Carbon-Supported Nickel Catalysts—Comparison in Alpha-Pinene Oxidation Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon (AC)
2.2. Preparation of Metallic Catalysts
2.2.1. Impregnation of Activated Carbon with Nickel Salt Solution and Reduction with Hydrogen
2.2.2. Hydrothermal Method of Preparation of the Catalysts in the Autoclave
2.3. Characteristics of the Catalysts Obtained from Biomass
2.4. Alpha-Pinene Oxidation Method
3. Results and Discussion
3.1. Characterization of the Obtained Ni-Modified Carbonaceous Materials
3.2. Activity of Carbon-Supported Nickel Catalysts
3.3. Determination of the Kinetics Parameters Determination of the Kinetics Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of Activated Carbon from Biomass and Its’ Applications in Water and Gas Purification, a Review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural Bio-Waste Materials as Potential Sustainable Precursors Used for Activated Carbon Production: A Review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Kamińska, A.; Niedoba, O.; Michalkiewicz, B. CO2 Adsorption on Activated Carbons Prepared from Molasses: A Comparison of Two and Three Parametric Models. Materials 2021, 14, 7458. [Google Scholar] [CrossRef] [PubMed]
- Adeyi, O. Proximate Composition of Some Agricultural Wastes in Nigeria and Their Potential Use in Activated Carbon Production. J. Appl. Sci. Environ. Manag. 2010, 14, 55–58. [Google Scholar] [CrossRef]
- González-García, P. Activated Carbon from Lignocellulosics Precursors: A Review of the Synthesis Methods, Characterization Techniques and Applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Kim, M.H.; Jeong, I.T.; Park, S.B.; Kim, J.W. Analysis of Environmental Impact of Activated Carbon Production from Wood Waste. Environ. Eng. Res. 2019, 24, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Danish, M.; Ahmad, T. A Review on Utilization of Wood Biomass as a Sustainable Precursor for Activated Carbon Production and Application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Zieliński, B.; Miądlicki, P.; Przepiórski, J. Development of Activated Carbon for Removal of Pesticides from Water: Case Study. Sci. Rep. 2022, 12, 20869. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A Study on Chemical Constituents and Sugars Extraction from Spent Coffee Grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Janissen, B.; Huynh, T. Chemical Composition and Value-Adding Applications of Coffee Industry by-Products: A Review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Low, J.H.; Rahman, W.A.W.A.; Jamaluddin, J. Structural Elucidation of Tannins of Spent Coffee Grounds by CP-MAS 13C NMR and MALDI-TOF MS. Ind. Crops Prod. 2015, 69, 456–461. [Google Scholar] [CrossRef] [Green Version]
- De Nicola, E.; Meriç, S.; Gallo, M.; Iaccarino, M.; Della Rocca, C.; Lofrano, G.; Russo, T.; Pagano, G. Vegetable and Synthetic Tannins Induce Hormesis/Toxicity in Sea Urchin Early Development and in Algal Growth. Environ. Pollut. 2007, 146, 46–54. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Yadav, S.K. Retardation in Seedling Growth and Induction of Early Senescence in Plants upon Caffeine Exposure Is Related to Its Negative Effect on Rubisco. Photosynthetica 2009, 47, 293–297. [Google Scholar] [CrossRef]
- Miyashira, C.H.; Tanigushi, D.G.; Gugliotta, A.M.; Santos, D.Y. Influence of Caffeine on the Survival of Leaf-Cutting Ants Atta Sexdens Rubropilosa and in Vitro Growth of Their Mutualistic Fungus. Pest Manag. Sci. 2012, 68, 935–940. [Google Scholar] [CrossRef]
- Sledz, W.; Los, E.; Paczek, A.; Rischka, J.; Motyka, A.; Zoledowska, S.; Piosik, J.; Lojkowska, E. Antibacterial Activity of Caffeine against Plant Pathogenic Bacteria. Acta Biochim. Pol. 2015, 62, 605–612. [Google Scholar] [CrossRef]
- Tsou, M.F.; Hung, C.F.; Lu, H.F.; Wu, L.T.; Chang, S.H.; Chang, H.L.; Chen, G.W.; Chung, J.G. Effects of Caffeic Acid, Chlorogenic Acid and Ferulic Acid on Growth and Arylamine N-Acetyltransferase Activity in Shigella Sonnei (Group D). Microbios 2000, 101, 37–46. [Google Scholar]
- Woldesenbet, A.G.; Woldeyes, B.; Chandravanshi, B.S. Bio-Ethanol Production from Wet Coffee Processing Waste in Ethiopia. Springerplus 2016, 5, 1903. [Google Scholar] [CrossRef] [Green Version]
- Adi, A.J.; Noor, Z.M. Waste Recycling: Utilization of Coffee Grounds and Kitchen Waste in Vermicomposting. Bioresour. Technol. 2009, 100, 1027–1030. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars Metabolism and Ethanol Production by Different Yeast Strains from Coffee Industry Wastes Hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Kamińska, A.; Miądlicki, P.; Kiełbasa, K.; Kujbida, M.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Wróblewska, A. Activated Carbons Obtained from Orange Peels, Coffee Grounds, and Sunflower Husks—Comparison of Physicochemical Properties and Activity in the Alpha-Pinene Isomerization Process. Materials 2021, 14, 7448. [Google Scholar] [CrossRef]
- Shanthilal, J.; Bhattacharya, S. Nanoparticles and Nanotechnology in Food. In Conventional and Advanced Food Processing Technologies; John Wiley & Sons, Ltd.: Chichester, UK, 2014; Volume 9781118406, pp. 567–594. ISBN 9781118406281. [Google Scholar]
- Ádám, A.A.; Szabados, M.; Varga, G.; Papp, Á.; Musza, K.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Ultrasound-Assisted Hydrazine Reduction Method for the Preparation of Nickel Nanoparticles, Physicochemical Characterization and Catalytic Application in Suzuki-Miyaura Cross-Coupling Reaction. Nanomaterials 2020, 10, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lestinsky, P.; Zikmund, Z.; Grycova, B.; Ryczkowski, R.; Grams, J.; Inayat, A. Production of Hydrogen over Ni/Carbonaceous Catalyst. Fuel 2020, 278, 118398. [Google Scholar] [CrossRef]
- Fidalgo, B.; Zubizarreta, L.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Synthesis of Carbon-Supported Nickel Catalysts for the Dry Reforming of CH4. Fuel Process. Technol. 2010, 91, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Malobela, L.J.; Heveling, J.; Augustyn, W.G.; Cele, L.M. Nickel-Cobalt on Carbonaceous Supports for the Selective Catalytic Hydrogenation of Cinnamaldehyde. Ind. Eng. Chem. Res. 2014, 53, 13910–13919. [Google Scholar] [CrossRef]
- Li, B.; Ren, Y.; Fan, Q.; Feng, A.; Dong, W. Preparation and Characterization of Spherical Nickel-Doped Carbonaceous Resin as Hydrogenation Catalysts I. Carbonization Procedures. Carbon 2004, 42, 2669–2676. [Google Scholar] [CrossRef]
- Salimi, M.; Tavasoli, A.; Balou, S.; Hashemi, H.; Kohansal, K. Influence of Promoted Bimetallic Ni-Based Catalysts and Micro/Mesopores Carbonaceous Supports for Biomass Hydrothermal Conversion to H2-Rich Gas. Appl. Catal. B Environ. 2018, 239, 383–397. [Google Scholar] [CrossRef]
- Altass, H.M.; Ahmed, S.A.; Salama, R.S.; Moussa, Z.; Jassas, R.S.; Alsantali, R.I.; Al-Rooqi, M.M.; Ibrahim, A.A.; Khder, M.A.; Morad, M.; et al. Low Temperature CO Oxidation Over Highly Active Gold Nanoparticles Supported on Reduced Graphene Oxide@Mg-BTC Nanocomposite. Catal. Lett. 2022, 153, 876–886. [Google Scholar] [CrossRef]
- Masruri; Pamungkas, K.K. Copper Nanoparticle Catalysed Aerobic Oxidation of α-Pinene. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042023. [Google Scholar] [CrossRef]
- Lei, J.; Lu, X.H.; Zhang, J.L.; Wei, X.L.; Zhou, D.; Xia, Q.H. Epoxidation of Mixed Bi-Olefins with Air over Nanosized Co 3O4 Assisted by Ultrasonic Waves. Indian J. Chem. Sect. A Inorg. Phys. Theor. Anal. Chem. 2013, 52, 709–716. [Google Scholar]
- Kamińska, A.; Maciejewska, N.; Miądlicki, P.; Kiełbasa, K.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Fe-Modified Activated Carbon Obtained from Biomass as a Catalyst for α-Pinene Autoxidation. Polish J. Chem. Technol. 2021, 23, 73–80. [Google Scholar] [CrossRef]
- Rauchdi, M.; Ait Ali, M.; Roucoux, A.; Denicourt-Nowicki, A. Novel Access to Verbenone via Ruthenium Nanoparticles-Catalyzed Oxidation of A-Pinene in Neat Water. Appl. Catal. A Gen. 2018, 550, 266–273. [Google Scholar] [CrossRef]
- Cánepa, A.L.; Chanquía, C.M.; Vaschetti, V.M.; Eimer, G.A.; Casuscelli, S.G. Biomass toward Fine Chemical Products: Oxidation of α-Pinene over Sieves Nanostructured Modified with Vanadium. J. Mol. Catal. A Chem. 2015, 404–405, 65–73. [Google Scholar] [CrossRef]
- Denicourt-Nowicki, A.; Roucoux, A. Odyssey in Polyphasic Catalysis by Metal Nanoparticles. Chem. Rec. 2016, 16, 2127–2141. [Google Scholar] [CrossRef]
- Zhu, S.J.; Xu, S.C.; Zhao, Z.D. An Efficient Synthesis Method Targeted to a Novel Aziridine Derivative of P-Menthane from Turpentine and Its Herbicidal Activity. Nat. Prod. Res. 2017, 31, 1536–1543. [Google Scholar] [CrossRef]
- Yoo, S.K.; Day, D.F. Bacterial Metabolism of α- and β-Pinene and Related Monoterpenes by Pseudomonas Sp. Strain PIN. Process Biochem. 2002, 37, 739–745. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential Oils Used in Aromatherapy: A Systemic Review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Wender, P.A.; Mucciaro, T.P. A New and Practical Approach to the Synthesis of Taxol and Taxol Analogues: The Pinene Path. J. Am. Chem. Soc. 1992, 114, 5878–5879. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Tobbala, D.E.; El-Bahy, S.M.; Nassan, M.A.; Salama, R.S. The Role of Phosphotungstic Acid in Enhancing the Catalytic Performance of UiO-66 (Zr) and Its Applications as an Efficient Solid Acid Catalyst for Coumarins and Dihydropyrimidinones Synthesis. Catal. Commun. 2022, 169, 106479. [Google Scholar] [CrossRef]
- Ananikov, V.P. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Kamińska, A.; Miądlicki, P.; Kiełbasa, K.; Serafin, J.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Wróblewska, A. FeCl3-Modified Carbonaceous Catalysts from Orange Peel for Solvent-Free Alpha-Pinene Oxidation. Materials 2021, 14, 7729. [Google Scholar] [CrossRef] [PubMed]
- Dongil, A.B.; Ghampson, I.T.; García, R.; Fierro, J.L.G.; Escalona, N. Hydrodeoxygenation of Guaiacol over Ni/Carbon Catalysts: Effect of the Support and Ni Loading. RSC Adv. 2016, 6, 2611–2623. [Google Scholar] [CrossRef]
- Rahman, M.M.; Muttakin, M.; Pal, A.; Shafiullah, A.Z.; Saha, B.B. A Statistical Approach to Determine Optimal Models for IUPAC-Classified Adsorption Isotherms. Energies 2019, 12, 4565. [Google Scholar] [CrossRef] [Green Version]
- Sing, K. The Use of Nitrogen Adsorption for the Characterisation of Porous Materials. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 3–9. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Pérez, L.; Buitrago-Sierra, R.; Sepúlveda-Escribano, A. CeO2-Promoted Ni/Activated Carbon Catalysts for the Water-Gas Shift (WGS) Reaction. Int. J. Hydrog. Energy 2014, 39, 17589–17599. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kamińska, A.; Miądlicki, P.; Wróblewska, A.; Kiełbasa, K.; Wróbel, R.J.; Serafin, J.; Michalkiewicz, B. Activated Carbon Modification towards Efficient Catalyst for High Value-Added Products Synthesis from Alpha-Pinene. Materials 2021, 14, 7811. [Google Scholar] [CrossRef]
- Iwu, K.O.; Lombardo, A.; Sanz, R.; Scirè, S.; Mirabella, S. Facile Synthesis of Ni Nanofoam for Flexible and Low-Cost Non-Enzymatic Glucose Sensing. Sens. Actuators B Chem. 2016, 224, 764–771. [Google Scholar] [CrossRef]
- Yuan, G.; Jiang, Z.; Aramata, A.; Gao, Y. Electrochemical Behavior of Activated-Carbon Capacitor Material Loaded with Nickel Oxide. Carbon 2005, 43, 2913–2917. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of Nanoscaled and Bulk NiO Structural and Environmental Characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Kalam, A.; Al-Sehemi, A.G.; Al-Shihri, A.S.; Du, G.; Ahmad, T. Synthesis and Characterization of NiO Nanoparticles by Thermal Decomposition of Nickel Linoleate and Their Optical Properties. Mater. Charact. 2012, 68, 77–81. [Google Scholar] [CrossRef]
- Rahdar, A.; Aliahmad, M.; Azizi, Y. NiO Nanoparticles: Synthesis and Characterization. J. Nanostruct. 2015, 5, 145–151. [Google Scholar] [CrossRef]
- O’Reilly, J.M.; Mosher, R.A. Functional Groups in Carbon Black by FTIR Spectroscopy. Carbon 1983, 21, 47–51. [Google Scholar] [CrossRef]
- Kemp, K.C.; Baek, S.B.; Lee, W.G.; Meyyappan, M.; Kim, K.S. Activated Carbon Derived from Waste Coffee Grounds for Stable Methane Storage. Nanotechnology 2015, 26, 385602. [Google Scholar] [CrossRef]
- Afriani, A.; Abdullah, I.; Krisnandi, Y.K. Synthesis of NiCl2 impregnated Mesoporous Carbon and Its Adsorption Activity on CO2. In Proceedings of the AIP Conference Proceedings, Bandung, Indonesia, 29–30 September 2021; Volume 2349, p. 020048. [Google Scholar]
- Cuenya, B.R. Synthesis and Catalytic Properties of Metal Nanoparticles: Size, Shape, Support, Composition, and Oxidation State Effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Karra, S.; Wooten, M.; Griffith, W.; Gorski, W. Morphology of Gold Nanoparticles and Electrocatalysis of Glucose Oxidation. Electrochim. Acta 2016, 218, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Jiang, K.; Wei, R.; Tahir, M.; Wu, X.; Shen, M.; Wang, X.; Cao, C. Popcorn-Derived Porous Carbon Flakes with an Ultrahigh Specific Surface Area for Superior Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 30626–30634. [Google Scholar] [CrossRef]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Zhang, Y.; Zhu, X.; Chen, B. Hollow Multi-Shelled Co3O4 as Nanoreactors to Activate Peroxymonosulfate for Highly Effective Degradation of Carbamazepine: A Novel Strategy to Reduce Nano-Catalyst Agglomeration. J. Hazard. Mater. 2022, 427, 127890. [Google Scholar] [CrossRef]
- Sekimoto, K.; Fukuyama, D.; Inomata, S. Accurate Identification of Dimers from α-Pinene Oxidation Using High-Resolution Collision-Induced Dissociation Mass Spectrometry. J. Mass Spectrom. 2020, 55, e4508. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Robles-Dutenhefner, P.; Menini, L.; Gusevskaya, E.V. Cobalt Catalyzed Autoxidation of Monoterpenes in Acetic Acid and Acetonitrile Solutions. J. Mol. Catal. A Chem. 2003, 201, 71–77. [Google Scholar] [CrossRef]
- Ancel, J.E.; Maksimchuk, N.V.; Simakova, I.L.; Semikolenov, V.A. Kinetic Peculiarities of α-Pinene Oxidation by Molecular Oxygen. Appl. Catal. A Gen. 2004, 272, 109–114. [Google Scholar] [CrossRef]
- Emanuel, N.M.; Knorre, D.G. Kurs Khimicheskoi Kinetiki (Course in Chemical Kinetics); Vysshaya Shkola: Moscow, Russia, 1962. [Google Scholar]
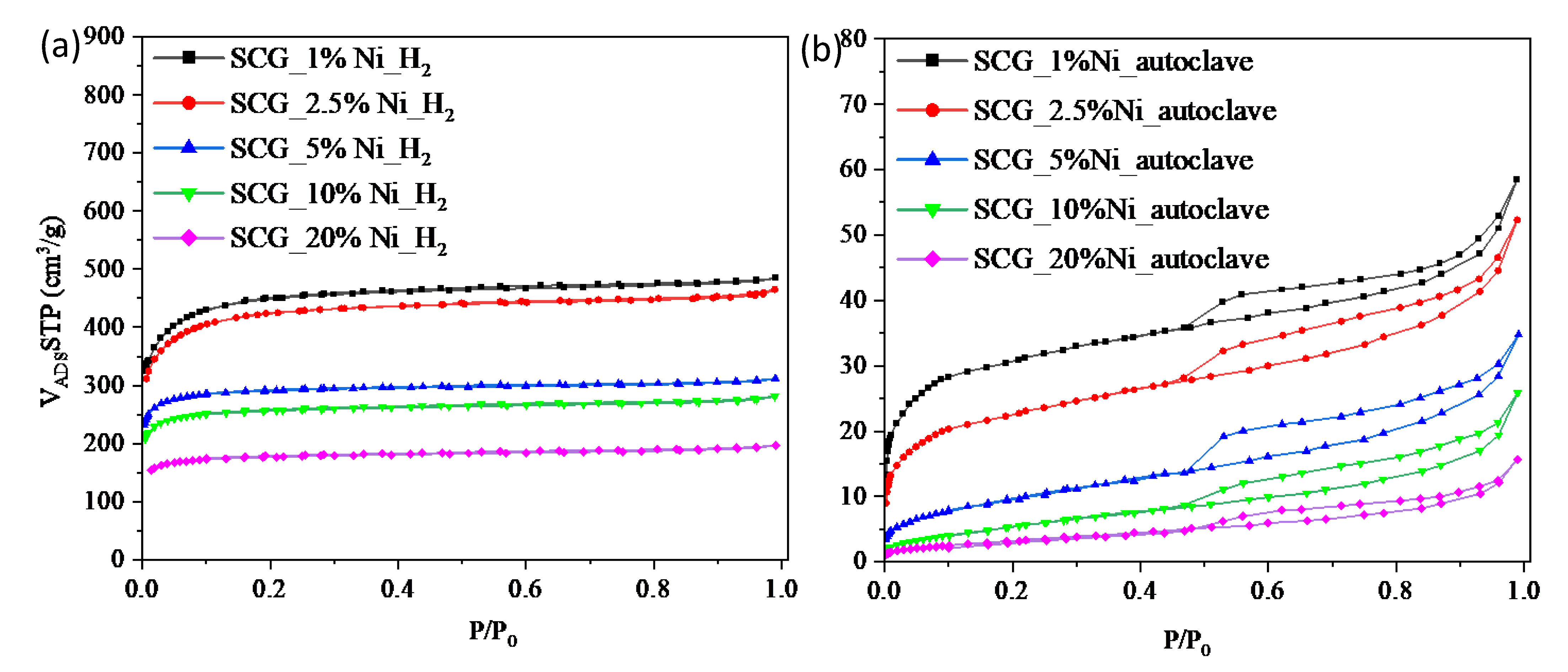














| Sample | SBET [m2/g] | Vtot [cm3/g] | Vmic [cm3/g] |
|---|---|---|---|
| SCG_1%Ni_H2 | 1534 | 0.752 | 0.563 |
| SCG_2.5%Ni_H2 | 1104 | 0.569 | 0.410 |
| SCG_5%Ni_H2 | 926 | 0.483 | 0.405 |
| SCG_10%Ni_H2 | 838 | 0.436 | 0.360 |
| SCG_20%Ni_H2 | 569 | 0.305 | 0.246 |
| SCG_1%Ni_autoclave | 105 | 0.091 | 0.074 |
| SCG_2.5%Ni_autoclave | 46 | 0.081 | 0.051 |
| SCG_5%Ni_autoclave | 35 | 0.054 | 0.038 |
| SCG_10%Ni_autoclave | 22 | 0.040 | 0.012 |
| SCG_20%Ni_autoclave | 13 | 0.023 | 0.011 |
| Sample | (wt%) | |||
|---|---|---|---|---|
| Si | Cl | K | Ni | |
| SCG_1%Ni_H2 | 0.42 | 0.44 | 0.75 | 0.92 |
| SCG_2.5%Ni_H2 | 0.88 | 0.51 | 0.23 | 2.50 |
| SCG_5%Ni_H2 | 0.62 | 0.91 | 0.34 | 4.91 |
| SCG_10%Ni_H2 | 0.52 | 0.97 | 0.19 | 9.90 |
| SCG_20%Ni_H2 | 0.29 | 1.00 | 0.16 | 19.99 |
| SCG_1%Ni_autoclave | 0.88 | - | - | 1.05 |
| SCG_2.5%Ni_autoclave | 0.53 | - | - | 2.57 |
| SCG_5%Ni_autoclave | 0.57 | - | - | 5.08 |
| SCG_10%Ni_autoclave | 0.93 | - | - | 10.31 |
| SCG_20%Ni_autoclave | 0.39 | - | - | 19.99 |
| Sample | Acid-Site Concentration (mmol/g) |
|---|---|
| SCG_1%Ni_H2 | 1.738 |
| SCG_2.5%Ni_H2 | 2.182 |
| SCG_5%Ni_H2 | 2.588 |
| SCG_10%Ni_H2 | 3.415 |
| SCG_20%Ni_H2 | 4.522 |
| SCG_1%Ni_autoclave | 1.549 |
| SCG_2.5%Ni_autoclave | 1.222 |
| SCG_5%Ni_autoclave | 1.289 |
| SCG_10%Ni_autoclave | 1.157 |
| SCG_20%Ni_autoclave | 1.109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, A.; Sreńscek-Nazzal, J.; Kiełbasa, K.; Grzeszczak, J.; Serafin, J.; Wróblewska, A. Carbon-Supported Nickel Catalysts—Comparison in Alpha-Pinene Oxidation Activity. Sustainability 2023, 15, 5317. https://doi.org/10.3390/su15065317
Kamińska A, Sreńscek-Nazzal J, Kiełbasa K, Grzeszczak J, Serafin J, Wróblewska A. Carbon-Supported Nickel Catalysts—Comparison in Alpha-Pinene Oxidation Activity. Sustainability. 2023; 15(6):5317. https://doi.org/10.3390/su15065317
Chicago/Turabian StyleKamińska, Adrianna, Joanna Sreńscek-Nazzal, Karolina Kiełbasa, Jadwiga Grzeszczak, Jarosław Serafin, and Agnieszka Wróblewska. 2023. "Carbon-Supported Nickel Catalysts—Comparison in Alpha-Pinene Oxidation Activity" Sustainability 15, no. 6: 5317. https://doi.org/10.3390/su15065317
APA StyleKamińska, A., Sreńscek-Nazzal, J., Kiełbasa, K., Grzeszczak, J., Serafin, J., & Wróblewska, A. (2023). Carbon-Supported Nickel Catalysts—Comparison in Alpha-Pinene Oxidation Activity. Sustainability, 15(6), 5317. https://doi.org/10.3390/su15065317









