Bacillus Thuringiensis Enhances the Ability of Ryegrass to Remediate Cadmium-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Design
2.3. Sample Collection and Processing
2.4. Determination Methods
2.4.1. Determination of Metals in Plants
2.4.2. Determination of Soil Enzyme Activity
2.5. Data Analysis
3. Results and Discussion
3.1. Changes in Soil Physical and Chemical Properties after Inoculation with SY
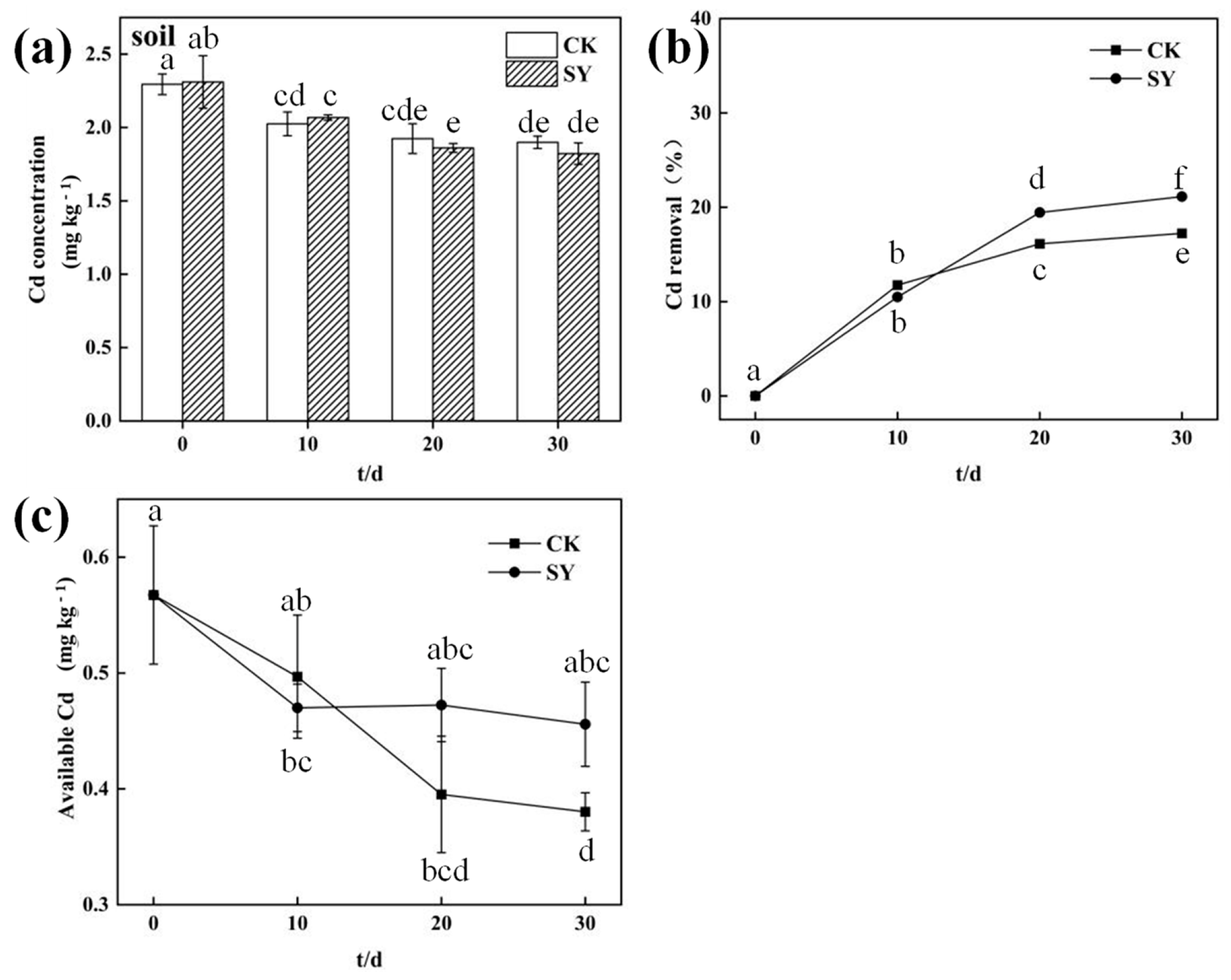
3.2. Effects of SY Inoculation on Total and Available Cd Content in Root Soil
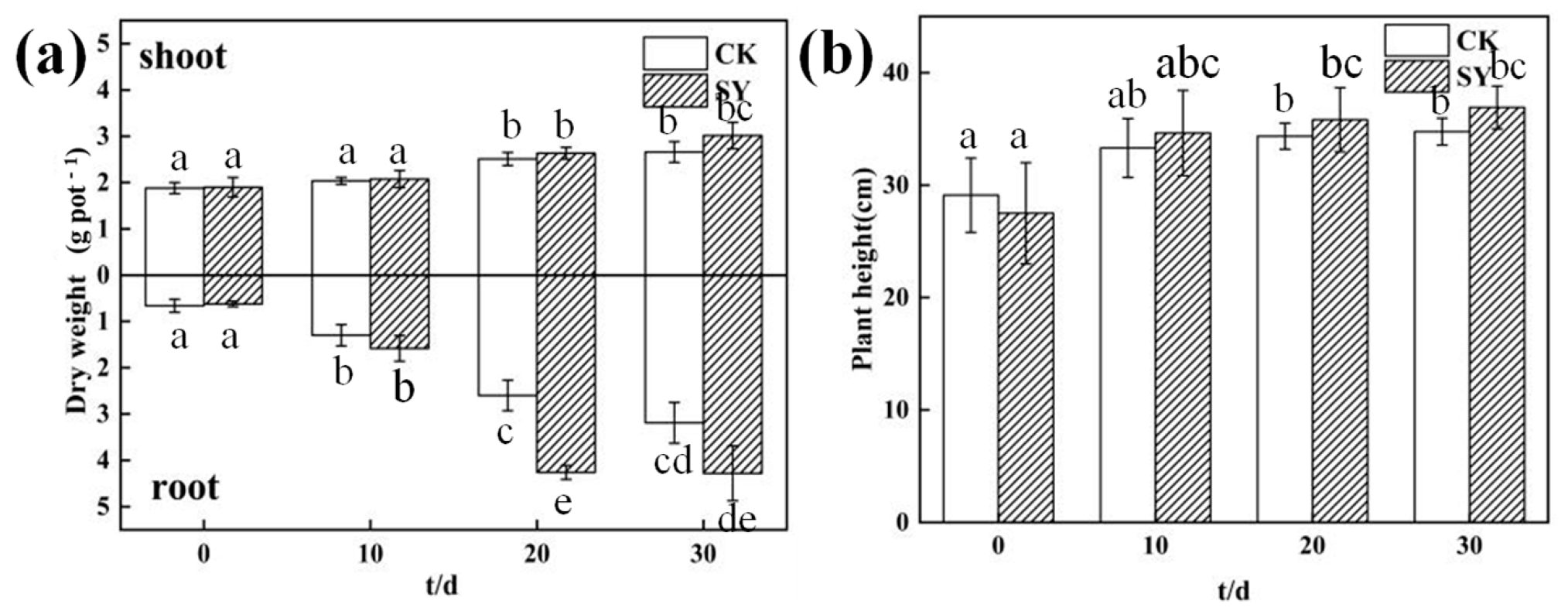
3.3. Effects of SY on the Growth of Ryegrass
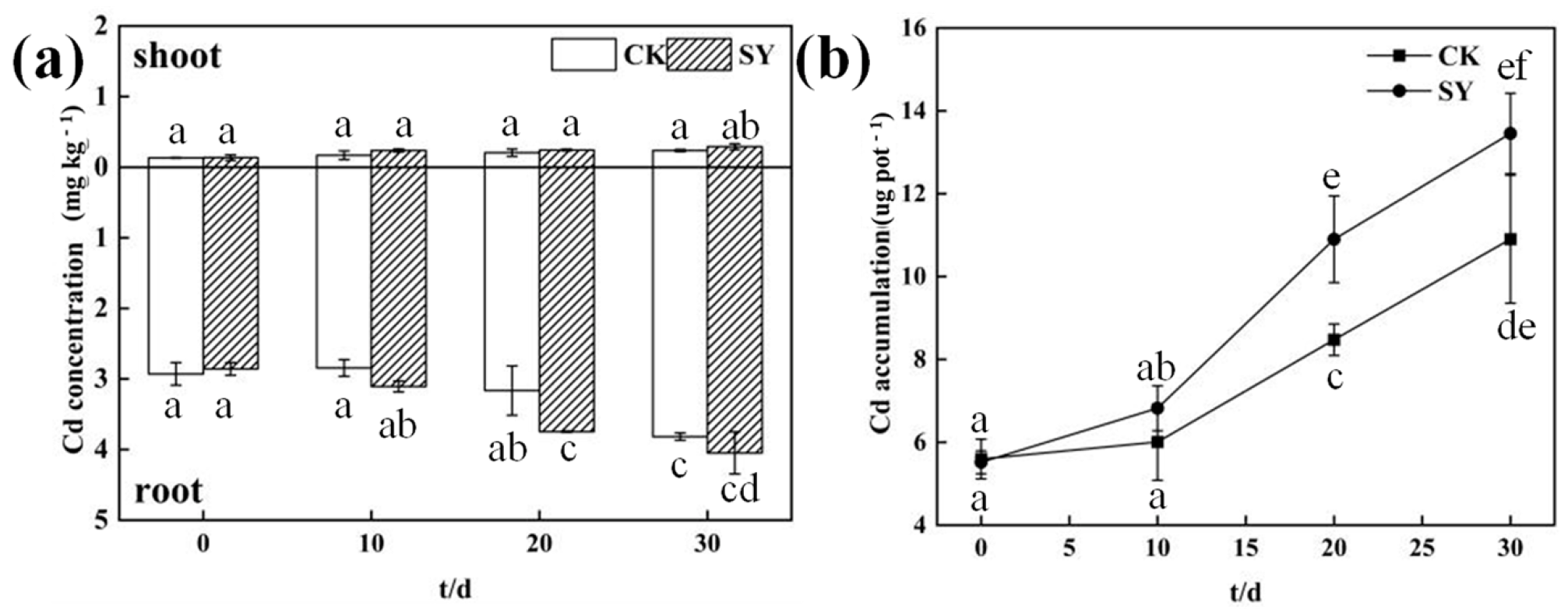
3.4. Effects of SY Inoculation on the Content and Accumulation of Cd in Ryegrass
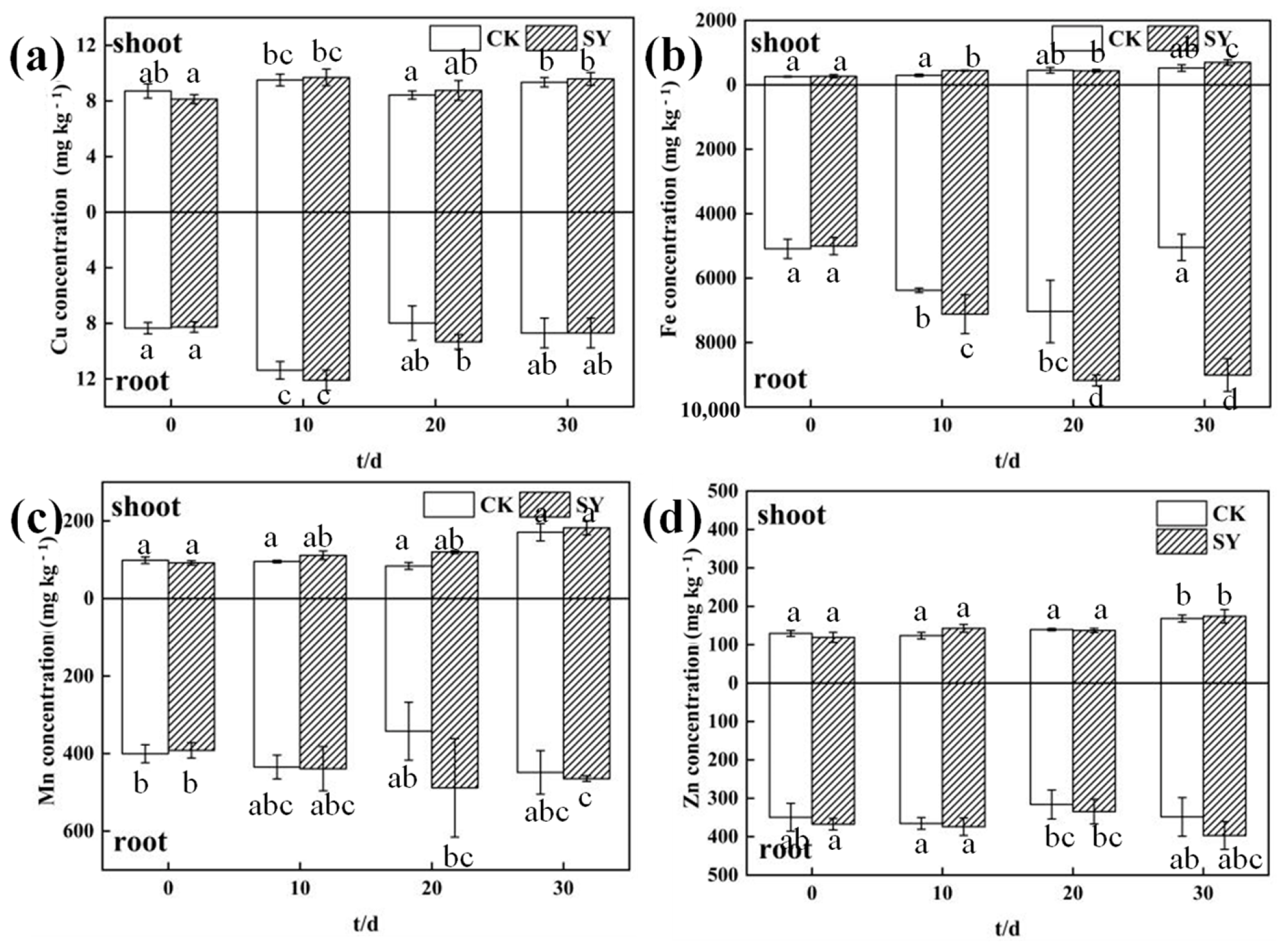
3.5. Effects of SY Inoculation on Trace Elements in Ryegrass
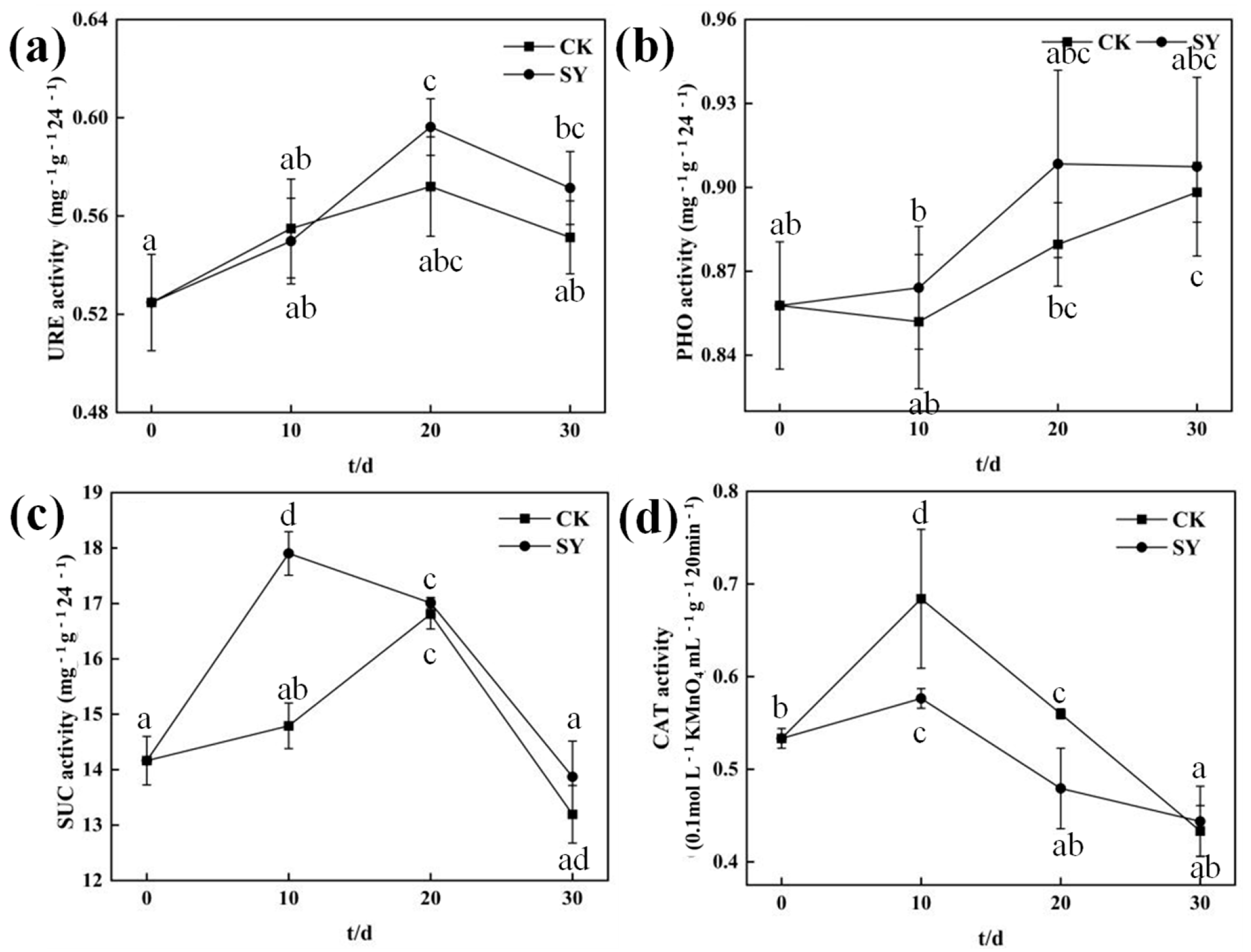
3.6. Changes in Soil Enzyme Activity after Inoculation with SY
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, L.; Huang, Y.; Huang, L.; Huang, Q.; Li, Y.; Li, X.; Yang, S.; Liu, C.; Liu, Z. Combined biochar and soda residues increases maize yields and decreases grain Cd/Pb in a highly Cd/Pb-polluted acid Udults soil. Agric. Ecosyst. Environ. 2021, 306, 107198. [Google Scholar] [CrossRef]
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.; Huang, M.; Chen, Q.; Shi, Z. Assessment of potentially toxic element pollution in soils and related health risks in 271 cities across China. Envrion. Pollut. 2021, 270, 116196. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, C.; Hong, X.; Kang, G.; Qin, M.; Yang, D.; Pang, B.; Li, Y.; He, J.; Dick, R. Risk assessment and source analysis of soil heavy metal pollution from lower reaches of Yellow River irrigation in China. Sci. Total Envrion. 2018, 633, 1136–1147. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Q.; Huang, R. Characteristics of Heavy Metal Pollution in Agricultural Soils and Bioaccumulation in Plants of Dabaoshan Mine. Soils 2017, 49, 141–149. [Google Scholar]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Purchase, D.; Mulla, S.; Saratale, G.; Bharagava, R. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. Vol. 2019, 249, 71–131. [Google Scholar]
- Huang, Y.; Huang, Y.; Hou, J.; Wu, L.; Christie, P.; Liu, W. Microbial community assembly of the hyperaccumulator plant Sedum plumbizincicola in two contrasting soil types with three levels of cadmium contamination. Sci. Total Environ. 2023, 863, 160917. [Google Scholar] [CrossRef]
- Xie, H.; Ma, Y.; Wang, Y.; Sun, F.; Liu, R.; Liu, X.; Xu, Y. Biological response and phytoremediation of perennial ryegrass to halogenated flame retardants and Cd in contaminated soils. J. Environ. Chem. Eng. 2021, 9, 106526. [Google Scholar] [CrossRef]
- Li, F.; Qiu, Y.; Xu, X.; Yang, F.; Wang, Z.; Feng, J.; Wang, J. EDTA-enhanced phytoremediation of heavy metals from sludge soil by Italian ryegrass (Lolium perenne L.). Ecotoxicol. Environ. Saf. 2020, 191, 110185. [Google Scholar] [CrossRef] [PubMed]
- Basant, K.N.; Singh, J.; Kumari, B.; Sinam, G.; Gautam, A.; Singh, G.; Swapnil; Mishra, K.; Mallick, S. Nickel and cadmium phytoextraction efficiencies of vetiver and lemongrass grown on Ni-Cd battery waste contaminated soil: A comparative study of linear and nonlinear models. J. Environ. Manag. 2021, 295, 113144. [Google Scholar]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.; Sahu, A.; Shukla, R.; Singh, B.; Rai, J.; Sharma, P.; Lade, H. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Huang, C.; Xu, W.; Qin, Y.; Li, Y.; Li, T.; Yang, M.; He, Z. Difference of cadmium bioaccumulation and transportation in two ryegrass varieties and the correlation between plant cadmium concentration and soil cadmium chemical forms. Wirel. Pers. Commun. 2020, 110, 291–307. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, L.; Peng, X.; Fan, L.; Chen, S.; Qin, P.; Zhang, J.; Chen, A.; Huang, H. Effect of different amounts of fruit peel-based activator combined with phosphate-solubilizing bacteria on enhancing phytoextraction of Cd from farmland soil by ryegrass. Environ. Pollut. 2023, 316, 120602. [Google Scholar] [CrossRef]
- Kumar, A.; Vandana, R.; Singh, M.; Pandey, K. Plant growth promoting rhizobacteria (PGPR). A promising approach for disease management. In Microbes and Environmental Management; Studium Press: New Delhi, India, 2015; pp. 195–209. [Google Scholar]
- Chen, B.; Zhang, Y.; Rafiq, M.; Khan, K.; Pan, F.; Yang, X.; Feng, Y. Improvement of cadmium uptake and accumulation in Sedum alfredii by endophytic bacteria Sphingomonas SaMR12: Effects on plant growth and root exudates. Chemosphere 2014, 117, 367–373. [Google Scholar] [CrossRef]
- El-Meihy, R.; Abou-Aly, H.; Youssef, A.; Tewfike, T.; El-Alkshar, E. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ. Exp. Bot. 2019, 162, 295–301. [Google Scholar] [CrossRef]
- Li, Y.; Han, H.; He, L.; Wang, Q.; Sheng, X. Inoculation with endophytic Bacillus megaterium H3 increases Cd phytostabilization and alleviates Cd toxicity to hybrid pennisetum in Cd-contaminated aquatic environments. Environ. Sci. Pollut. Res. 2017, 24, 1416–1423. [Google Scholar] [CrossRef]
- Deng, Y.-Q.; Cao, X.-Y.; Tan, C.-Y.; Sun, L.-J.; Peng, X.; Bai, J.; Huang, S.-P. Strengthening the effect of Bacillus megaterium on remediation of Cd-contaminated farmland soil by Sedum plumbizincicola. J. Appl. Ecol. 2020, 31, 3111–3118. [Google Scholar]
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid Pennisetum. Biotechnol. Rep. 2019, 24, e00374. [Google Scholar] [CrossRef]
- Abdallah, D.; Frikha-Gargouri, O.; Tounsi, S. Rizhospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol. Control 2018, 124, 61–67. [Google Scholar] [CrossRef]
- Delfim, J.; Gerding, M.; Zagal, E. Phosphorus fractions in Andisol and Ultisol inoculated with Bacillus thuringiensis and phosphorus uptake by wheat. J. Plant Nutr. 2020, 43, 2728–2739. [Google Scholar] [CrossRef]
- Din, B.; Rafique, M.; Javed, M.; Kamran, M.; Mehmood, S.; Khan, M.; Sultan, T.; Munis, M.; Chaudhary, H. Assisted phytoremediation of chromium spiked soils by Sesbania Sesban in association with Bacillus xiamenensis PM14: A biochemical analysis. Plant Physiol. Biochem. 2020, 146, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Shaaban, M.; Hussain, Q.; Mehmood, S.; Zhu, J.; Fu, Q.; Aziz, O.; Hu, H. Influence of organic and inorganic passivators on Cd and Pb stabilization and microbial biomass in a contaminated paddy soil. J. Soils Sediments 2018, 18, 2948–2959. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.; Guo, J.; Kang, Z.; Babar, M. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, V.; Sidhu, G.; Datta, S.; Dhanjal, D.; Koul, B.; Janeja, H.; Singh, J. Plant growth promoting rhizobacteria from heavy metal contaminated soil promote growth attributes of Pisum sativum L. Biocatal. Agric. Biotechnol. 2019, 17, 665–671. [Google Scholar] [CrossRef]
- Jo, H.; Tagele, S.; Pham, H.; Kim, M.-C.; Choi, S.-D.; Kim, M.-J.; Park, Y.-J.; Ibal, J.; Park, G.-S.; Shin, J.-H. Response of soil bacterial community and pepper plant growth to application of Bacillus thuringiensis KNU-07. Agronomy 2020, 10, 551. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Liu, Q.; Vestergård, M.; Topalovic, O.; Wang, Q.; Zhou, Q.; Huang, L.; Yang, X.; Feng, Y. The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J. Hazard. Mater. 2020, 395, 122661. [Google Scholar] [CrossRef]
- Rout, G.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhao, Y.; Fan, L.; Jin, Q.; Yang, G.; Xu, Z. Improvement of manganese phytoremediation by Broussonetia papyrifera with two plant growth promoting (PGP) Bacillus species. Chemosphere 2020, 260, 127614. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Sheng, X.; Hu, J.; He, L.; Wang, Q. Metal-immobilizing Serratia liquefaciens CL-1 and Bacillus thuringiensis X30 increase biomass and reduce heavy metal accumulation of radish under field conditions. Ecotoxicol. Environ. Saf. 2018, 161, 526–533. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Guo, Z.-H.; Xiao, X.-Y.; Wang, S.; Jiang, Z.-C.; Zeng, P. Phytostabilisation potential of giant reed for metals contaminated soil modified with complex organic fertiliser and fly ash: A field experiment. Sci. Total Environ. 2017, 576, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Appl. Soil Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Fu, S.-L.; Zhang, X.-P.; Zhao, K.; Chen, H. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Nascimento, F.; Hernandez, A.; Glick, B.; Rossi, M. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef]
| Treatment | pH | Available K (mg kg−1) | Available P (mg kg−1) | Available N (mg kg−1) | Organic Matter (g kg−1) |
|---|---|---|---|---|---|
| CK-0 | 7.68 ± 0.02 a | 55.28 ± 1.70 a | 7.75 ± 1.08 a | 88.45 ± 2.45 a | 29.20 ± 1.03 a |
| SY-0 | 7.65 ± 0.01 a | 69.91 ± 1.22 b | 7.98 ± 0.76 a | 89.98 ± 0.51 a | 29.71 ± 0.61 a |
| CK-30 | 7.51 ± 0.01 b | 34.64 ± 2.48 c | 5.42 ± 1.03 b | 87.43 ± 6.34 a | 26.08 ± 0.78 a |
| SY-30 | 7.46 ± 0.01 c | 34.98 ± 0.01 c | 5.84 ± 0.16 b | 102.26 ± 6.43 b | 31.02 ± 1.10 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Mi, R.; Li, Q.; Lang, J.; Lan, Y.; Huang, N.; Yang, G. Bacillus Thuringiensis Enhances the Ability of Ryegrass to Remediate Cadmium-Contaminated Soil. Sustainability 2023, 15, 5177. https://doi.org/10.3390/su15065177
Jin J, Mi R, Li Q, Lang J, Lan Y, Huang N, Yang G. Bacillus Thuringiensis Enhances the Ability of Ryegrass to Remediate Cadmium-Contaminated Soil. Sustainability. 2023; 15(6):5177. https://doi.org/10.3390/su15065177
Chicago/Turabian StyleJin, Jiyuan, Ruidong Mi, Qiao Li, Jian Lang, Yushu Lan, Na Huang, and Gang Yang. 2023. "Bacillus Thuringiensis Enhances the Ability of Ryegrass to Remediate Cadmium-Contaminated Soil" Sustainability 15, no. 6: 5177. https://doi.org/10.3390/su15065177





