Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives
Abstract
:1. Introduction
2. Immobilized Algae Bioremediation Technology
2.1. Methodology
2.2. Mechanisms of Heavy Metal Removal by Algae
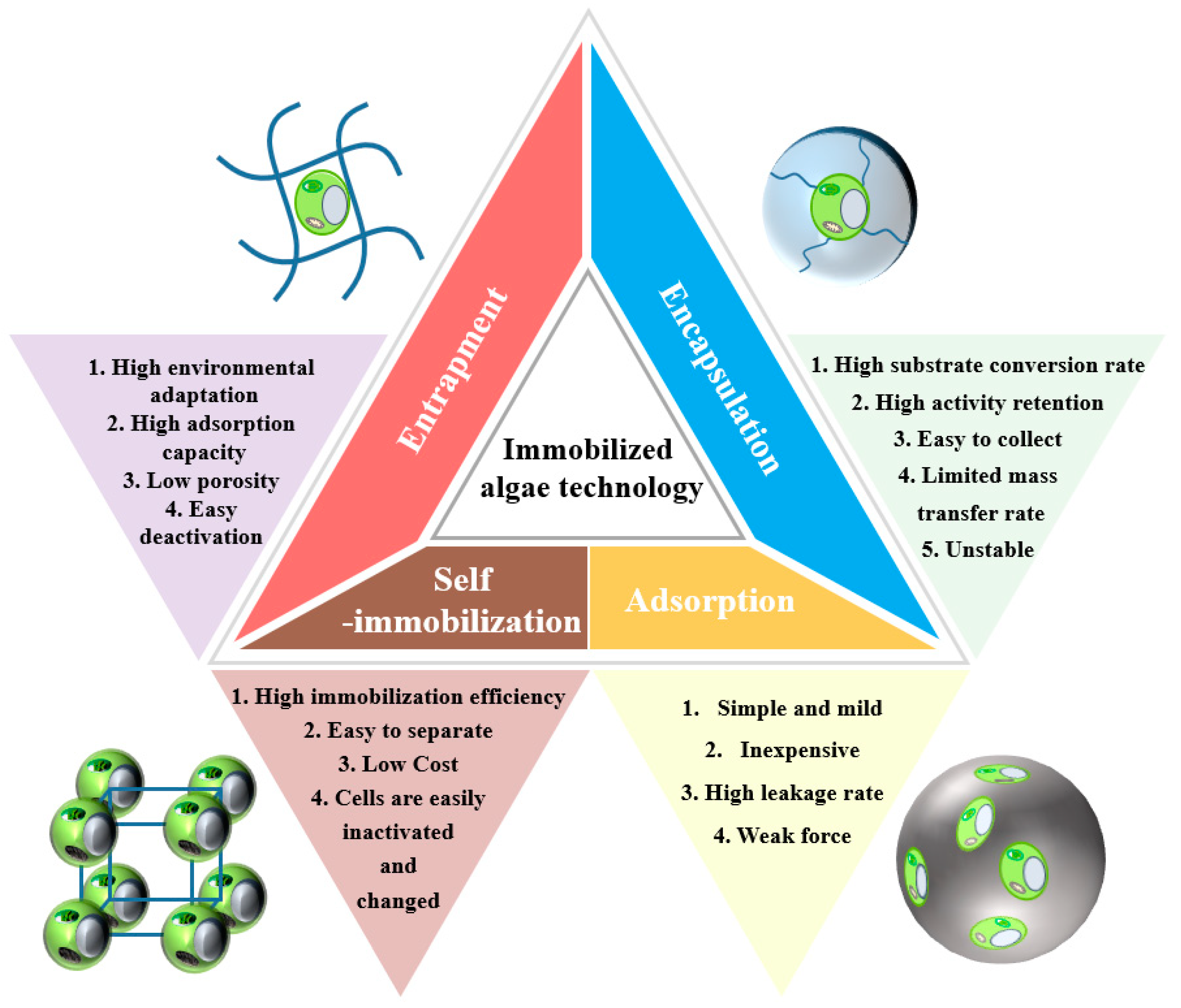
2.3. Immobilized Algae Technology
2.3.1. Adsorption
2.3.2. Encapsulation
2.3.3. Entrapment
2.3.4. Self-Immobilization
3. Immobilization Parameters
3.1. Adsorbent Dosage
3.2. Initial Metal Concentration and Type
3.3. Temperature
3.4. pH Value
3.5. Contact Time
3.6. Metal Systems
3.7. Algae Type
3.8. Immobilized Carriers
4. Life Cycle Assessment and Economic Evaluation
- the production of algae cultivation and the production of immobilized carriers,
- the production of immobilized algal systems and their transport to wastewater treatment,
- the production of various solvents included,
- the production of electricity and water,
- the adsorption of heavy metal ions, and
- the regeneration and reuse process of immobilized adsorbents.
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Cai, L.-M.; Xu, Z.-C.; Qi, J.-Y.; Feng, Z.-Z.; Xiang, T.-S. Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 2015, 127, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Zhong, T. Pollution and health risk assessment of heavy metals in urban soil in China. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 424–434. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Nateras-Ramírez, O.; Martínez-Macias, M.R.; Sánchez-Machado, D.I.; López-Cervantes, J.; Aguilar-Ruiz, R.J. An overview of microalgae for Cd2+ and Pb2+ biosorption from wastewater. Bioresour. Technol. Rep. 2022, 17, 100932. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2023, 81, 1130–1136. [Google Scholar] [CrossRef]
- Liu, T.; Lawluvy, Y.; Shi, Y.; Ighalo, J.O.; He, Y.; Zhang, Y.; Yap, P.-S. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J. Environ. Chem. Eng. 2022, 10, 106502. [Google Scholar] [CrossRef]
- Khan, Z.U.; Khan, W.U.; Ullah, B.; Ali, W.; Ahmad, B.; Ali, W.; Yap, P.-S. Graphene oxide/PVC composite papers functionalized with p-Phenylenediamine as high-performance sorbent for the removal of heavy metal ions. J. Environ. Chem. Eng. 2021, 9, 105916. [Google Scholar] [CrossRef]
- Syeda, H.I.; Sultan, I.; Razavi, K.S.; Yap, P.-S. Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: A review. J. Water Process Eng. 2022, 46, 102446. [Google Scholar] [CrossRef]
- Syeda, H.I.; Yap, P.-S. A review on three-dimensional cellulose-based aerogels for the removal of heavy metals from water. Sci. Total Environ. 2022, 807, 150606. [Google Scholar] [CrossRef]
- Min, K.J.; Kim, J.M.; Park, K.Y. Characteristics of heavy metal separation and determination of limiting current density in a pilot-scale electrodialysis process for plating wastewater treatment. Sci. Total Environ. 2021, 757, 143762. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Assefi, M.; Maroufi, S.; Sahajwalla, V. Selective isolation of heavy metals from spent electronic waste solution by macroporous ion-exchange resins. J. Hazard. Mater. 2019, 371, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano-fibrous metal-organic framework membrane filtration process. Sci. Total Environ. 2019, 674, 355–362. [Google Scholar] [CrossRef] [PubMed]
- El Gaayda, J.; Rachid, Y.; Titchou, F.E.; Barra, I.; Hsini, A.; Yap, P.-S.; Oh, W.-D.; Swanson, C.; Hamdani, M.; Akbour, R.A. Optimizing removal of chromium (VI) ions from water by coagulation process using central composite design: Effectiveness of grape seed as a green coagulant. Sep. Purif. Technol. 2023, 307, 122805. [Google Scholar] [CrossRef]
- El-Gaayda, J.; Titchou, F.E.; Oukhrib, R.; Yap, P.-S.; Liu, T.; Hamdani, M.; Ait Akbour, R. Natural flocculants for the treatment of wastewaters containing dyes or heavy metals: A state-of-the-art review. J. Environ. Chem. Eng. 2021, 9, 106060. [Google Scholar] [CrossRef]
- Liu, C.; Wu, T.; Hsu, P.-C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; et al. Direct/Alternating Current Electrochemical Method for Removing and Recovering Heavy Metal from Water Using Graphene Oxide Electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, L.; Song, S.; Farías, M.E.; Li, Y.; Tang, C. Cadmium removal from diluted wastewater by using high-phosphorus-culture modified microalgae. Chem. Phys. Lett. 2021, 771, 138561. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, Y.; Li, C.; Lv, X.; Wang, D. Biosorption of cadmium(II) from aqueous solution by chitosan encapsulated Zygosaccharomyces rouxii. Environ. Prog. Sustain. Energy 2013, 32, 1101–1110. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.H.; Buang, A. Enhanced biosorption of transition metals by living Chlorella vulgaris immobilized in Ca-alginate beads. Environ. Technol. 2019, 40, 1793–1809. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Chen, C.-Y.; Guo, W.-Q.; Chang, H.-W.; Chen, W.-M.; Lee, D.-J.; Huang, C.-C.; Ren, N.-Q.; Chang, J.-S. Fixed-bed biosorption of cadmium using immobilized Scenedesmus obliquus CNW-N cells on loofa (Luffa cylindrica) sponge. Bioresour. Technol. 2014, 160, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, H.; Song, S. Cell surface characterization of some oleaginous green algae. J. Appl. Phycol. 2016, 28, 2323–2332. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Bhatt, K.; Simsek, H. Microalgae-based removal of pollutants from wastewaters: Occurrence, toxicity and circular economy. Chemosphere 2022, 306, 135576. [Google Scholar] [CrossRef]
- Mandotra, S.K.; Lolu, A.J.; Kumar, S.; Ramteke, P.W.; Ahluwalia, A.S. Integrated Approach for Bioremediation and Biofuel Production Using Algae. In Restoration of Wetland Ecosystem: A Trajectory towards a Sustainable Environment; Upadhyay, A.K., Singh, R., Singh, D.P., Eds.; Springer: Singapore, 2020; pp. 145–160. [Google Scholar]
- Covarrubias, S.A.; de-Bashan, L.E.; Moreno, M.; Bashan, Y. Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 2012, 93, 2669–2680. [Google Scholar] [CrossRef]
- Sankhla, M.; Kumar, R.; Biswas, A. Dynamic nature of heavy metal toxicity in water and sediments of Ayad River with climatic change. Int. J. Hydro. 2019, 3, 339–343. [Google Scholar] [CrossRef]
- Appenroth, K.-J. Definition of “Heavy Metals” and Their Role in Biological Systems. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–29. [Google Scholar]
- Ameri, A.; Tamjidi, S.; Dehghankhalili, F.; Farhadi, A.; Saati, M.A. Application of algae as low cost and effective bio-adsorbent for removal of heavy metals from wastewater: A review study. Environ. Technol. Rev. 2020, 9, 85–110. [Google Scholar] [CrossRef]
- Priatni, S.; Ratnaningrum, D.; Warya, S.; Audina, E. Phycobiliproteins production and heavy metals reduction ability of Porphyridium sp. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012006. [Google Scholar] [CrossRef]
- Balaji, S.; Kalaivani, T.; Sushma, B.; Pillai, C.V.; Shalini, M.; Rajasekaran, C. Characterization of sorption sites and differential stress response of microalgae isolates against tannery effluents from ranipet industrial area—An application towards phycoremediation. Int. J. Phytoremediat. 2016, 18, 747–753. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Pandey, A.; Pathak, V.V.; Tyagi, V.V.; Kothari, R. Phycoremediation: Algae as Eco-friendly Tools for the Removal of Heavy Metals from Wastewaters. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 53–76. [Google Scholar]
- Park, D.M.; Reed, D.W.; Yung, M.C.; Eslamimanesh, A.; Lencka, M.M.; Anderko, A.; Fujita, Y.; Riman, R.E.; Navrotsky, A.; Jiao, Y. Bioadsorption of Rare Earth Elements through Cell Surface Display of Lanthanide Binding Tags. Environ. Sci. Technol. 2016, 50, 2735–2742. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, K. Biosorption and bioaccumulation–The prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Sargın, İ.; Arslan, G.; Kaya, M. Efficiency of chitosan–algal biomass composite microbeads at heavy metal removal. React. Funct. Polym. 2016, 98, 38–47. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Darwish, H.; Alharthi, S.; Al-Zaban, M.I.; Noureldeen, A.; Hassan, S.H.A. Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box–Behnken experimental design. Sci. Rep. 2022, 12, 3256. [Google Scholar] [CrossRef] [PubMed]
- Saba, S. Biosorption of Heavy Metals. In Biosorption; Jan, D., Branislav, V., Eds.; IntechOpen: Rijeka, Italy, 2018; p. Ch. 2. [Google Scholar]
- Aswathi Mohan, A.; Robert Antony, A.; Greeshma, K.; Yun, J.-H.; Ramanan, R.; Kim, H.-S. Algal biopolymers as sustainable resources for a net-zero carbon bioeconomy. Bioresour. Technol. 2022, 344, 126397. [Google Scholar] [CrossRef]
- Greeshma, K.; Kim, H.-S.; Ramanan, R. The emerging potential of natural and synthetic algae-based microbiomes for heavy metal removal and recovery from wastewaters. Environ. Res. 2022, 215, 114238. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.-H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038. [Google Scholar] [CrossRef]
- Pereira, S.B.; Mota, R.; Vieira, C.P.; Vieira, J.; Tamagnini, P. Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci. Rep. 2015, 5, 14835. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Yang, Y.; Wang, P.; Wang, C.; Miao, L.; Wang, X.; Lv, B.; You, G.; Liu, Z. Effects of CeO2, CuO, and ZnO nanoparticles on physiological features of Microcystis aeruginosa and the production and composition of extracellular polymeric substances. Environ. Sci. Pollut. Res. 2017, 24, 226–235. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Liu, Y.; Huang, F.; Zhang, C. Effect of extracellular polymeric substances on arsenic accumulation in Chlorella pyrenoidosa. Sci. Total Environ. 2020, 704, 135368. [Google Scholar] [CrossRef]
- Zohoorian, H.; Ahmadzadeh, H.; Molazadeh, M.; Shourian, M.; Lyon, S. Chapter 41–Microalgal bioremediation of heavy metals and dyes. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 659–674. [Google Scholar]
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal Bioremediation of heavy metals: An insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129. [Google Scholar] [CrossRef]
- Tripathi, S.; Poluri, K.M. Metallothionein-and Phytochelatin-Assisted Mechanism of Heavy Metal Detoxification in Microalgae. In Approaches to the Remediation of Inorganic Pollutants; Hasanuzzaman, M., Ed.; Springer: Singapore, 2021; pp. 323–344. [Google Scholar]
- Ahmad, J.; Ali, A.A.; Baig, M.A.; Iqbal, M.; Haq, I.; Irfan Qureshi, M. Chapter 8–Role of Phytochelatins in Cadmium Stress Tolerance in Plants. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 185–212. [Google Scholar]
- García-García, J.D.; Sánchez-Thomas, R.; Moreno-Sánchez, R. Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnol. Adv. 2016, 34, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Bhuyan, P.P.; Nayak, R.; Patra, S.; Behera, C.; Ki, J.-S.; Ragusa, A.; Lukatkin, A.S.; Jena, M. Microalgal Phycoremediation: A Glimpse into a Sustainable Environment. Toxics 2022, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Chen, P.-W.; Hsu, C.-Y.; Lee, L. The use of autotrophic Chlorella vulgaris in chromium (VI) reduction under different reduction conditions. J. Taiwan Inst. Chem. Eng. 2017, 74, 1–6. [Google Scholar] [CrossRef]
- Sarojini, G.; Venkatesh Babu, S.; Rajamohan, N.; Senthil Kumar, P.; Rajasimman, M. Surface modified polymer-magnetic-algae nanocomposite for the removal of chromium- equilibrium and mechanism studies. Environ. Res. 2021, 201, 111626. [Google Scholar] [CrossRef] [PubMed]
- Raize, O.; Argaman, Y.; Yannai, S. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol. Bioeng. 2004, 87, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel -Aty, A.M.; Ammar, N.S.; Abdel Ghafar, H.H.; Ali, R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013, 4, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Rensing, C.; Zhu, Y.-G. Cyanobacteria-Mediated Arsenic Redox Dynamics Is Regulated by Phosphate in Aquatic Environments. Environ. Sci. Technol. 2014, 48, 994–1000. [Google Scholar] [CrossRef]
- Badisa, V.L.D.; Latinwo, L.M.; Odewumi, C.O.; Ikediobi, C.O.; Badisa, R.B.; Ayuk-Takem, L.T.; Nwoga, J.; West, J. Mechanism of DNA damage by cadmium and interplay of antioxidant enzymes and agents. Environ. Toxicol. 2007, 22, 144–151. [Google Scholar] [CrossRef]
- Al-Hasawi, Z.M.; Abdel-Hamid, M.I.; Almutairi, A.W.; Touliabah, H.E. Response of Pseudokirchneriella subcapitata in Free and Alginate Immobilized Cells to Heavy Metals Toxicity. Molecules 2020, 25, 2847. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Smith, S.M.; Raston, C.L. Application of Various Immobilization Techniques for Algal Bioprocesses. In Biomass and Biofuels from Microalgae: Advances in Engineering and Biology; Moheimani, N.R., McHenry, M.P., de Boer, K., Bahri, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–44. [Google Scholar]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Sánchez-Saavedra, M.d.P.; Molina-Cárdenas, C.A.; Castro-Ochoa, F.Y.; Castro-Ceseña, A.B. Protective effect of glycerol and PEG-methyl ether methacrylate coatings on viability of alginate-immobilized Synechococcus elongatus after cold storage. J. Appl. Phycol. 2019, 31, 2289–2297. [Google Scholar] [CrossRef]
- Fu, M.; Liang, J.; Wang, S.; Geng, C.; Zhang, W.; Wu, T. The Response of Microalgae Chlorella sp. to Free and Immobilized ZrO2 and Mg(OH)2 Nanoparticles: Perspective from the Growth Characteristics. Environ. Eng. Sci. 2020, 37, 429–438. [Google Scholar] [CrossRef]
- Xie, B.; Gong, W.; Yu, H.; Tang, X.; Yan, Z.; Luo, X.; Gan, Z.; Wang, T.; Li, G.; Liang, H. Immobilized microalgae for anaerobic digestion effluent treatment in a photobioreactor-ultrafiltration system: Algal harvest and membrane fouling control. Bioresour. Technol. 2018, 268, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; Silva, E.A. Biosorption of nickel(II) and copper(II) ions in batch and fixed-bed columns by free and immobilized marine algae Sargassum sp. J. Clean. Prod. 2017, 150, 58–64. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, X.; Su, G.; Danquah, M.K.; Zhang, S.; Lu, Y.; Sun, Y.; Lin, L. Bioprocess considerations for microalgal-based wastewater treatment and biomass production. Renew. Sustain. Energy Rev. 2015, 42, 1385–1392. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017, 120, 174–184. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, D.; Im, S.; Jang, A. Optimization of alginate bead size immobilized with Chlorella vulgaris and Chlamydomonas reinhardtii for nutrient removal. Bioresour. Technol. 2020, 302, 122891. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Fermentative ethanol production from Madhuca indica flowers using immobilized yeast cells coupled with solar driven direct contact membrane distillation with commercial hydrophobic membranes. Energy Convers. Manag. 2019, 181, 593–607. [Google Scholar] [CrossRef]
- Murujew, O.; Whitton, R.; Kube, M.; Fan, L.; Roddick, F.; Jefferson, B.; Pidou, M. Recovery and reuse of alginate in an immobilized algae reactor. Environ. Technol. 2021, 42, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Ho, S.-H. Immobilized microalgal system: An achievable idea for upgrading current microalgal wastewater treatment. Environ. Sci. Ecotechnol. 2023, 14, 100227. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Li, Z.; Fan, L.; Chen, R.; Wu, X.; Li, J.; Zeng, W. A high-efficiency Fe2O3@Microalgae composite for heavy metal removal from aqueous solution. J. Water Process Eng. 2020, 33, 101026. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Li, Y.; Yu, Z.; Chen, Z.; Ye, T.; Wang, X.; Hu, Z.; Liu, S.; Xiao, B.; et al. Cultivation of algal biofilm using different lignocellulosic materials as carriers. Biotechnol. Biofuels 2017, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Vasilieva, S.; Lobakova, E.; Grigoriev, T.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Gotovtsev, P.; Antipova, C.; Zagoskin, Y.; Scherbakov, P.; et al. Bio-inspired materials for nutrient biocapture from wastewater: Microalgal cells immobilized on chitosan-based carriers. J. Water Process Eng. 2021, 40, 101774. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdou, A.E.H.; Mohamed, S.M.S.; Osman, M.M. Engineered staphylococcus aureus via immobilization on magnetic Fe3O4-phthalate nanoparticles for biosorption of divalent ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3810–3824. [Google Scholar] [CrossRef]
- Whitton, R.; Ometto, F.; Villa, R.; Pidou, M.; Jefferson, B. Influence of light regime on the performance of an immobilised microalgae reactor for wastewater nutrient removal. Algal Res. 2019, 44, 101648. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Pan, F.; Zhou, H.; Yang, X.; Li, W.; Liu, H. Adsorption Properties toward Trivalent Rare Earths by Alginate Beads Doping with Silica. Ind. Eng. Chem. Res. 2013, 52, 3453–3461. [Google Scholar] [CrossRef]
- Qin, L.; Gao, M.; Zhang, M.; Feng, L.; Liu, Q.; Zhang, G. Application of encapsulated algae into MBR for high-ammonia nitrogen wastewater treatment and biofouling control. Water Res. 2020, 187, 116430. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Li, F.; Ho, S.-H. Data-driven analysis on immobilized microalgae system: New upgrading trends for microalgal wastewater treatment. Sci. Total Environ. 2022, 852, 158514. [Google Scholar] [CrossRef]
- Maswanna, T.; Phunpruch, S.; Lindblad, P.; Maneeruttanarungroj, C. Enhanced hydrogen production by optimization of immobilized cells of the green alga Tetraspora sp. CU2551 grown under anaerobic condition. Biomass Bioenergy 2018, 111, 88–95. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Kube, M.; Spedding, B.; Gao, L.; Fan, L.; Roddick, F. Nutrient removal by alginate-immobilized Chlorella vulgaris: Response to different wastewater matrices. J. Chem. Technol. Biotechnol. 2020, 95, 1790–1799. [Google Scholar] [CrossRef]
- Saxena, A.; Mishra, B.; Tiwari, A. Development of diatom entrapped alginate beads and application of immobilized cells in aquaculture. Environ. Technol. Innov. 2021, 23, 101736. [Google Scholar] [CrossRef]
- Giese, E.C.; Silva, D.D.V.; Costa, A.F.M.; Almeida, S.G.C.; Dussán, K.J. Immobilized microbial nanoparticles for biosorption. Crit. Rev. Biotechnol. 2020, 40, 653–666. [Google Scholar] [CrossRef]
- Zheng, Z.; Ali, A.; Su, J.; Zhang, S.; Fan, Y.; Sun, Y. Self-immobilized biochar fungal pellet combined with bacterial strain H29 enhanced the removal performance of cadmium and nitrate. Bioresour. Technol. 2021, 341, 125803. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Vasilieva, S.; Shibzukhova, K.; Morozov, A.; Solovchenko, A.; Bessonov, I.; Kopitsyna, M.; Lukyanov, A.; Chekanov, K.; Lobakova, E. Immobilization of microalgae on the surface of new cross-linked polyethylenimine-based sorbents. J. Biotechnol. 2018, 281, 31–38. [Google Scholar] [CrossRef]
- Tran, N.-A.T.; Seymour, J.R.; Siboni, N.; Evenhuis, C.R.; Tamburic, B. Photosynthetic carbon uptake induces autoflocculation of the marine microalga Nannochloropsis oculata. Algal Res. 2017, 26, 302–311. [Google Scholar] [CrossRef]
- Alam, M.A.; Wan, C.; Guo, S.-L.; Zhao, X.-Q.; Huang, Z.-Y.; Yang, Y.-L.; Chang, J.-S.; Bai, F.-W. Characterization of the flocculating agent from the spontaneously flocculating microalga Chlorella vulgaris JSC-7. J. Biosci. Bioeng. 2014, 118, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, S.; Lobakova, E.; Solovchenko, A. Biotechnological Applications of Immobilized Microalgae. In Environmental Biotechnology; Gothandam, K.M., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 3, pp. 193–220. [Google Scholar]
- Martins, S.C.S.; Martins, C.M.; Fiúza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar] [CrossRef]
- Petrovič, A.; Simonič, M. Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int. J. Environ. Sci. Technol. 2016, 13, 1761–1780. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shim, E.; Yun, H.-S.; Park, Y.-T.; Kim, D.; Ji, M.-K.; Kim, C.-K.; Shin, W.-S.; Choi, J. Biosorption of Cu(II) by immobilized microalgae using silica: Kinetic, equilibrium, and thermodynamic study. Environ. Sci. Pollut. Res. 2016, 23, 1025–1034. [Google Scholar] [CrossRef]
- Rinanti, A.; Fachrul, M.F.; Hadisoebroto, R. Improving Biosorption of Cu (II)-ion on Artificial Wastewater by Immobilized Biosorbent of Tropical Microalgae. GEOMATE J. 2017, 13, 6–10. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, W.; Li, H.; Ho, S.-H.; Chen, J.; Xie, Y.; Shi, X. Enhancing cadmium bioremediation by a complex of water-hyacinth derived pellets immobilized with Chlorella sp. Bioresour. Technol. 2018, 257, 157–163. [Google Scholar] [CrossRef]
- El Bestawy, E. Efficiency of immobilized cyanobacteria in heavy metals removal from industrial effluents. Desalination Water Treat 2019, 159, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Lieswito, N.A.; Rinanti, A.; Fachrul, M.F. Removal of heavy metal (Cu2+) by immobilized microalgae biosorbent with effect of temperature and contact time. J. Phys. Conf. Ser. 2019, 1402, 022106. [Google Scholar] [CrossRef] [Green Version]
- Byamba, T.; Hasegawa, K.; Maeda, I. Removal of Pb (II) from Aqueous Solution by a Pectin-Producing Alga, Penium margaritaceum, Immobilized on Filter Paper. Microbiol. Res. 2022, 13, 1007–1017. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El Sabagh, S.; Abou El-Souod, G.; Elbeltagy, A. Biosorption of Cadmium from Aqueous Solution by Free and Immobilized Dry Biomass of Chlorella vulgaris. Int. J. Environ. Res. 2019, 13, 511–521. [Google Scholar] [CrossRef]
- Erkaya, I.A.; Arica, M.Y.; Akbulut, A.; Bayramoglu, G. Biosorption of uranium(VI) by free and entrapped Chlamydomonas reinhardtii: Kinetic, equilibrium and thermodynamic studies. J. Radioanal. Nucl. Chem. 2014, 299, 1993–2003. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; da Silva, E.A. Biosorption of nickel and copper ions from synthetic solution and electroplating effluent using fixed bed column of immobilized brown algae. J. Water Process Eng. 2019, 32, 100904. [Google Scholar] [CrossRef]
- Kwak, H.W.; Kim, M.K.; Lee, J.Y.; Yun, H.; Kim, M.H.; Park, Y.H.; Lee, K.H. Preparation of bead-type biosorbent from water-soluble Spirulina platensis extracts for chromium (VI) removal. Algal Res. 2015, 7, 92–99. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Q.; Cui, L.; Wu, X.; Li, J.; Zeng, W.; Shen, L. Adsorption characteristics of Cr(VI) on microalgae immobilized by different carriers. Int. J. Phytoremediat. 2022, 24, 704–720. [Google Scholar] [CrossRef]
- Benaisa, S.; Arhoun, B.; Villen-Guzman, M.; El Mail, R.; Rodriguez-Maroto, J.M. Immobilization of Brown Seaweeds Sargassum vulgare for Fe3+ Removal in Batch and Fixed-Bed Column. Water Air Soil Pollut. 2019, 230, 19. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Jiménez, C.; Rodriguez-Maroto, J.M. Batch and Fixed-Bed Biosorption of Pb (II) Using Free and Alginate-Immobilized Spirulina. Processes 2021, 9, 466. [Google Scholar] [CrossRef]
- Mokone, J.G.; Tutu, H.; Chimuka, L.; Cukrowska, E.M. Optimization and Characterization of Cladophora sp. Alga Immobilized in Alginate Beads and Silica Gel for the Biosorption of Mercury from Aqueous Solutions. Water Air Soil Pollut. 2018, 229, 215. [Google Scholar] [CrossRef]
- Do Nascimento, W.J.; Landers, R.; Gurgel Carlos da Silva, M.; Vieira, M.G.A. Equilibrium and desorption studies of the competitive binary biosorption of silver(I) and copper(II) ions on brown algae waste. J. Environ. Chem. Eng. 2021, 9, 104840. [Google Scholar] [CrossRef]
- Leon-Vaz, A.; Cubero-Cardoso, J.; Trujillo-Reyes, Á.; Fermoso, F.G.; León, R.; Funk, C.; Vigara, J.; Urbano, J. Enhanced wastewater bioremediation by a sulfur-based copolymer as scaffold for microalgae immobilization (AlgaPol). Chemosphere 2023, 315, 137761. [Google Scholar] [CrossRef]
- Sari, B.G.; Olivoto, T.; Diel, M.I.; Krysczun, D.K.; Lúcio, A.D.C.; Savian, T.V. Nonlinear Modeling for Analyzing Data from Multiple Harvest Crops. Agron. J. 2018, 110, 2331–2342. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Pandey, L.K.; Gaur, J.P. Metal sorption by algal biomass: From batch to continuous system. Algal Res. 2016, 18, 95–109. [Google Scholar] [CrossRef]
- Akbari, M.; Hallajisani, A.; Keshtkar, A.R.; Shahbeig, H.; Ali Ghorbanian, S. Equilibrium and kinetic study and modeling of Cu(II) and Co(II) synergistic biosorption from Cu(II)-Co(II) single and binary mixtures on brown algae C. indica. J. Environ. Chem. Eng. 2015, 3, 140–149. [Google Scholar] [CrossRef]
- Edris, G.; Alhamed, Y.; Alzahrani, A. Biosorption of Cadmium and Lead from Aqueous Solutions by Chlorella vulgaris Biomass: Equilibrium and Kinetic Study. Arab. J. Sci. Eng. 2014, 39, 87–93. [Google Scholar] [CrossRef]
- Rezaei, H. Biosorption of chromium by using Spirulina sp. Arab. J. Chem. 2016, 9, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Bhat, A.H.; Buang, A. Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: Kinetic and equilibrium modeling. J. Clean. Prod. 2018, 171, 1361–1375. [Google Scholar] [CrossRef]
- Sibi, G. Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. Green Energy Environ. 2016, 1, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Schiewer, S. Multi-resistance kinetic models for biosorption of Cd by raw and immobilized citrus peels in batch and packed-bed columns. Chem. Eng. J. 2014, 244, 105–116. [Google Scholar] [CrossRef]
- Zaib, M.; Athar, M.M.; Saeed, A.; Farooq, U.; Salman, M.; Makshoof, M.N. Equilibrium, kinetic and thermodynamic biosorption studies of Hg(II) on red algal biomass of Porphyridium cruentum. Green Chem. Lett. Rev. 2016, 9, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Davarnejad, R.; Panahi, P. Cu(II) and Ni(II) removal from aqueous solutions by adsorption on Henna and optimization of effective parameters by using the response surface methodology. J. Ind. Eng. Chem. 2016, 33, 270–275. [Google Scholar] [CrossRef]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Cho, J.-S.; Heo, J.-S.; Delaune, R.D.; Seo, D.-C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Tofan, L. Polymeric Biomass Derived Adsorbents for Co(II) Remediation, Recycling and Analysis. Polymers 2022, 14, 1647. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae–A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Namal, O.O.; Kalipci, E. Adsorption kinetics of methylene blue using alkali and microwave-modified apricot stones. Sep. Sci. Technol. 2019, 54, 1722–1738. [Google Scholar] [CrossRef]
- Ng, F.-L.; Phang, S.-M.; Periasamy, V.; Yunus, K.; Fisher, A.C. Enhancement of Power Output by using Alginate Immobilized Algae in Biophotovoltaic Devices. Sci. Rep. 2017, 7, 16237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Qi, Y.; Li, Y.; Zhang, Y.; He, X.; Wang, Y. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium(IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour. Technol. 2013, 142, 611–619. [Google Scholar] [CrossRef]
- Di Natale, F.; Lancia, A.; Molino, A.; Musmarra, D. Removal of chromium ions form aqueous solutions by adsorption on activated carbon and char. J. Hazard. Mater. 2007, 145, 381–390. [Google Scholar] [CrossRef]
- Kuczajowska-Zadrożna, M.; Filipkowska, U.; Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Kumar, A.; Sidharth, S.; Kandasubramanian, B. A review on algal biosorbents for heavy metal remediation with different adsorption isotherm models. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Taelman, S.E.; Champenois, J.; Edwards, M.D.; De Meester, S.; Dewulf, J. Comparative environmental life cycle assessment of two seaweed cultivation systems in North West Europe with a focus on quantifying sea surface occupation. Algal Res. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Fan, L.; Cui, L.; Zhang, Y.; Cheng, J.; Wu, X.; Zeng, W.; Tian, Q.; Shen, L. Construction of fungi-microalgae symbiotic system and adsorption study of heavy metal ions. Sep. Purif. Technol. 2021, 268, 118689. [Google Scholar] [CrossRef]
- Olguín, E.J. Dual purpose microalgae–bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a Biorefinery. Biotechnol. Adv. 2012, 30, 1031–1046. [Google Scholar] [CrossRef]
- Ozkan, A.; Kinney, K.; Katz, L.; Berberoglu, H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012, 114, 542–548. [Google Scholar] [CrossRef]
- Abinandan, S.; Praveen, K.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Life Cycle Assessment for the Environmental Sustainability of the Immobilized Acid-Adapted Microalgal Technology in Iron Removal from Acid Mine Drainage. ACS Sustain. Chem. Eng. 2020, 8, 15670–15677. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chen, C.-Y.; Chang, J.-S. Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour. Technol. 2020, 302, 122817. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Najim, A.A.; Al-Musawi, T.J.; Alwared, A.I. Adsorptive performance of a mixture of three nonliving algae classes for nickel remediation in synthesized wastewater. J. Environ. Health Sci. Eng. 2019, 17, 529–538. [Google Scholar] [CrossRef]
- Kube, M.; Mohseni, A.; Fan, L.; Roddick, F. Impact of alginate selection for wastewater treatment by immobilised Chlorella vulgaris. Chem. Eng. J. 2019, 358, 1601–1609. [Google Scholar] [CrossRef]
- Praveen, K.; Abinandan, S.; Venkateswarlu, K.; Megharaj, M. Sustainability Evaluation of Immobilized Acid-Adapted Microalgal Technology in Acid Mine Drainage Remediation following Emergy and Carbon Footprint Analysis. Molecules 2022, 27, 1015. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, T.; Yang, Z. Immobilizing unicellular microalga on pellet-forming filamentous fungus: Can this provide new insights into the remediation of arsenic from contaminated water? Bioresour. Technol. 2019, 284, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Samal, D.P.K.; Sukla, L.B.; Pattanaik, A.; Pradhan, D. Role of microalgae in treatment of acid mine drainage and recovery of valuable metals. Mater. Today Proc. 2020, 30, 346–350. [Google Scholar] [CrossRef]
- García de Llasera, M.P.; León Santiago, M.; Loera Flores, E.J.; Bernal Toris, D.N.; Covarrubias Herrera, M.R. Mini-bioreactors with immobilized microalgae for the removal of benzo(a)anthracene and benzo(a)pyrene from water. Ecol. Eng. 2018, 121, 89–98. [Google Scholar] [CrossRef]

| Algae Type | Immobilized Carriers | Heavy Metal Types and Systems | Immobilization Method | Adsorbent Dosage (g/L) | Optimal Initial Metal Concentration (mg/L) | Optimal pH | Optimal Temperature (°C) | Optimal Contact Time | Maximum Adsorption Capacity (mg/g) | Maximum Adsorption Efficiency | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorella sorokiniana | Alginate | Cu(II) | Encapsulation | - | 25 | 5.0 | 40 | 180 min | 150.07 | 97.10% | [92] |
| Cd(II) | 25 | 5.0 | 20 | 180 min | 48.87 | 50.94% | |||||
| Ni(II) | 25 | 5 | 20 | 180 min | 101.73 | 74% | |||||
| Cu(II)/Ni(II) | 30 | 5.0 | 40 | 180 min | Cu(II):21.47 Ni(II):11.15 | Cu(II):89.68% Ni(II):39.66% | |||||
| Cu(II)/Cd(II) | 50 | 5.0 | 20 | 180 min | Cu(II):39.13 Cd(II):15.11 | Cu(II):91.53% Cd(II):32.64% | |||||
| Cd(II)/Ni(II) | 30 | 5.0 | 20 | 180 min | Cd(II):15.10 Ni(II):11.77 | Cd(II):63.03% Ni(II):42.08% | |||||
| Cu(II)/Ni(II)/Cd(II) | 30 | 5.0 | 40 | 180 min | Cu(II):24.30 Ni(II):12.59 Cd(II):8.25 | Cu(II):84.51% Ni(II):47.41% Cd(II):32.43% | |||||
| Chlorella vulgaris | Calcium alginate beads | Fe(II) | Adsorption | 0.6 | 250 | 6 | 25 | 450 min | 43.43 | - | [21] |
| Mn(II) | 0.6 | 250 | 6 | 25 | 450 min | 40.98 | - | ||||
| Zn(II) | 0.6 | 250 | 6 | 25 | 450 min | 37.43 | - | ||||
| Micractinium reisseri KGE33 | Silicon dioxide | Cu(II) | Entrapment | 100 | - | 5 | 40 | 24 h | 1.710 | 87.1% | [93] |
| Synechocystis sp. PCC6803 | Fe2O3 | Cr(VI) | Adsorption | 0.5 | 100 | 2.0 | 29.85 | 30 min | 69.77 | 88.37% | [72] |
| Cu(II) | 0.5 | 100 | 5.0 | 29.85 | 60 min | 38.68 | 78.89% | ||||
| Pb(II) | 0.5 | 100 | 5.0 | 29.85 | 30 min | 62.63 | 88.89% | ||||
| Cd(II) | 0.5 | 100 | 5.0 | 29.85 | 30 min | 42.12 | 88.89% | ||||
| Chlorella sp. (FACHB-31) | Biochar | Cd(II) | Surface adsorption, polymer matrix | 1.0 | 100 | 6.0 | 26 | 50 min | 217.41 | 86.57 ± 0.61% | [42] |
| Cladophora sp. | Chitosan | Cd(II) | Crosslinking | 0.2 g | 10 | 6.0 | 25 | 60 min | 0.240 mmol/g | - | [37] |
| Cr(III) | 0.2 g | 10 | 5.0 | 25 | 360 min | 1.128 mmol/g | - | ||||
| Cu(II) | 0.2 g | 10 | 5.0 | 25 | 360 min | 1.059 mmol/g | - | ||||
| Ni(II) | 0.2 g | 10 | 6.0 | 25 | 60 min | 0.239 mmol/g | - | ||||
| Zn(II) | 0.2 g | 10 | 5.0 | 25 | 60 min | 0.310 mmol/g | - | ||||
| Chlorella sp., Ankistrodesmus braunii, and Scenedesmus quadricauda var quadri-spina | Sodium alginate | Cu(II) | Entrapment | 10 g | 50 | 3.0 | 28 ± 2 | 180 min | - | 43.19% | [94] |
| Chlorella sp. (FACHB-31) | Water-hyacinth leaf pelle | Cd(II) | Surface adsorption | 1.3 | 10 | 6.0 | - | 5 d | - | 48% | [95] |
| Water-hyacinth root pellet | 1.3 | 10 | 6.0 | - | 5 d | - | 35% | ||||
| Water-hyacinth leaf biochar pellets | 1.3 | 10 | 6.0 | - | 5 d | 13.81 ± 0.94 | 92.45 ± 0.5% | ||||
| Water-hyacinth root biochar pellets | 1.3 | 10 | 6.0 | - | 5 d | - | 60% | ||||
| Anabaena variabilis | Water-hyacinth leaf pelle | Fe(II) | Entrapment | - | 13.88 | - | - | 6 h | - | 94.45% | [96] |
| Anabaena variabilis | Zn(II) | 5.1 | 6 h | 98.98% | |||||||
| Anabaena variabilis and Tolypthrix ceytonica | Zn(II) | 5.1 | 6 h | 98.63% | |||||||
| Tolypthrix ceytonica | Zn(II) | 5.1 | 6 h | 98.61% | |||||||
| Anabaena variabilis and Tolypthrix ceytonica | Pb(II) | 4.5 | 6 h | 94.22% | |||||||
| Anabaena variabilis | Cu(II) | 0.15 | 6 h | 93.33% | |||||||
| Tolypthrix ceytonica | Cu(II) | 0.15 | 6 h | 91.33% | |||||||
| Chlorella sorokiniana and Monoraphidium sp. | Sodium alginate beads | Cu(II) | Entrapment | 0.5 g | 20 | 4.0 | 35 | 180 min | - | 96.4% | [97] |
| Sargassum sp. | Calcium alginate beads | Ni(II) | Entrapment | 0.1 g | 50 | 5.0 | 30 | 4 h | 1.69 mmol/g | - | [64] |
| Cu(II) | 0.1 g | 50 | 5.0 | 30 | 6 h | 2.06 mmol/g | - | ||||
| Penium margaritaceum | Filter paper | Pb(II) | Adsorption | 1.0 | 1.0 | - | 25 | 8 h | 3.4 | 55.4% | [98] |
| Chlorella vulgaris | Calcium alginate beads | Cd(II) | Entrapment | 0.5 | 75 | 6.0 | 25 | 105 min | 1.168 | 76.448% | [99] |
| Chlamydomonas reinhardtii | Carboxymethyl cellulose beads | U(VI) | Entrapment | - | 1 | 4.5 | 25 | 60 min | 218.3 | 92.4% | [100] |
| Sargassum sp. | Sodium alginate | Ni(II) | Entrapment | - | 1 mmol/L | 4.5 | 30 | - | 1.404 mmol/L | - | [101] |
| Cu(II) | Entrapment | - | 1 mmol/L | 4.5 | 30 | - | 1.656 mmol/L | - | |||
| Spirulina platensis | Beads | Cr(VI) | Entrapment | 1.0 | 250 | 3.0 | 25 | - | 49 | 75% | [102] |
| Turbinaria ornata | Sodium alginate beads | Cd(II) | Entrapment | 5.04 | 25.2 | 5.06 | 25 | 90 min | - | 98.65% | [38] |
| Synechocystis sp. PCC6803 | Sodium alginate | Cr(VI) | Adsorption-crosslinking | 1.5 | 40 | 7.0 | 30 | 30 min | 7.6 | - | [103] |
| Chitosan | Cr(VI) | Adsorption | 1.5 | 40 | 7.0 | 30 | 30 min | 37.1 | - | ||
| Cu(II) | 1.5 | 40 | 7.0 | 30 | 30 min | 25.98 | - | ||||
| Pb(II) | 1.5 | 40 | 7.0 | 30 | 30 min | 25.06 | - | ||||
| Cd(II) | 1.5 | 40 | 7.0 | 30 | 30 min | 24.62 | - | ||||
| Carrageenan | Cr(VI) | Polymer matrix | 1.5 | 40 | 7.0 | 30 | 30 min | 19.7 | - | ||
| Diatomite | Cr(VI) | Adsorption | 1.5 | 40 | 7.0 | 30 | 30 min | 8.0 | - | ||
| Quartz sand | Cr(VI) | Entrapment | 1.5 | 40 | 7.0 | 30 | 30 min | 6.2 | - | ||
| Polyvinyl alcohol | Cr(VI) | - | 1.5 | 40 | 7.0 | 30 | 30 min | 24.2 | - | ||
| Sargassum vulgare | Calcium alginate beads | Fe(III) | Entrapment | 20 | 50 | 2.0 | 25 | 120 min | 17.09 | 86.07% | [104] |
| Spirulina | Calcium alginate beads | Pb(II) | Entrapment | 10 | 5.63 | 5.2 | 25 | 72 h | 282.17 | - | [105] |
| Cladophora sp. alga | Calcium alginate beads | Hg(II) | Entrapment | 10 | 100 | 5.0 | 16 | 60 min | 43.87 | - | [106] |
| Silicone | Hg(II) | Entrapment | 10 | 100 | 5.0 | 16 | 60 min | 39.47 | - | ||
| Sargassum filipendula | Sodium alginate | Cu(II) | Entrapment | 0.1 g | - | 5.0 | 30 | - | 3.60 mmol/g | - | [107] |
| Ag(I) | 0.1 g | - | 5.0 | 30 | - | 8.67 mmol/g | - | ||||
| Chlorella sorokiniana | Sulfur-Sigma-Aldrich’s castor oil copolymer | Cd(II) | Adsorption | 1 | 50 | 6.0 | 27 | 24 h | - | 80% | [108] |
| Sulfur- Castor oil copolymer | 1 | 50 | 6.0 | 27 | 24 h | - | 90% | ||||
| Sulfur and Sigma-Aldrich’s castor oil copolymer | Cu(II); Cd(II) | 1 | 8 | 6.0 | 27 | 24 h | - | Cu(II):92%; Cd(II):90% | |||
| Sulfur- Castor oil copolymer | 1 | 8 | 6.0 | 27 | 24 h | - | Cu(II):95%; Cd(II):90% |
| Algae Types | Immobilization Carriers | Adsorption of Heavy Metals | Comparison Method | Life Cycle Assessment | Economic Analysis | Reference |
|---|---|---|---|---|---|---|
| Desmodesmus sp. MAS and Heterochlorella sp. MAS3 | Alginate beads | Fe(II) | Eggshell-microalgae method | GWP: three-fold reduction; 51.53 kg/m3 CO2 reduction from transport; 3.397 kg/m3 CO2 reduction from coal-fired power generation | 50% reduction in fossil fuel consumption | [132] |
| Limestone systems | GWP: seven-fold reduction, 3.207 kg/m3 CO2 reduction from coal-fired power generation | 50% reduction in fossil fuel consumption | ||||
| Desmodesmus sp. MAS1 and Heterochlorella sp. MAS3 | Alginate beads | Fe(II) | Passive handling systems | 5% reduction in CO2 emissions | 80% reduction in renewable energy reduction rate | [136] |
| Active handling systems | 80% reduction in CO2 emissions | Renewable energy reduction rate reduced by 9% | ||||
| Chlorella sp. (FACHB-31) | Water hyacinth leaf biochar pellets | Cd(II) | - | - | Removal efficiency: 91.1% (3 cycles) | [95] |
| C. vulgaris | Calcium alginate | Fe(II) | - | - | Removal efficiency: 3.56% reduction (5 cycles) | [114] |
| Mn(II) | - | Removal efficiency: 4.32% reduction (5 cycles) | ||||
| Zn(II) | - | Removal efficiency: 4.87% reduction (5 cycles) | ||||
| Sargassum vulgare | Calcium alginate beads | Fe(III) | - | - | Removal efficiency: 22% (5 cycles) | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Osman, A.I.; Rooney, D.W.; Oh, W.-D.; Yap, P.-S. Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives. Sustainability 2023, 15, 5128. https://doi.org/10.3390/su15065128
Chen Z, Osman AI, Rooney DW, Oh W-D, Yap P-S. Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives. Sustainability. 2023; 15(6):5128. https://doi.org/10.3390/su15065128
Chicago/Turabian StyleChen, Zhonghao, Ahmed I. Osman, David W. Rooney, Wen-Da Oh, and Pow-Seng Yap. 2023. "Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives" Sustainability 15, no. 6: 5128. https://doi.org/10.3390/su15065128
APA StyleChen, Z., Osman, A. I., Rooney, D. W., Oh, W.-D., & Yap, P.-S. (2023). Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives. Sustainability, 15(6), 5128. https://doi.org/10.3390/su15065128










