Biocrude Production Using a Novel Cyanobacterium: Pilot-Scale Cultivation and Lipid Extraction via Hydrothermal Liquefaction
Abstract
:1. Introduction
2. Methods
2.1. Strain and Culture Conditions
2.2. Quantification of Pigments
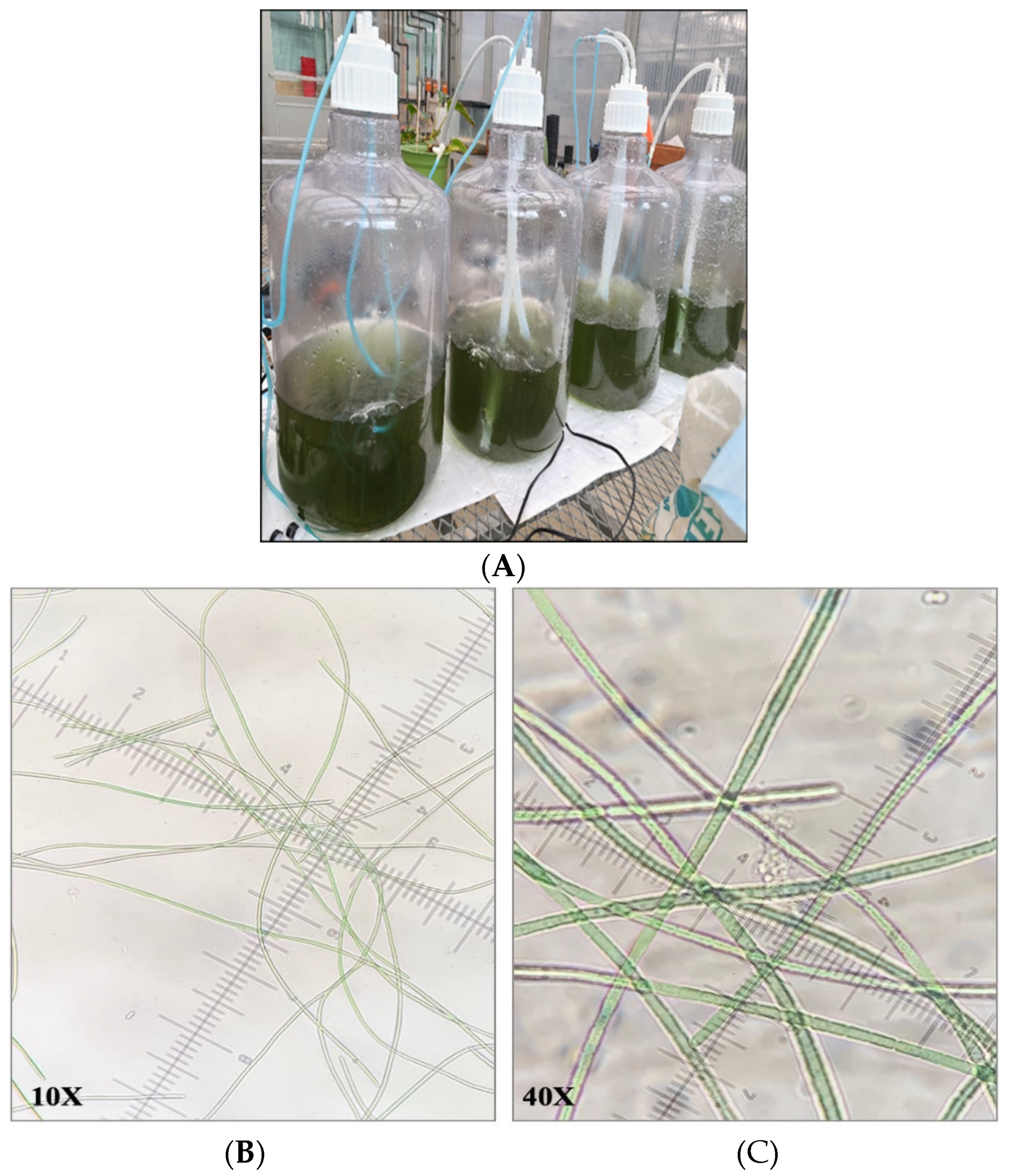
2.3. Optimization of Indoor Reactor Systems
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Patents
| Licensed Intellectual Properties from Morgan State University | ||
|---|---|---|
| Issue # | Invention Title | Status |
| 10,626,363 | Engineered cyanobacteria with enhanced salt tolerance | Issued U.S. Patent |
| 10,793,883 | Engineered cyanobacteria with enhanced lipid production | Issued U.S. Patent |
| 11,162,067 | Composition and method for enhancing photosynthetic efficiency of microorganisms | Issued U.S. Patent |
| 16/712,125 | Iron nanoparticle-mediated enhancement of lipids in the cyanobacterium Fremyella diplosiphon | Utility Patent Application |
| 17/891,482 | Engineered cyanobacteria with enhanced UV tolerance | Non-provisional Application |
| 63/408,920 | Cyanobacteria as anti-inflammatory and nutritional feed | Provisional Application |
References
- Schroeder, D.C.; Truxell, R.W.; Bartus, M.J.; Sequest, L.L.C. Mass Production of Aquatic Plants. U.S. Patent Application 12/327,178, 3 December 2008. [Google Scholar]
- Wargo, J.; Wargo, L.; Alderman, N. Harmful Effects of Vehicle Exhaust. 2016. Available online: http://www.ehhi.org/reports/exhaust/summary.shtml (accessed on 9 September 2021).
- Xu, Z.; Sheffield, P.E.; Hu, W.; Su, H.; Yu, W.; Qi, X.; Tong, S. Climate change and children’s health—A call for research on what works to protect children. Int. J. Environ. Res. Public Health 2012, 9, 3298–3316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Energy Agency (IEA). Weo-2016 Special Report Energy and Air Pollution; IEA: Paris, France, 2016. [Google Scholar]
- Behera, S.; Singh, R.; Arora, R.; Sharma, N.K.; Shukla, M.; Kumar, S. Scope of algae as third generation biofuels. Front. Bioeng. Biotech. 2015, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Fargioni, J.; Hill, J.; Plosky, S.; Hawthorne, P. Land clearing and biofuel carbon debt. Science 2008, 319, 1236–1238. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.; Nelson, E.; Tilman, D.; Poloski, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol fuels. Proc. Natl. Acad. Sci. USA 2006, 30, 11206–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venteris, E.R.; Skaggs, R.L.; Coleman, A.M.; Wigmosta, M.S. A GIS cost model to assess the availability of freshwater, seawater, and saline groundwater for algal biofuel production in the United States. Environ. Sci. Tech. 2013, 47, 4840–4849. [Google Scholar] [CrossRef]
- Weyer, K.M.; Bush, D.R.; Darzins, A.; Willson, B.D. Theoretical maximum algal oil production. Bioenergy Res. 2010, 3, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Kesharvani, S.; Dwivedi, G. Algae as a feedstock for biodiesel production in Indian perspective. Mater. Today Proc. 2021, 47, 5873–5880. [Google Scholar] [CrossRef]
- Tabatabai, B.; Chen, H.; Lu, J.; Giwa-Otusajo, J.; McKenna, A.M.; Shrivastava, A.K.; Sitther, V. Fremyella diplosiphon as a biodiesel agent: Identification of fatty acid methyl esters via microwave-assisted direct in situ transesterification. Bioenergy Res. 2018, 11, 528–537. [Google Scholar] [CrossRef]
- Ross, A.B.; Biller, P.; Kubacki, M.L.; Li, H.; Lea-Langton, A.; Jones, J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, F.; Liu, Y.; Meng, Y.; Wang, W.; Liu, C.; Zhang, J.; Du, H.; Li, J. Preparation and application of ZrO2–SiO2 complex oxide for efficient biocrude generation by hydrothermal liquefaction of Spirulina. Fuel 2022, 317, 123325. [Google Scholar] [CrossRef]
- Goswami, G.; Makut, B.B.; Das, D. Sustainable production of bio-crude oil via hydrothermal liquefaction of symbiotically grown biomass of microalgae-bacteria coupled with effective wastewater treatment. Sci. Rep. 2019, 9, 15016. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.J. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Roháček, K.; Barták, M. Technique of the modulated chlorophyll fluorescence: Basic concepts, useful parameters, and some applications. Photosynthetica 2020, 37, 339–363. [Google Scholar] [CrossRef]
- Kahn, K.; Mazel, D.; Houmard, J.; Tandeau de Marsac, N.; Schaefer, M. A role for cpeYZ in cyanobacterial phycoerythrin biosynthesis. J. Bacteriol. 1997, 179, 998–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandeau de Marsac, N.; Houmard, J. Complementary chromatic adaptation: Physiological conditions and action spectra. Methods Enzymol. 1988, 167, 318–328. [Google Scholar]
- Whitaker, M.J.; Bordowitz, J.R.; Montgomery, B.L. CpcF-dependent regulation of pigmentation and development in Fremyella diplosiphon. Biochem. Biophys. Res. Comm. 2009, 389, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Sitther, V.; Tabatabai, B.; Fathabad, S.G.; Gichuki, S.; Chen, H.; Arumanayagam, A.C.S. Cyanobacteria as a biofuel source: Advances and applications. In Advances in Cyanobacterial Biology; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 18; pp. 269–289. [Google Scholar]
- Ferliandi, F.; Budiman, A.; Suyono, E.A.; Dewayanto, N. Application of analytic hierarchy process in the selection of Botryococcus braunii cultivation technology for bio-crude oil production. Front. Renew. Energy 2022, 1, 23–30. [Google Scholar]
- Koley, S.; Khadase, M.S.; Mathimani, T.; Raheman, H.; Mallick, N. Catalytic and non-catalytic hydrothermal processing of Scenedesmus obliquus biomass for bio-crude production—A sustainable energy perspective. Energy Convers. Manag. 2018, 163, 111–121. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Zhang, B.; Li, R.; Shahbazi, A. Hydrothermal liquefaction enhanced by various chemicals as a means of sustainable dairy manure treatment. Sustainability 2018, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Frišták, V.; Laughinghouse, H.D., IV; Bell, S.M. The use of biochar and pyrolysed materials to improve water quality through microcystin sorption separation. Water 2020, 12, 2871. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Oezaslan, M.; Dosche, C. Surface characterization of oxidized biochar fibers derived from Luffa cylindrica and lanthanide binding. J. Environ. Chem. Eng. 2017, 5, 4069–4074. [Google Scholar] [CrossRef]
- Mishra, K.P.; Mukherji, S. Biosorption of diesel and lubricating oil on algal biomass. 3Biotech 2012, 2, 301–310. [Google Scholar] [CrossRef] [Green Version]
- van Thor, J.J.; Fisher, N.; Rich, P.R. Assignments of the Pfr−Pr FTIR difference spectrum of cyanobacterial phytochrome Cph1 ysing 15N and 13C isotopically labeled phycocyanobilin chromophore. J. Phys. Chem. B 2005, 109, 20597–20604. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Montgomery, B.L. Salinity impacts photosynthetic pigmentation and cellular morphology changes by distinct mechanisms in Fremyella diplosiphon. Biochem. Biophys. Res. Commun. 2013, 433, 84–89. [Google Scholar] [CrossRef]
- Pattanaik, B.; Busch, A.W.; Hu, P.; Chen, J.; Montgomery, B.L. Responses to iron limitation are impacted by light quality and regulated by RcaE in the chromatically acclimating cyanobacterium Fremyella diplosiphon. Microbiology 2014, 160, 992–1005. [Google Scholar] [CrossRef]
- Walters, K.J.; Whitaker, M.J.; Singh, S.P.; Montgomery, B.L. Light intensity and reactive oxygen species are centrally involved in photoregulatory responses during complementary chromatic adaptation in Fremyella diplosiphon. Comm. Integr. Biol. 2013, 6, e25005. [Google Scholar] [CrossRef]
- Pattanaik, B.; Whitaker, M.J.; Montgomery, B.L. Light quantity affects the regulation of cell shape in Fremyella diplosiphon. Front. Microbiol. 2012, 3, 170. [Google Scholar] [CrossRef] [Green Version]








| # | Component | Retention Time (min) | Application | Main Mass Fragment (m/z) |
|---|---|---|---|---|
| 1 | Hexadecane | 17.234 | Additive cetane number | 70 |
| 2 | Heptadecane | 19.53 | Additive cetane number, oxidative stability | 57 |
| 3 | Methyl heptadecane | 21.5 | Biological marker/standard | 57 |
| 4 | Pentadecanoic acid | 25.174 | Biodiesel precursor | 82 |
| 5 | Palmitic acid | 28.202 | Biodiesel precursor | 73 |
| 6 | Eicosatrienoic acid | 33.458 | Biodiesel precursor | 79 |
| 7 | Docosanoic acid | 33.751 | Biodiesel precursor | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gichuki, S.; Tabatabai, B.; Sitther, V. Biocrude Production Using a Novel Cyanobacterium: Pilot-Scale Cultivation and Lipid Extraction via Hydrothermal Liquefaction. Sustainability 2023, 15, 4878. https://doi.org/10.3390/su15064878
Gichuki S, Tabatabai B, Sitther V. Biocrude Production Using a Novel Cyanobacterium: Pilot-Scale Cultivation and Lipid Extraction via Hydrothermal Liquefaction. Sustainability. 2023; 15(6):4878. https://doi.org/10.3390/su15064878
Chicago/Turabian StyleGichuki, Samson, Behnam Tabatabai, and Viji Sitther. 2023. "Biocrude Production Using a Novel Cyanobacterium: Pilot-Scale Cultivation and Lipid Extraction via Hydrothermal Liquefaction" Sustainability 15, no. 6: 4878. https://doi.org/10.3390/su15064878
APA StyleGichuki, S., Tabatabai, B., & Sitther, V. (2023). Biocrude Production Using a Novel Cyanobacterium: Pilot-Scale Cultivation and Lipid Extraction via Hydrothermal Liquefaction. Sustainability, 15(6), 4878. https://doi.org/10.3390/su15064878






