Influence of Radioactive Sludge Content on Vitrification of High-Level Liquid Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Simulated HLW and Sludge
2.1.2. Basic Glass
2.2. Glass Preparation
2.3. Sample Characterization
3. Results
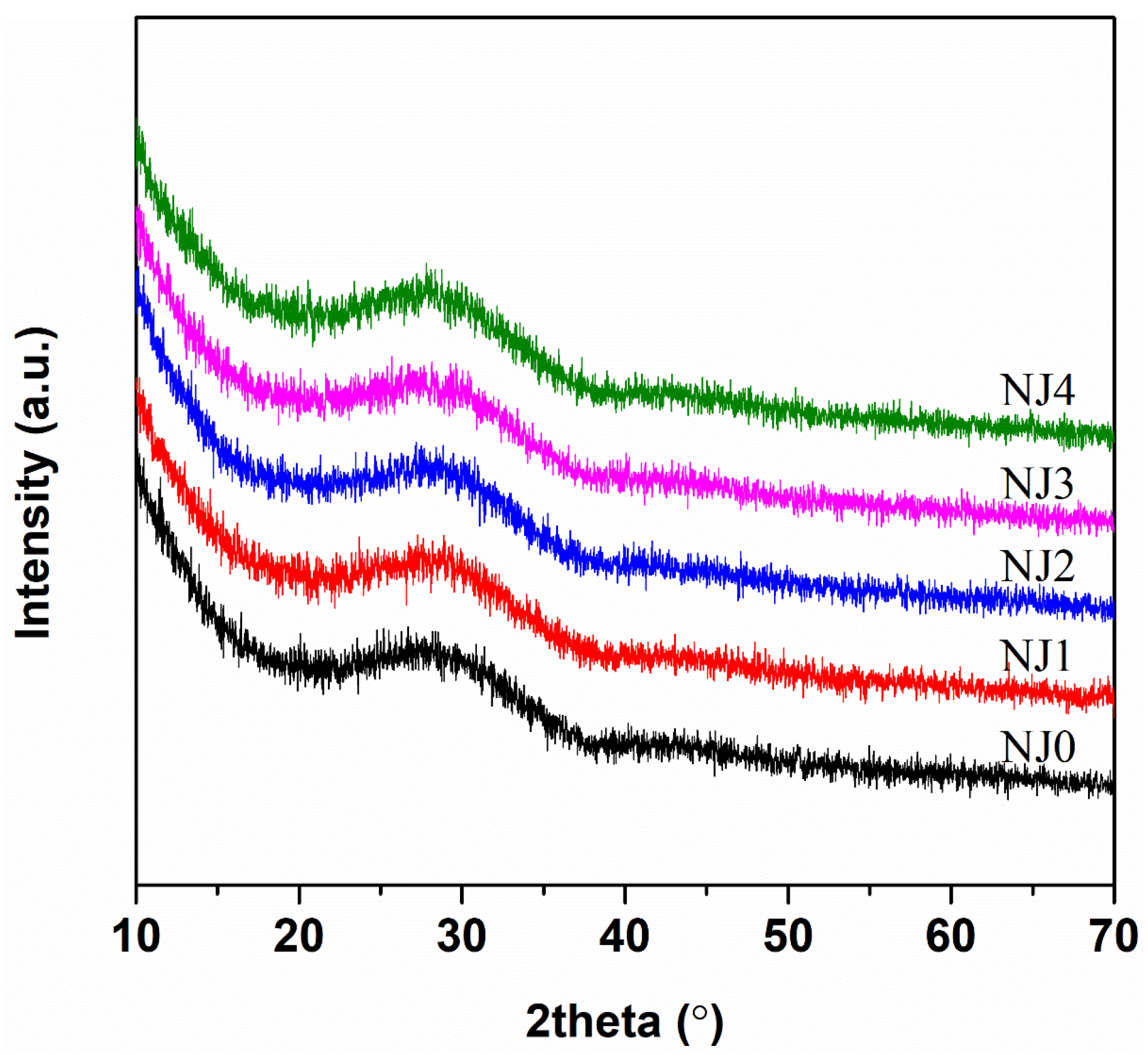
3.1. Appearance and Homogeneity
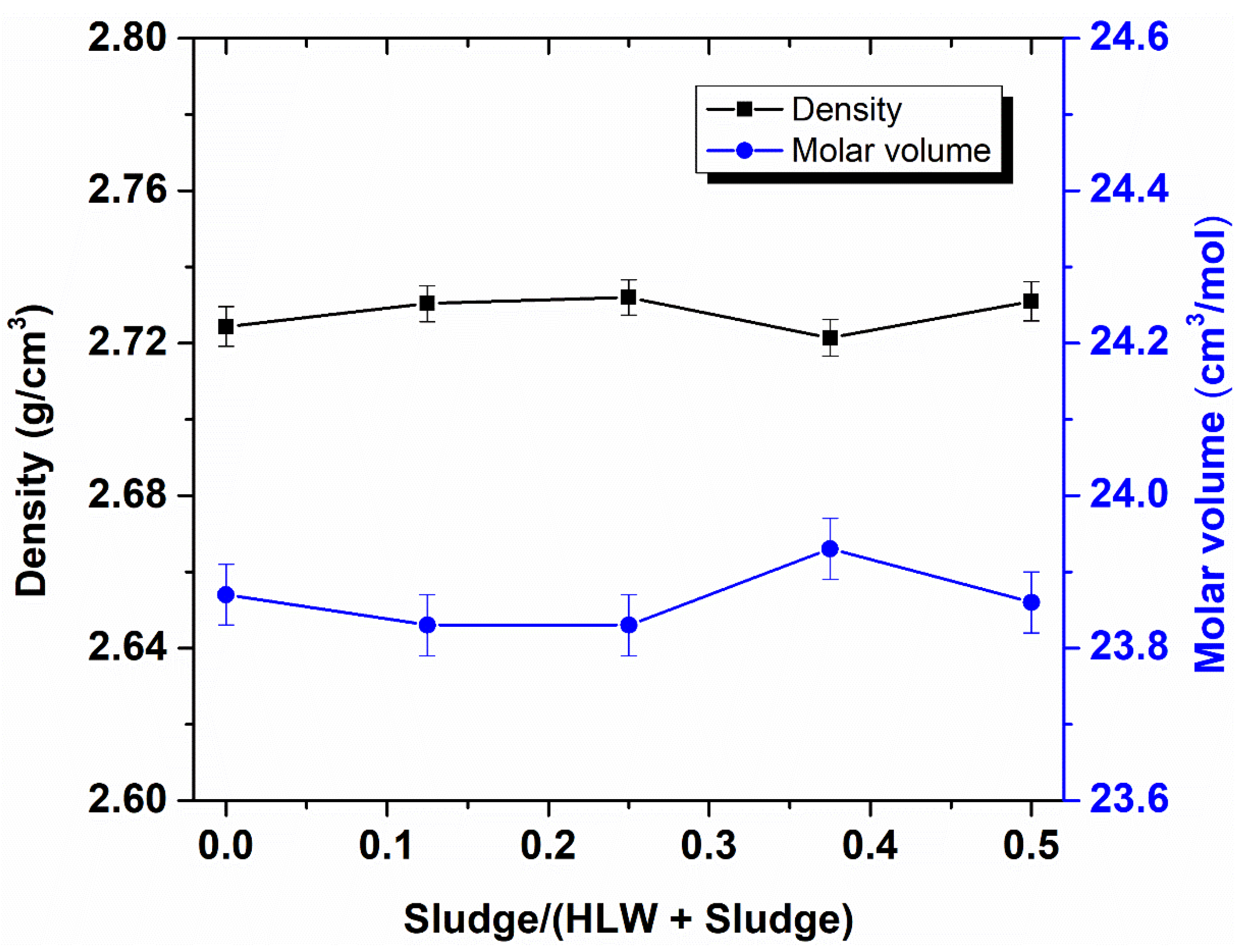
3.2. Glass Composition and Density
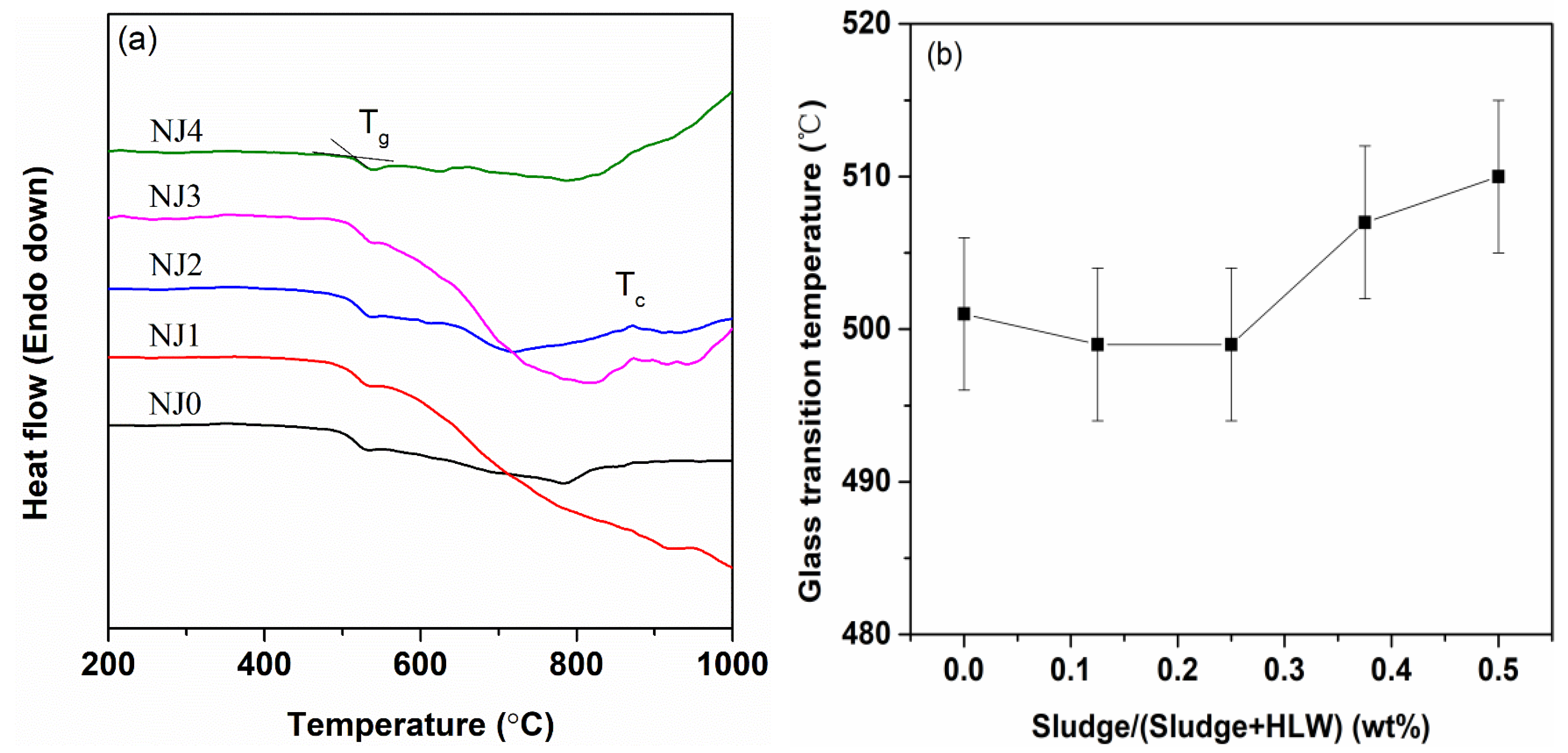
3.3. DSC Curves
3.4. Viscosity
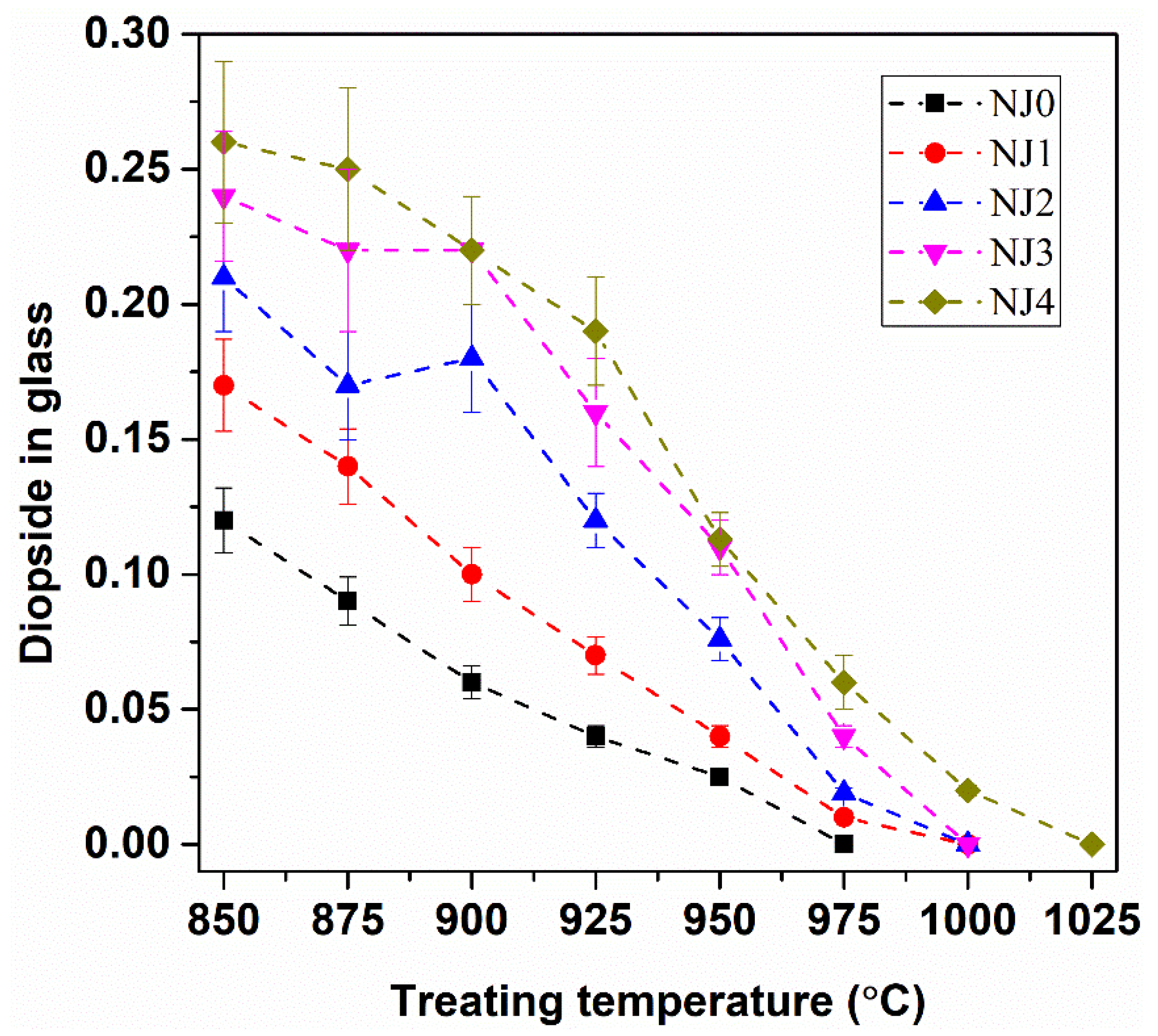
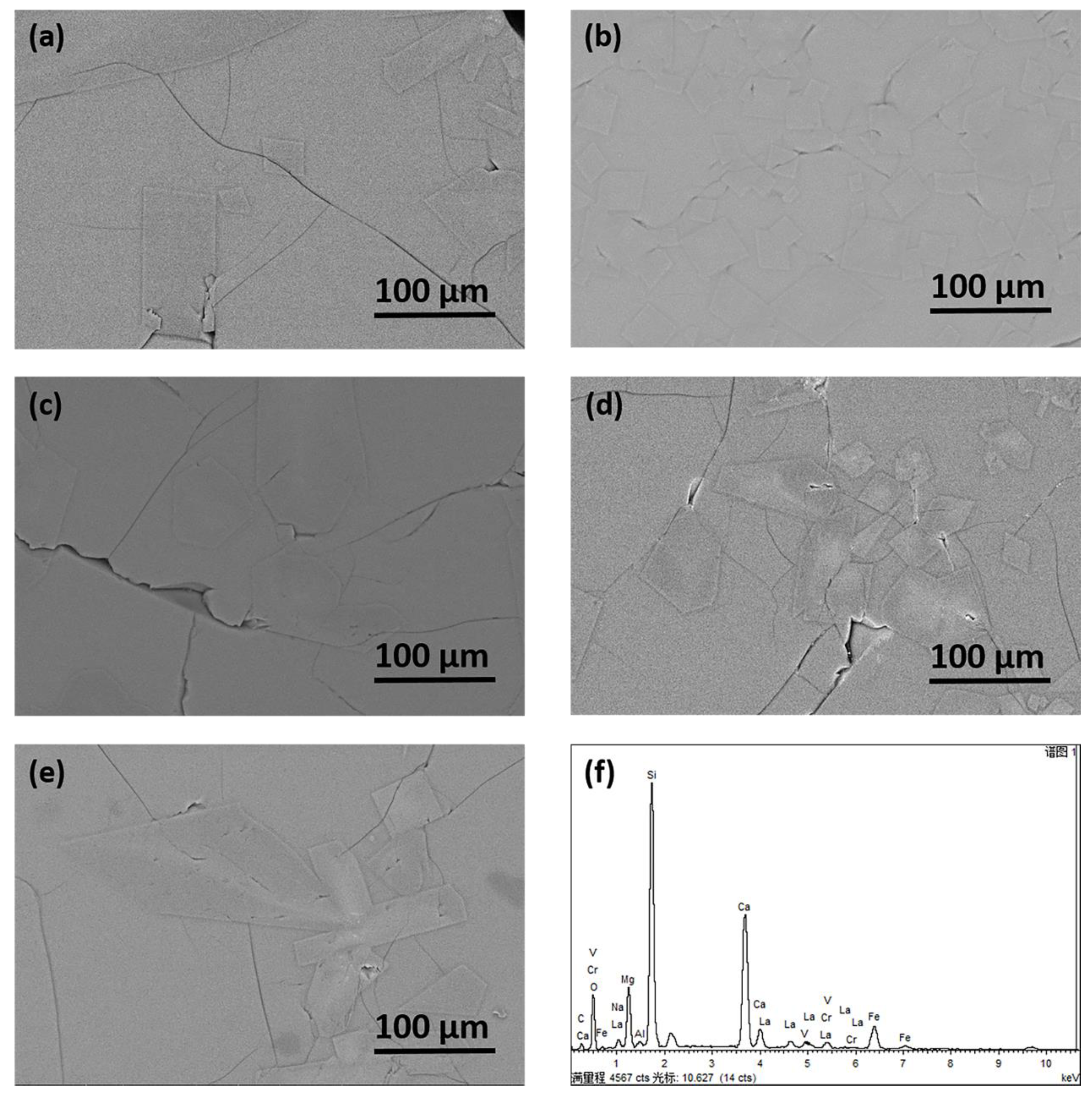
3.5. Glass-Crystallization Features
3.6. PCT-B Test
4. Discussion
4.1. Influence of Sludge Content on Glass Products’ Properties
4.2. Influence of Sludge Content on Glass Melting
5. Conclusions
- The substitution of sludge for HLW was conducive to minor changes in the glass density, molar volume, glass-transition temperature, and sulfur retention.
- The substitution of sludge for HLW gave rise to a notable increase in the viscosity and chemical durability of the glasses. Upon heat treatment, the crystallinity increased with the increasing sludge content in the glass, although the formed crystalline phases in the glasses remained the same.
- The glasses NJ0–NJ2 were proposed to vitrify the HLW blended with the sludge from the perspective of melter processing; that is, the sludge content in the overall waste should not exceed 25 wt %.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojovan, M.I. Immobilisation of Radioactive Wastes in Glass. In An Introduction to Nuclear Waste Immobilisation, 3rd ed.; Ojovan, M.I., Lee, W.E., Kalmykov, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–368. [Google Scholar]
- Aloy, A.S. Calcination and vitrification processes for conditioning of radioactive wastes. In Handbook of Advanced Radioactive Waste Conditioning Technologies, 1st ed.; Ojovan, M.I., Ed.; Woodhead Publishing: Oxford, UK, 2011; pp. 136–158. [Google Scholar]
- Tan, S. Glass-based stabilization/solidification of radioactive waste. In Low Carbon Stabilization and Solidification of Hazardous Wastes; Tsang, D., Wang, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 433–447. [Google Scholar]
- Wang, X.; Tuo, X.; Zhou, H.; Zhang, W.; Li, Z.; Chen, X.; Luo, H. Glass formulation development on high sodium and high sulfur bearing high level liquid waste for vitrification process. J. Nucl. Radiochem. 2013, 35, 180–192. (In Chinese) [Google Scholar]
- Wu, L.; Xiao, J.; Wang, X.; Teng, Y.; Li, Y.; Liao, Q. Crystalline phase, microstructure, and aqueous stability of zirconolite–barium borosilicate glass-ceramics for immobilization of simulated sulfate bearing high-level liquid waste. J. Nucl. Mater. 2018, 498, 241–248. [Google Scholar] [CrossRef]
- Liang, J.; Song, C.; Pan, C.; Ding, G.; Yang, W. The Preliminery Study of High-Level Radioactive Sludge I. Preparation and Property Study of Simulated High Level Radioactive Sludge. J. Nucl. Radiochem. 2000, 22, 37–44. (In Chinese) [Google Scholar]
- Fox, K.M.; Edwards, T.B.; Peeler, D.K. High Level Waste (HLW) Sludge Batch (SB4)-Selecting Glasses for a Variability Study; Savannah River National Laboratory: Aiken, SC, USA, 2006; p. 29. [Google Scholar]
- Sayenko, S.Y.; Shkuropatenko, V.A.; Pylypenko, O.V.; Karsim, S.O.; Zykova, A.V.; Kutnii, D.V.; Wagh, A.S. Radioactive waste immobilization of Hanford sludge in magnesium potassium phosphate ceramic forms. Prog. Nucl. Energy 2022, 152, 104315. [Google Scholar] [CrossRef]
- Donald, I.W. Waste Immobilisation in Glass and Ceramic Based Hosts: Radioactive, Toxic and Hazardous Wastes; Wiley-Blackwell: Chichester, UK, 2010; p. 527. [Google Scholar]
- Heath, P.G.; Stewart, M.W.A.; Moricca, S.; Hyatt, N.C. Hot-isostatically pressed wasteforms for Magnox sludge immobilisation. J. Nucl. Mater. 2018, 499, 233–241. [Google Scholar] [CrossRef]
- Tan, S.; Kirk, N.; Marshall, M.; McGann, O.; Hand, R.J. Vitrification of an intermediate level Magnox sludge waste. J. Nucl. Mater. 2019, 515, 392–400. [Google Scholar] [CrossRef]
- Amamoto, I.; Kobayashi, H.; Kitamura, N.; Takebe, H.; Mitamura, N.; Tsuzuki, T.; Fukayama, D.; Nagano, Y.; Jantzen, T.; Hack, K. Research on vitrification technology to immobilize radioactive sludge generated from Fukushima Daiichi power plant: Enhanced glass medium. J. Nucl. Sci. Technol. 2016, 53, 1467–1475. [Google Scholar] [CrossRef]
- Piovesan, V.; Bardez-Giboire, I.; Fournier, M.; Frugier, P.; Jollivet, P.; Montouillout, V.; Pellerin, N.; Gin, S. Chemical durability of peraluminous glasses for nuclear waste conditioning. NPJ Mater. Degrad. 2018, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mohd Fadzil, S.; Hrma, P.; Schweiger, M.J.; Riley, B.J. Component effects on crystallization of RE-containing aluminoborosilicate glass. J. Nucl. Mater. 2016, 478, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Mysen, B.; Richet, P. Silicate Glasses and Melts, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 720. [Google Scholar]
- Fox, K.M.; Peeler, D.K.; Edwards, T.B.; Best, D.R.; Reamer, I.A.; Workman, R.J.; Marra, J.C.; Riley, B.J.; Vienna, J.D.; Crum, J.V.; et al. International Study of Aluminum Impacts on Crystallization in U.S. High Level Waste Glass; Savannah River National Laboratory: Aiken, SC, USA, 2008; p. 172. [Google Scholar]
- Cizman, A.; Idczak, K.; Krupinski, M.; Girsova, M.; Zarzycki, A.; Rysiakiewicz-Pasek, E.; Zielony, E.; Staniorowski, P.; Wrzesinska, P.; Perlikowski, I.; et al. Comprehensive studies of activity of Ni in inorganic sodium borosilicate glasses doped with nickel oxide. Appl. Surf. Sci. 2021, 558, 149891. [Google Scholar] [CrossRef]
- Ren, M.; Yang, P.; Xu, J.; Zhang, Q.; Chen, K.; Tang, D.; Zhuang, H.; Sa, B.; Zhang, T. Effect of nickel doping on structure and suppressing boron volatility of borosilicate glass sealants in solid oxide fuel cells. J. Eur. Ceram. Soc. 2019, 39, 2179–2185. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sudarsan, K.V.; Sengupta, P.; Vatsa, R.K.; Tyagi, A.K.; Kaushik, C.P.; Das, D.; Raj, K. Role of Sulfate in Structural Modifications of Sodium Barium Borosilicate Glasses Developed for Nuclear Waste Immobilization. J. Am. Ceram Soc. 2008, 91, 3903–3907. [Google Scholar] [CrossRef]
- Liu, L.; Qie, D.; Zhou, H.; Li, B.; Li, Y.; Xu, J.; Zhang, H. Verification experiment research of glass formualtion for high sodium and high sulfur bearing HLLW. J. Nucl. Radiochem. 2014, 36, 163–168. (In Chinese) [Google Scholar]
- Goel, A.; McCloy, J.S.; Pokorny, R.; Kruger, A.A. Challenges with vitrification of Hanford High-Level Waste (HLW) to borosilicate glass–An overview. J. Non-Cryst. Solids X 2019, 4, 100033. [Google Scholar] [CrossRef]
- Ojovan, M.I. Viscosity and Glass Transition in Amorphous Oxides. Adv. Condens. Matter Phys. 2008, 2008, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Hrma, P.; Kruger, A.A. High-temperature viscosity of many-component glass melts. J. Non-Cryst. Solids 2016, 437, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Meng, B.; Zhu, Y.; Yang, D.; Cui, Z.; Jiao, Y.; Liu, H.; Dai, C. Effect of alkaline earth metal oxide content on crystallization behavior of simulated high level liquid waste glass. Bull. Chin. Ceram. Soc. 2022, 41, 4081–4088. [Google Scholar]
- Yang, Y.-G.; Zhang, Z.-T.; Hua, X.-H.; Wang, L. Crystallization of the simulated HLW glass. J. Nucl. Radiochem. 2014, 36, 317–320. (In Chinese) [Google Scholar]
- Manara, D.; Grandjean, A.; Pinet, O.; Dussossoy, J.L.; Neuville, D.R. Sulfur behavior in silicate glasses and melts: Implications for sulfate incorporation in nuclear waste glasses as a function of alkali cation and V2O5 content. J. Non-Cryst. Solids 2007, 353, 12–23. [Google Scholar] [CrossRef]
- Lenoir, M.; Grandjean, A.; Poissonnet, S.; Neuville, D.R. Quantitation of sulfate solubility in borosilicate glasses using Raman spectroscopy. J. Non-Cryst. Solids 2009, 355, 1468–1473. [Google Scholar] [CrossRef]
- Billings, A.L.; Fox, K.M. Retention of Sulfate in Savannah River Site High-Level Radioactive Waste Glass. Int. J. Appl. Glass Sci. 2010, 1, 388–400. [Google Scholar] [CrossRef]
- Lorier, T.H.; Reamer, I.A.; Workman, R.J. Initial Sulfate Solubility Study for Sludge Batch 4 (Sb4); Savannah River National Laboratory: Aiken, SC, USA, 2005; p. 22. [Google Scholar]
- McClane, D.L.; Amoroso, J.W.; Fox, K.M.; Kruger, A.A. Nepheline crystallization behavior in simulated high-level waste glasses. J. Non-Cryst. Solids 2019, 505, 215–224. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Effects of nickel on network structure and thermal properties of a new solid oxide cell seal glass. J. Power Sources 2008, 185, 993–1000. [Google Scholar] [CrossRef]
- Hrma, P.; Han, S.-S. Effect of glass composition on activation energy of viscosity in glass-melting-temperature range. J. Non-Cryst. Solids 2012, 358, 1818–1829. [Google Scholar] [CrossRef]
- Kroll, J.O.; Nelson, Z.J.; Skidmore, C.H.; Dixon, D.R.; Vienna, J.D. Formulation of high-Al2O3 waste glasses from projected Hanford waste compositions. J. Non-Cryst. Solids 2019, 517, 17–25. [Google Scholar] [CrossRef]
- Hrma, P.; Vienna, J.; Crum, J.; Piepel, G.; Mika, M. Liquidus Temperature of High-Level Waste Borosilicate Glasses With Spinel Primary Phase. MRS Online Proc. Libr. 1999, 608, 671. [Google Scholar] [CrossRef]
- Jantzen, C.M.; Brown, K.G. Predicting the Spinel?Nepheline Liquidus for Application to Nuclear Waste Glass Processing. Part I: Primary Phase Analysis, Liquidus Measurment, and Quasicrystalline Approach. J. Am. Ceram Soc. 2007, 90, 1866–1879. [Google Scholar] [CrossRef]




| Simulated HLW | Simulated Sludge | ||
|---|---|---|---|
| Oxide | Content (wt %) | Oxide | Content (wt %) |
| CeO2 | 0.35 | ||
| Cr2O3 | 2.02 | ||
| Cs2O | 0.09 | ||
| K2O | 0.61 | ||
| La2O3 | 11.63 | La2O3 | 5.0 |
| Fe2O3 | 21.46 | Fe2O3 | 20.0 |
| Al2O3 | 8.77 | Al2O3 | 25.0 |
| BaO | 0.13 | BaO | 5.0 |
| Na2O | 45.41 | Na2O | 20.0 |
| MoO3 | 0.78 | ||
| Nd2O3 | 0.69 | ||
| NiO | 2.56 | NiO | 20.0 |
| P2O5 | 0.44 | ||
| SO3 | 4.62 | SO3 | 5.0 |
| SrO | 0.05 | ||
| TiO2 | 0.32 | ||
| Y2O3 | 0.09 | ||
| Total | 100.00 | Total | 100.00 |
| Oxide | NJ0 | NJ1 | NJ2 | NJ3 | NJ4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal | Measured | Nominal | Measured | Nominal | Measured | Nominal | Measured | Nominal | Measured | |
| SiO2 | 44.89 | 45.20 | 44.89 | 44.70 | 44.89 | 45.25 | 44.89 | 44.76 | 44.89 | 44.60 |
| B2O3 * | 12.26 | 12.26 | 12.26 | 12.26 | 12.26 | 12.26 | 12.26 | 12.26 | 12.26 | 12.26 |
| Al2O3 | 5.10 | 6.15 | 5.43 | 6.44 | 5.75 | 6.74 | 6.08 | 6.99 | 6.40 | 7.23 |
| Li2O * | 2.18 | 2.18 | 2.18 | 2.18 | 2.18 | 2.18 | 2.18 | 2.18 | 2.18 | 2.18 |
| Na2O | 11.65 | 11.10 | 11.14 | 10.67 | 10.63 | 10.11 | 10.12 | 9.78 | 9.61 | 9.25 |
| MgO | 4.37 | 4.24 | 4.37 | 4.08 | 4.37 | 4.28 | 4.37 | 4.35 | 4.37 | 4.45 |
| CaO | 6.72 | 6.31 | 6.72 | 6.68 | 6.72 | 6.53 | 6.72 | 6.56 | 6.72 | 6.87 |
| BaO | 3.51 | 3.39 | 3.61 | 3.37 | 3.71 | 3.53 | 3.80 | 3.55 | 3.90 | 3.75 |
| Fe2O3 | 3.43 | 3.58 | 3.40 | 3.47 | 3.38 | 3.35 | 3.35 | 3.43 | 3.32 | 3.28 |
| La2O3 | 1.86 | 2.01 | 1.73 | 2.06 | 1.60 | 1.73 | 1.46 | 1.58 | 1.33 | 1.21 |
| NiO | 0.41 | 0.32 | 0.76 | 0.82 | 1.11 | 1.05 | 1.46 | 1.35 | 1.80 | 1.84 |
| V2O5 | 1.50 | 1.54 | 1.50 | 1.43 | 1.50 | 1.38 | 1.50 | 1.53 | 1.50 | 1.55 |
| SO3 | 0.74 | 0.54 | 0.75 | 0.62 | 0.75 | 0.57 | 0.76 | 0.61 | 0.77 | 0.53 |
| Sb2O3 | 0.50 | 0.56 | 0.50 | 0.59 | 0.50 | 0.53 | 0.50 | 0.56 | 0.50 | 0.48 |
| Others ** | 0.88 | 0.62 | 0.76 | 0.63 | 0.65 | 0.51 | 0.55 | 0.58 | 0.45 | 0.52 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Sample | Atomic Content (Normalized to O = 6; Fe Assumed to Be Present as Fe2+) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Ca | Mg | Fe | Al | Cr | Ni | La | O | |
| NJ0 | 2.05 | 0.77 | 0.80 | 0.20 | 0.02 | 0.02 | 0.01 | 0.03 | 6.00 |
| NJ1 | 2.07 | 0.78 | 0.77 | 0.17 | 0.04 | 0.01 | 0.02 | 0.03 | 6.00 |
| NJ2 | 2.07 | 0.76 | 0.78 | 0.19 | 0.03 | 0.02 | 0.02 | 0.02 | 6.00 |
| NJ3 | 2.07 | 0.78 | 0.74 | 0.18 | 0.03 | 0.02 | 0.04 | 0.03 | 6.00 |
| NJ4 | 2.06 | 0.75 | 0.77 | 0.18 | 0.04 | 0.01 | 0.04 | 0.04 | 6.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Chang, J.; Liu, X.; Sun, S.; Xian, L.; Zhang, S. Influence of Radioactive Sludge Content on Vitrification of High-Level Liquid Waste. Sustainability 2023, 15, 4937. https://doi.org/10.3390/su15064937
Tan S, Chang J, Liu X, Sun S, Xian L, Zhang S. Influence of Radioactive Sludge Content on Vitrification of High-Level Liquid Waste. Sustainability. 2023; 15(6):4937. https://doi.org/10.3390/su15064937
Chicago/Turabian StyleTan, Shengheng, Jiong Chang, Xiao Liu, Shikuan Sun, Liang Xian, and Shengdong Zhang. 2023. "Influence of Radioactive Sludge Content on Vitrification of High-Level Liquid Waste" Sustainability 15, no. 6: 4937. https://doi.org/10.3390/su15064937
APA StyleTan, S., Chang, J., Liu, X., Sun, S., Xian, L., & Zhang, S. (2023). Influence of Radioactive Sludge Content on Vitrification of High-Level Liquid Waste. Sustainability, 15(6), 4937. https://doi.org/10.3390/su15064937







