, 0 l | 0 ♀, 0 ♂, 17 n, 6 l |
| 600 | EFDF | Ir | 0 ♀, 2 ♂, 16 n, 10 l | 0 ♀, 0 ♂, 1 n, 0 l | 4 ♀, 2 ♂, 38 n, 21 l | 1 ♀, 0 ♂, 12 n, 5 l | 0 ♀, 0 ♂, 4 n, 0 l | 0 ♀, 0 ♂, 13 n, 6 l | |
| 800 | DBF | Ir | 1 ♀, 0 ♂, 24 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 2 ♂, 30 n, 383 l | 0 ♀, 0 ♂, 0 n, 0 l | 2 ♀, 1 ♂, 5 n, 0 l | 2 ♀, 0 ♂, 1 n, 0 l | |
| 1000 | MPF | Ir | 1 ♀, 3 ♂, 21 n, 330 l | 0 ♀, 0 ♂, 0 n, 0 l | 1♀, 1 ♂, 20 n, 62 l | 0 ♀, 0 ♂, 6 n, 18 l | 0 ♀, 1 ♂, 4 n, 0 l | 2 ♀, 5 ♂, 51 n, 410 l | |
| MED | 200 | POEH | Ir | 2 ♀, 3 ♂, 15 n, 3 l | 0 ♀, 1 ♂, 2 n, 7 l | 1♀, 1 ♂, 13 n, 28 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 4 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l |
| If | 0 ♀, 0 ♂, 1 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | |||
| 400 | EBW | Ir | 5 ♀, 9 ♂, 18 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 4 ♀, 2 ♂, 52 n, 14 l | 1 ♀, 1 ♂, 6 n, 0 l | 15 ♀, 15 ♂, 15 n, 0 l | 0 ♀, 2 ♂, 3 n, 0 l | |
| 600 | EBW | Ir | 3 ♀, 12 ♂, 26 n, 0 l | 0 ♀, 0 ♂, 2 n, 0 l | 0 ♀, 0 ♂, 48 n, 24 l | 0 ♀, 0 ♂, 1 n, 0 l | 0 ♀, 1 ♂, 26 n, 1 l | 1 ♀, 1 ♂, 4 n, 0 l | |
| 800 | EBW | Ir | 4 ♀, 6 ♂, 98 n, 0 l | 0 ♀, 0 ♂, 9 n, 20 l | 1 ♀, 1 ♂, 22 n, 195 l | 1 ♀, 1 ♂, 47 n, 0 l | 1 ♀, 7 ♂, 42 n, 6 l | 1 ♀, 3 ♂, 19 n, 4 l | |
| 1000 | PBF | Ir | 3 ♀, 2 ♂, 36 n, 1 l | 0 ♀, 0 ♂, 0 n, 3 l | 2 ♀, 2 ♂, 117 n, 5 l | 0 ♀, 0 ♂, 5 n, 0 l | 10 ♀, 12 ♂, 39 n, 0 l | 0 ♀, 0 ♂, 11 n, 0 l | |
| PAP | 200 | SOFD | Ir | 0 ♀, 0 ♂, 18 n, 57 l | 0 ♀, 1 ♂, 5 n, 0 l | 1 ♀, 0 ♂, 62 n, 21 l | 0 ♀, 0 ♂, 0 n, 0 l | 2 ♀, 1 ♂, 1 n, 0 l | 0 ♀, 1 ♂, 3 n, 0 l |
| Hc | 0 ♀, 0 ♂, 2 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 1 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | |||
| 400 | EBW | Ir | 1 ♀, 1 ♂, 5 n, 10 l | 0 ♀, 1 ♂, 1 n, 0 l | 0 ♀, 0 ♂, 23 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | |
| 600 | EBW | Ir | 0 ♀, 0 ♂, 3 n, 0 l | 0 ♀, 0 ♂, 3 n, 0 l | 0 ♀, 0 ♂, 13 n, 63 l | 0 ♀, 0 ♂, 3 n, 6 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 1 n, 0 l | |
| Hc | 0 ♀, 0 ♂, 0 n, 1 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 0 n, 0 l | |||
| 800 | MEBBLA | Ir | 0 ♀, 0 ♂, 2 n, 6 l | 0 ♀, 0 ♂, 0 n, 0 l | 0 ♀, 0 ♂, 27 n, 20 l | 0 ♀, 0 ♂, 4 n, 3 l | 0 ♀, 0 ♂, 3 n, 0 l | 0 ♀, 1 ♂, 5 n, 1 l | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vucelja, M.; Krčmar, S.; Habuš, J.; Perko, V.M.; Boljfetić, M.; Bjedov, L.; Margaletić, J. Altitudinal Distribution, Seasonal Dynamics and Borrelia burgdorferi Sensu Lato Infections in Hard Ticks (Acari: Ixodidae) in Different Forest Communities in Inland Croatia. Sustainability 2023, 15, 4862. https://doi.org/10.3390/su15064862
Vucelja M, Krčmar S, Habuš J, Perko VM, Boljfetić M, Bjedov L, Margaletić J. Altitudinal Distribution, Seasonal Dynamics and Borrelia burgdorferi Sensu Lato Infections in Hard Ticks (Acari: Ixodidae) in Different Forest Communities in Inland Croatia. Sustainability. 2023; 15(6):4862. https://doi.org/10.3390/su15064862
Chicago/Turabian StyleVucelja, Marko, Stjepan Krčmar, Josipa Habuš, Vesna Mojčec Perko, Marko Boljfetić, Linda Bjedov, and Josip Margaletić. 2023. "Altitudinal Distribution, Seasonal Dynamics and Borrelia burgdorferi Sensu Lato Infections in Hard Ticks (Acari: Ixodidae) in Different Forest Communities in Inland Croatia" Sustainability 15, no. 6: 4862. https://doi.org/10.3390/su15064862
APA StyleVucelja, M., Krčmar, S., Habuš, J., Perko, V. M., Boljfetić, M., Bjedov, L., & Margaletić, J. (2023). Altitudinal Distribution, Seasonal Dynamics and Borrelia burgdorferi Sensu Lato Infections in Hard Ticks (Acari: Ixodidae) in Different Forest Communities in Inland Croatia. Sustainability, 15(6), 4862. https://doi.org/10.3390/su15064862






