Early Strength-Promoting Mechanism of Inorganic Salts on Limestone-Calcined Clay Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Preparation and Methods
3. Results and Discussion
3.1. Compressive Strength
3.2. Hydration Heat Evolution
3.3. XRD
3.4. FTIR
3.5. Pore Structure
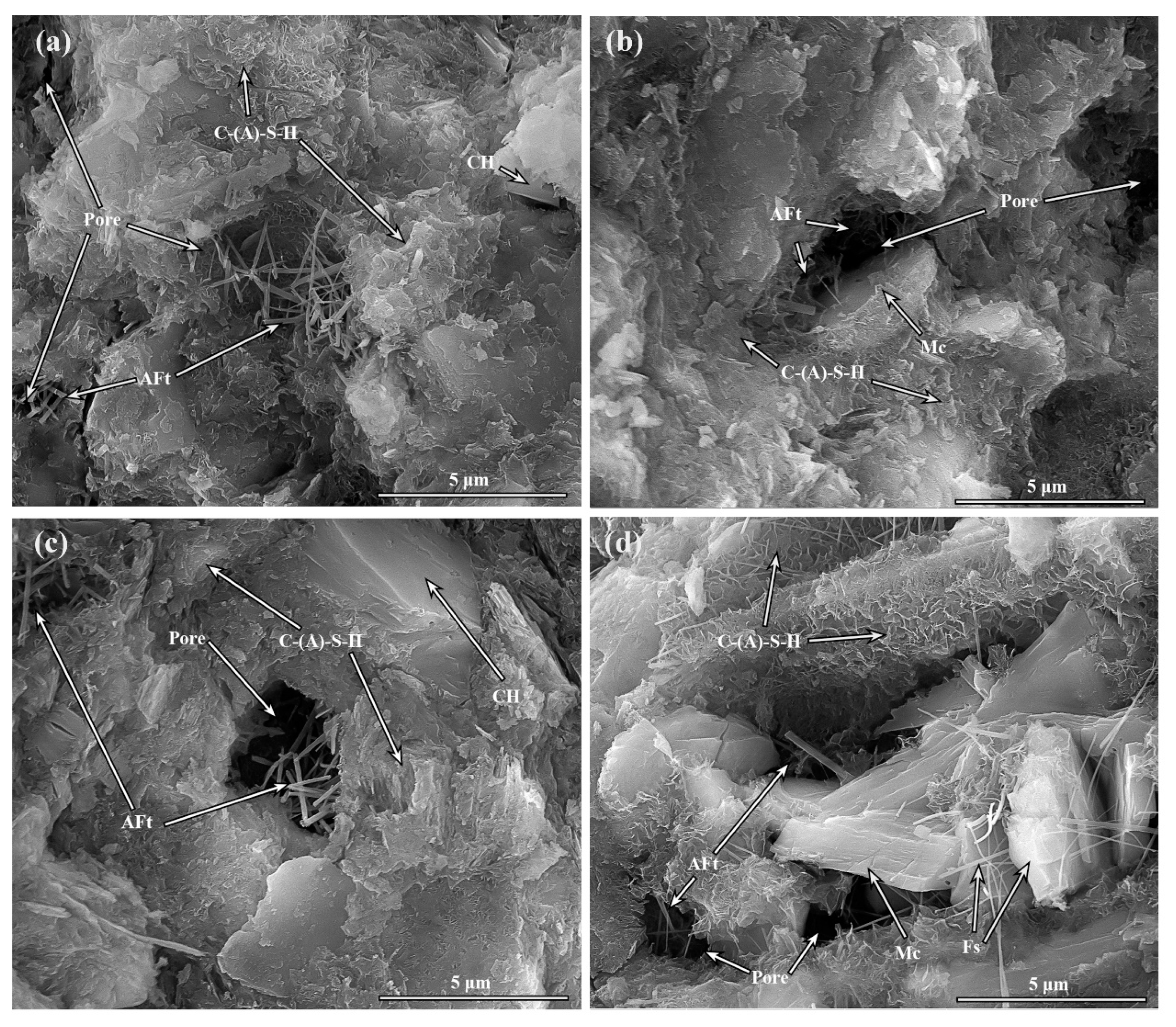
3.6. Microstructure Morphology
4. Conclusions
- (1)
- The carbonate ions in the solution react with solid gypsum, accelerating the precipitation of sulfate ions from gypsum, which are rapidly adsorbed on C-(A)-S-H so that the aluminate reaction strengthened. Then, the pozzolanic reaction occurs, improved by nano-calcium carbonate and an alkaline environment that enhances the solubility.
- (2)
- Supplementary sulfate ion from sodium sulfate reacted with alumina from C3A or metakaolin at the beginning, generating additional ettringite and promoting the early strength of LC3.
- (3)
- The sodium chloride reacts with alumina from tricalcium aluminate and metakaolin in the pozzolanic reaction, generating Friedel’s salt, thus refining the pore size and enhancing the early strength of LC3.
- (4)
- At the early stage in LC3 system with NaCl, the formation of carbo-aluminate phases had a competitive relationship with the formation of Friedel’s salt. The pozzolanic reaction products of metakaolin in LC3 were preferentially Friedel’s salts, and the generated carbo-aluminate phases were also converted into Friedel’s salt. At the later stage, the Friedel’s salt phase and the carbo-aluminate phase together played a role in refining pore size and increasing strength, which was one of the reasons why LC3-NaCl still had a high strength after 28 days.
- (5)
- A combination of XRD and MIP analysis confirmed that excessive Hc and Mc phases resulted in rapid porosity refinement, and then the lack of capillary pores of the binder affected the subsequent pozzolanic reaction and had a negative impact on the strength development.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, M.; Bishnoi, S.; Martirena, F.; Scrivener, K. Limestone Calcined Clay Cement and Concrete: A State-of-the-Art Review. Cem. Concr. Res. 2021, 149, 106564. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.M.C.; Lund, J.; Herfort, D. Influence of Fineness of Raw Clay and Calcination Temperature on the Performance of Calcined Clay-Limestone Blended Cements. Appl. Clay Sci. 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Cardinaud, G.; Roziere, E.; Martinage, O.; Loukili, A.; Barnes-Davin, L.; Paris, M.; Deneele, D. Calcined Clay—Limestone Cements: Hydration Processes with High and Low-Grade Kaolinite Clays. Constr. Build. Mater. 2021, 277, 122271. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the Calcined Kaolinite Content on the Hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical Properties and Durability Performance of Concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Briki, Y.; Avet, F.; Zajac, M.; Bowen, P.; Ben Haha, M.; Scrivener, K. Understanding of the Factors Slowing down Metakaolin Reaction in Limestone Calcined Clay Cement (LC3) at Late Ages. Cem. Concr. Res. 2021, 146, 106477. [Google Scholar] [CrossRef]
- Sui, S.; Wu, M.; Yang, Z.; Wang, F.; Liu, Z.; Jiang, J. An Investigation on the Formation of Friedel’s Salt in Tricalcium Silicate Combined with Metakaolin and Limestone Systems. Constr. Build. Mater. 2021, 284, 122855. [Google Scholar] [CrossRef]
- Shi, Z.; Ferreiro, S.; Lothenbach, B.; Geiker, M.R.; Kunther, W.; Kaufmann, J.; Herfort, D.; Skibsted, J. Sulfate Resistance of Calcined Clay—Limestone—Portland Cements. Cem. Concr. Res. 2019, 116, 238–251. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting Factors and Properties of Limestone Calcined Clay Cements (LC3). Green Mater. 2019, 7, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Ferreiro, S.; Herfort, D.; Damtoft, J.S. Effect of Raw Clay Type, Fineness, Water-to-Cement Ratio and Fly Ash Addition on Workability and Strength Performance of Calcined Clay—Limestone Portland Cements. Cem. Concr. Res. 2017, 101, 1–12. [Google Scholar] [CrossRef]
- Li, C.; Ideker, J.H.; Drimalas, T. The Efficacy of Calcined Clays on Mitigating Alkali-Silica Reaction (ASR) in Mortar and Its Influence on Microstructure. RILEM Bookseries 2015, 10, 211–217. [Google Scholar]
- Avet, F.; Scrivener, K. Influence of PH on the Chloride Binding Capacity of Limestone Calcined Clay Cements (LC3). Cem. Concr. Res. 2020, 131, 106031. [Google Scholar] [CrossRef]
- Avet, F.; Boehm-Courjault, E.; Scrivener, K. Investigation of C-A-S-H Composition, Morphology and Density in Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2019, 115, 70–79. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Microstructural Developments of Limestone Calcined Clay Cement (LC3) Pastes after Long-Term (3 Years) Hydration. Cem. Concr. Res. 2022, 153, 106693. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, D.; Wang, T.; McBain, M.; Li, V.C. Intrinsic Self-Stressing and Low Carbon Engineered Cementitious Composites (ECC) for Improved Sustainability. Cem. Concr. Res. 2021, 149, 106580. [Google Scholar] [CrossRef]
- Wang, L.; Rehman, N.U.; Curosu, I.; Zhu, Z.; Beigh, M.A.B.; Liebscher, M.; Chen, L.; Tsang, D.C.W.; Hempel, S.; Mechtcherine, V. On the Use of Limestone Calcined Clay Cement (LC3) in High-Strength Strain-Hardening Cement-Based Composites (HS-SHCC). Cem. Concr. Res. 2021, 144, 106421. [Google Scholar] [CrossRef]
- Chen, Y.; He, S.; Zhang, Y.; Wan, Z.; Copuroglu, O.; Schlangen, E. 3D Printing of Calcined Clay-Limestone-Based Cementitious Materials. Cem. Concr. Res. 2021, 149, 106553. [Google Scholar] [CrossRef]
- Chen, Y.; Rodriguez, C.R.; Li, Z.; Chen, B.; Copuroglu, O.; Schlangen, E. Effect of Different Grade Levels of Calcined Clays on Fresh and Hardened Properties of Ternary-Blended Cementitious Materials for 3D Printing. Cem. Concr. Compos. 2020, 114, 103708. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, B.; Tan, H.; Qi, H.; Shi, T. Effect of Sodium Carbonate and Sodium Phosphate on Hydration of Cement Paste. J. Build. Eng. 2022, 45, 103577. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Dell’Aversano, R.; Gazzaniga, G.; Pastore, T. Influence of Lithium Carbonate and Sodium Carbonate on Physical and Elastic Properties and on Carbonation Resistance of Calcium Sulphoaluminate-Based Mortars. Appl. Sci. 2020, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhang, Y.; Jia, Y.; She, W.; Liu, G.; Yang, Z.; Zhang, Y.; Zhang, W.; Sun, W. Effects of Sodium Sulfate on the Hydration and Properties of Lime-Based Low Carbon Cementitious Materials. J. Clean. Prod. 2019, 220, 677–687. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, L.; Chen, B.; Wu, J. Effect of Pre-Introduced Sodium Chloride on Cement Hydration Process. Adv. Cem. Res. 2021, 33, 526–539. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm Phase in Portland Cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. The Reaction between Metakaolin and Limestone and Its Effect in Porosity Refinement and Mechanical Properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Avet, F.; Hanpongpun, W.; Scrivener, K. Strength-Promoting Mechanism of Alkanolamines on Limestone-Calcined Clay Cement and the Role of Sulfate. Cem. Concr. Res. 2021, 147, 106527. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; De Weerdt, K.; Østnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of Calcium on Chloride Binding in Hydrated Portland Cement–Metakaolin–Limestone Blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Li, R.; Ye, H. Influence of Alkalis on Natural Carbonation of Limestone Calcined Clay Cement Pastes. Sustainability 2021, 13, 12833. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental Studies and Thermodynamic Modeling of the Carbonation of Portland Cement, Metakaolin and Limestone Mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Lei, L.; Palacios, M.; Plank, J.; Jeknavorian, A.A. Interaction between Polycarboxylate Superplasticizers and Non-Calcined Clays and Calcined Clays: A Review. Cem. Concr. Res. 2022, 154, 106717. [Google Scholar] [CrossRef]
- Damasceno Costa, A.R.; Goncalves, J.P. Accelerated Carbonation of Ternary Cements Containing Waste Materials. Constr. Build. Mater. 2021, 302, 124159. [Google Scholar] [CrossRef]
- Mota, B.; Matschei, T.; Scrivener, K. Impact of NaOH and Na2SO4 on the Kinetics and Microstructural Development of White Cement Hydration. Cem. Concr. Res. 2018, 108, 172–185. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Shang, H.; Zhao, T. Molecular Dynamics Study on the Tri-Calcium Silicate Hydration in Sodium Sulfate Solution: Interface Structure, Dynamics and Dissolution Mechanism. Constr. Build. Mater. 2018, 170, 402–417. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, A.; Bishnoi, S. Impact of Alkali Salts on the Hydration of Ordinary Portland Cement and Limestone-Calcined Clay Cement. J. Mater. Civ. Eng. 2021, 33, 04021223. [Google Scholar] [CrossRef]
- Cardoso, T.C.; de Matos, P.R.; Py, L.; Longhi, M.; Cascudo, O.; Kirchheim, A.P. Ternary Cements Produced with Non-Calcined Clay, Limestone, and Portland Clinker. J. Build. Eng. 2022, 45, 103437. [Google Scholar] [CrossRef]
- Ruan, Y.; Jamil, T.; Hu, C.; Gautam, B.P.; Yu, J. Microstructure and Mechanical Properties of Sustainable Cementitious Materials with Ultra-High Substitution Level of Calcined Clay and Limestone Powder. Constr. Build. Mater. 2022, 314, 125416. [Google Scholar] [CrossRef]
- “Metakaolin-Slag-Clinker Blends.” The Role of Na+ or K+ as Alkaline Activators of Theses Ternary Blends. Available online: https://ceramics.onlinelibrary.wiley.com/doi/epdf/10.1111/jace.12272 (accessed on 30 July 2022).
- Zhou, Y.; Lu, J.; Li, J.; Cheeseman, C.; Poon, C.S. Effect of NaCl and MgCl2 on the Hydration of Lime-Pozzolan Blend by Recycling Sewage Sludge Ash. J. Clean. Prod. 2021, 313, 127759. [Google Scholar] [CrossRef]
- Paul, G.; Boccaleri, E.; Buzzi, L.; Canonico, F.; Gastaldi, D. Friedel’s Salt Formation in Sulfoaluminate Cements: A Combined XRD and 27Al MAS NMR Study. Cem. Concr. Res. 2015, 67, 93–102. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; Lothenbach, B.; De Weerdt, K.; Garzón, S.F.; Enemark-Rasmussen, K.; Skibsted, J. Friedel’s Salt Profiles from Thermogravimetric Analysis and Thermodynamic Modelling of Portland Cement-Based Mortars Exposed to Sodium Chloride Solution. Cem. Concr. Compos. 2017, 78, 73–83. [Google Scholar] [CrossRef]
- Han, Y.; Lin, R.; Wang, X.-Y. Performance and Sustainability of Quaternary Composite Paste Comprising Limestone, Calcined Hwangtoh Clay, and Granulated Blast Furnace Slag. J. Build. Eng. 2021, 43, 102655. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Chen, L.; Huang, J.; Ma, T. The Influence of Two Types of Alkali Activators on the Microstructure and Performance of Supersulfated Cement Concrete: Mitigating the Strength and Carbonation Resistance. Cem. Concr. Compos. 2021, 118, 103947. [Google Scholar] [CrossRef]
- Kim, T. The Effects of Polyaluminum Chloride on the Mechanical and Microstructural Properties of Alkali-Activated Slag Cement Paste. Cem. Concr. Compos. 2019, 96, 46–54. [Google Scholar] [CrossRef]
- Zhiguang Huan; Jiang Chang Effect of Sodium Carbonate Solution on Self-Setting Properties of Tricalcium Silicate Bone Cement. J. Biomater. Appl. 2008, 23, 247–262. [CrossRef] [PubMed]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in Microstructure Characteristics of Cement Paste on Carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Tang, X.; Huang, J. Influence of NaCl on Mechanical Properties and Microstructure of Cement Stone. AMR 2013, 700, 136–139. [Google Scholar] [CrossRef]






| Oxide Composition (wt.%) | OPC | CC | LS | Gypsum |
|---|---|---|---|---|
| CaO | 60.70 | 0.02 | 53.29 | 29.93 |
| SiO2 | 22.32 | 46.12 | 3.10 | 4.08 |
| Al2O3 | 6.88 | 51.15 | 1.05 | 1.63 |
| Fe2O3 | 4.94 | 0.90 | 0.16 | 0.67 |
| MgO | 1.95 | 0.11 | 0.35 | 0.92 |
| Na2O | 0.13 | 0.06 | ||
| K2O | 1.11 | 1.06 | 0.05 | |
| SO3 | 0.57 | 0.11 | 0.03 | 38.20 |
| TiO2 | 0.83 | 0.46 | 0.00 | |
| P2O5 | 0.07 | 0.03 | 0.01 | |
| LOI | 0.50 | 0.05 | 41.90 | 1.06 |
| Mix Code (wt.%) | Binder | CC | LS | Gypsum | Na2CO3 | Na2SO4 | NaCl |
|---|---|---|---|---|---|---|---|
| LC3-Control | 50 | 30 | 15 | 5 | |||
| LC3-Na2CO3 | 50 | 30 | 15 | 5 | 1.00 | ||
| LC3-Na2SO4 | 50 | 30 | 15 | 5 | 1.34 | ||
| LC3-NaCl | 50 | 30 | 15 | 5 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Wu, S.; Chen, H. Early Strength-Promoting Mechanism of Inorganic Salts on Limestone-Calcined Clay Cement. Sustainability 2023, 15, 5286. https://doi.org/10.3390/su15065286
Zhou W, Wu S, Chen H. Early Strength-Promoting Mechanism of Inorganic Salts on Limestone-Calcined Clay Cement. Sustainability. 2023; 15(6):5286. https://doi.org/10.3390/su15065286
Chicago/Turabian StyleZhou, Weijie, Shuanglei Wu, and Huxing Chen. 2023. "Early Strength-Promoting Mechanism of Inorganic Salts on Limestone-Calcined Clay Cement" Sustainability 15, no. 6: 5286. https://doi.org/10.3390/su15065286




