Biomass–Coal Hybrid Fuel: A Route to Net-Zero Iron Ore Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermal Analysis
2.2. Calorific Values
2.3. Proximate Analysis
2.4. Volatile Matter Analysis
3. Results
3.1. Calorific Values
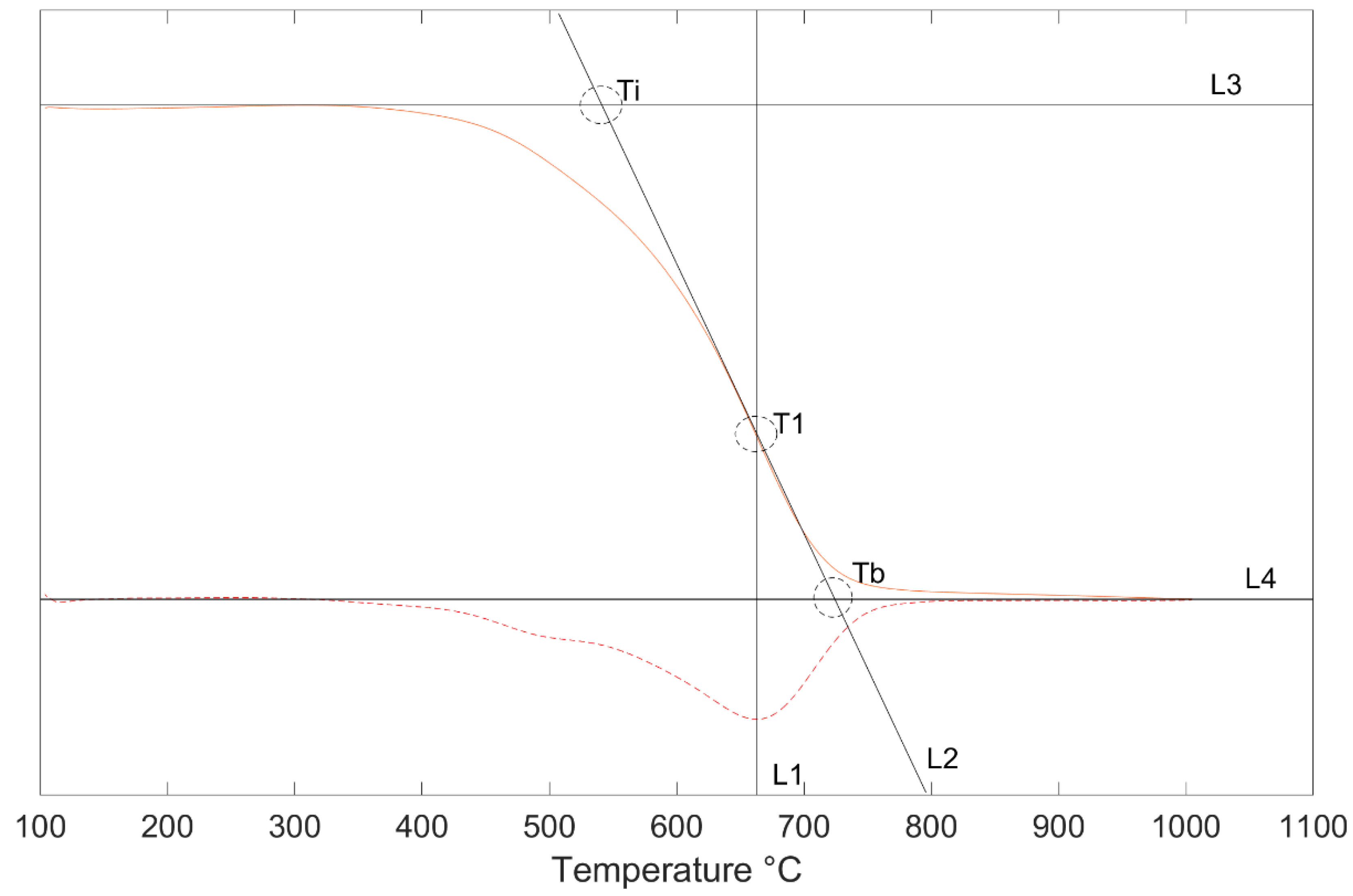
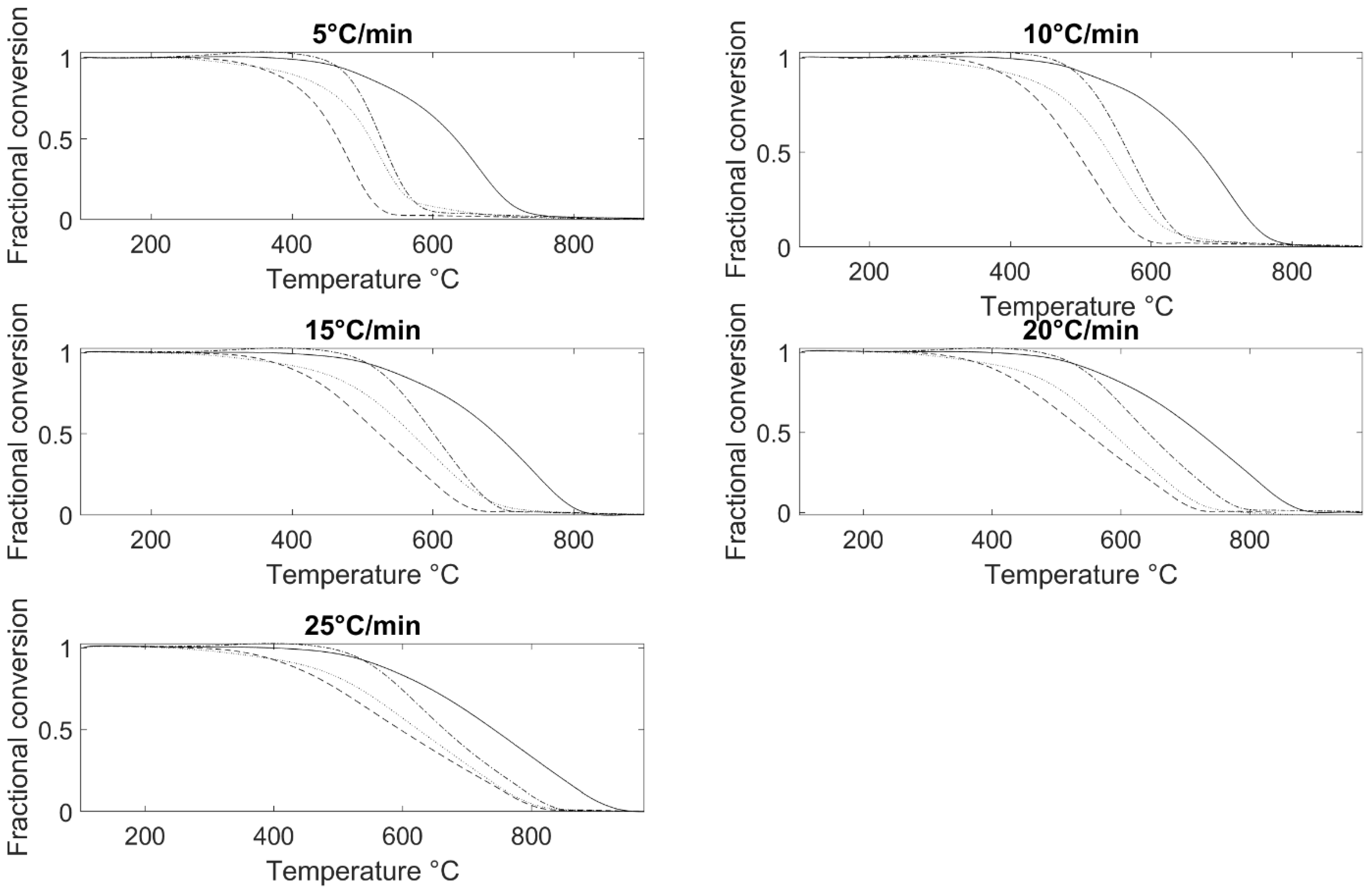
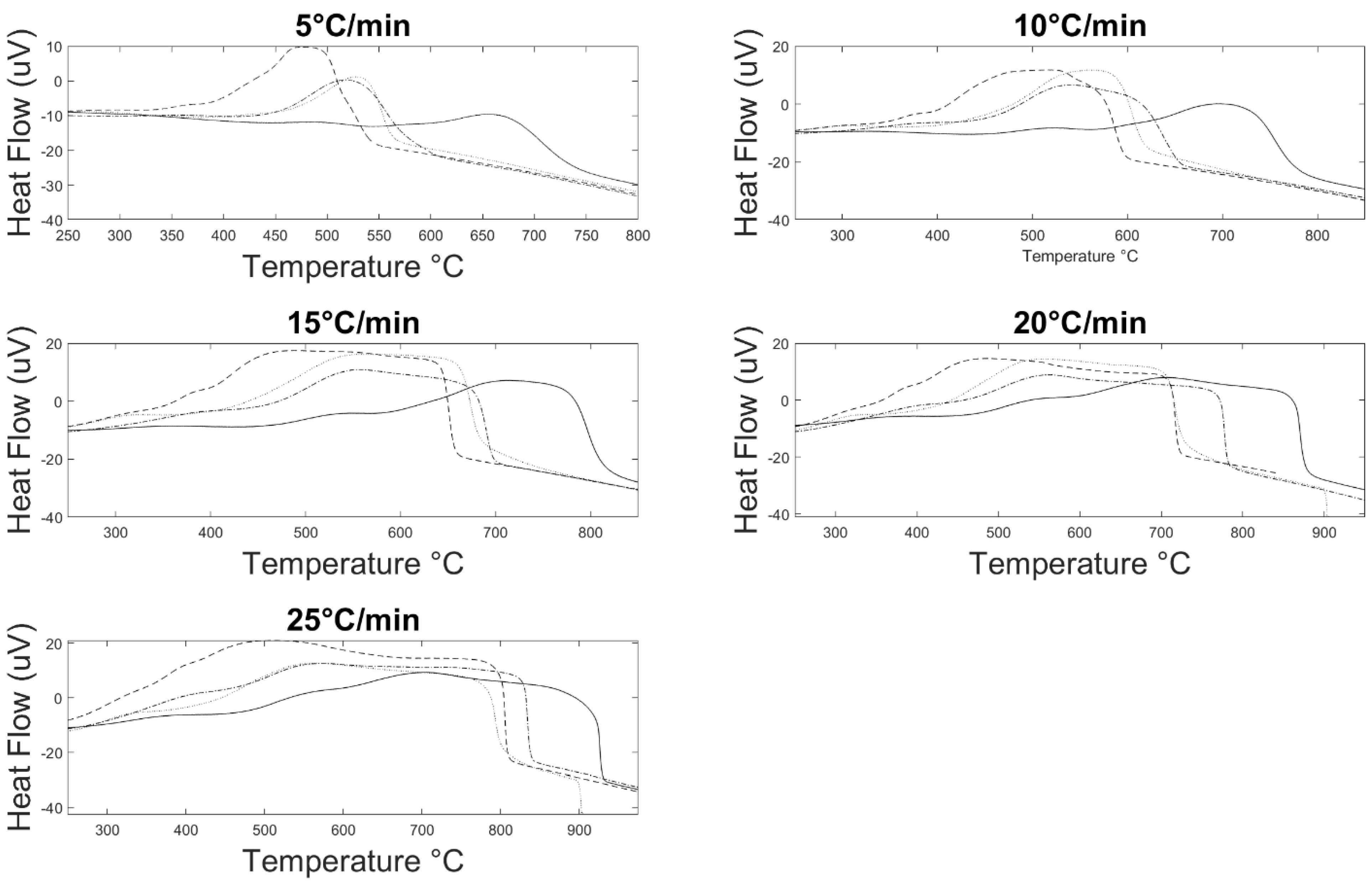
3.2. Burnout Kinetics
3.3. Volatile Matter Analysis
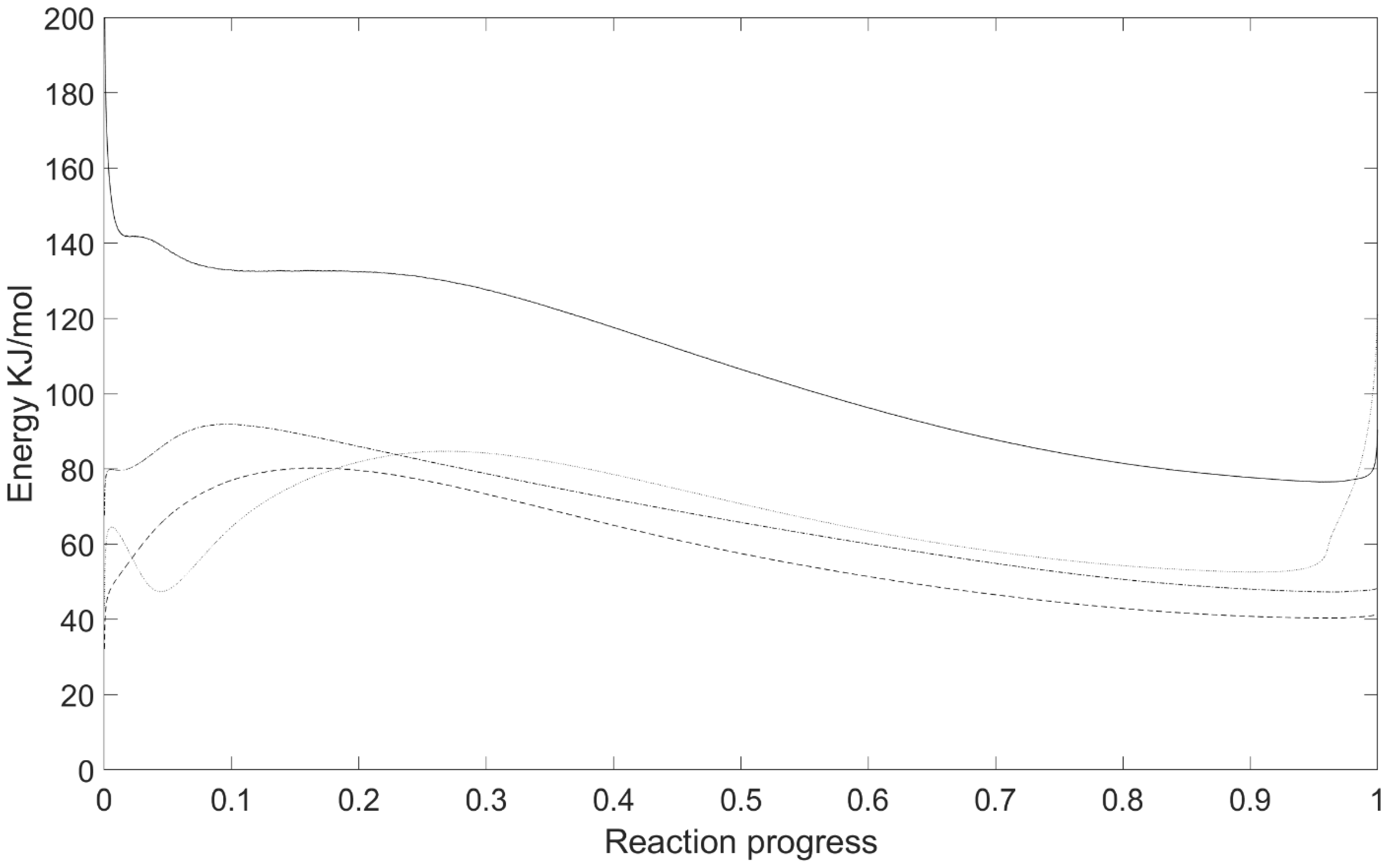
3.4. Isoconversional Kinetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Weight % | ||||
|---|---|---|---|---|
| Element | ecoke® A | ecoke® B | Coal A | PT Breeze |
| C K | 86.32 | 86.58 | 90.5 | 91.83 |
| O K | 11.17 | 10.65 | 6.83 | - |
| Na K | 0.26 | - | - | 0.12 |
| Al K | 0.16 | 0.21 | 0.86 | 2.94 |
| Si K | 0.23 | 0.62 | 1.02 | 3.11 |
| S K | 0.54 | 0.39 | 0.46 | 0.6 |
| Cl K | 0.11 | 0.19 | 0.21 | - |
| K K | 0.34 | 0.51 | - | 0.35 |
| Ca K | 0.35 | 0.71 | - | 0.33 |
| Fe K | 0.51 | 0.15 | 0.12 | 0.4 |
| Ti K | - | - | - | 0.28 |
| Mg K | - | - | - | 0.04 |
| Sample | Calorific Value (MJ/kg) |
|---|---|
| Ash Sawdust | 17.58 (0.5) |
| Walnut Sawdust | 18.24 (0.5) |
| Teak Sawdust | 18.14 (0.7) |

References
- Babich, A.; Senk, D. 13—Coke in the iron and steel industry. In New Trends in Coal Conversion; Suárez-Ruiz, I., Diez, M.A., Rubiera, F., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 367–404. [Google Scholar]
- UK Goverment. COP26-Presidency-Outcomes-The-Climate-Pact. 2021. Available online: https://ukcop26.org/wp-content/uploads/2021/11/COP26-Presidency-Outcomes-The-Climate-Pact.pdf (accessed on 14 September 2022).
- Cheng, Z.; Yang, J.; Zhou, L.; Liu, Y.; Wang, Q. Characteristics of charcoal combustion and its effects on iron-ore sintering performance. Appl. Energy 2016, 161, 364–374. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking—Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef] [Green Version]
- Zandi, M.; Martinez-Pacheco, M.; Fray, T.A.T. Biomass for iron ore sintering. Miner. Eng. 2010, 23, 1139–1145. [Google Scholar] [CrossRef]
- Nicol, S.; Chen, J.; Pownceby, M.; Webster, A. A review of the chemistry, structure and formation conditions of silico-ferrite of calcium and aluminum (‘SFCS’) phases. ISIJ Int. 2018, 58, 1111–1112. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Adam, M.; Kilburn, M.; Hapugoda, S.; Somerville, M.; Jahanshahi, S.; Mathieson, J.G. Substitution of Charcoal for Coke Breeze in Iron Ore Sintering. ISIJ Int. 2013, 53, 1607–1616. [Google Scholar] [CrossRef] [Green Version]
- Science, W. 2022 Thermogravimetric Analysis: Document Search. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 14 September 2022).
- Nyakuma, B.B.; Wong, S.L.; Oladokun, O.; Bello, A.A.; Hambali, H.U.; Abdullah, T.A.T.; Wong, K.Y. Review of the fuel properties, characterisation techniques, and pre-treatment technologies for oil palm empty fruit bunches. Biomass Convers. Biorefinery 2020, 13, 471–497. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 340–341, 53–68. [Google Scholar] [CrossRef]
- AKTS. Thermokinetics Software Thermal Analysis Isoconversional Model Fitting Dsc-Tg Detailed Description. Available online: https://www.akts.com/tk/thermokinetics-software-thermal-analysis-isoconversional-model-fitting-dsc-tg-detailed-description/ (accessed on 4 November 2022).
- Gerassimidou, S.; Velis, C.A.; Williams, P.T.; Komilis, D. Characterisation and composition identification of waste-derived fuels obtained from municipal solid waste using thermogravimetry: A review. Waste Manag. Res. 2020, 38, 942–965. [Google Scholar] [CrossRef]
- Skreiberg, A.; Skreiberg, Ø.; Sandquist, J.; Sørum, L. TGA and macro-TGA characterisation of biomass fuels and fuel mixtures. Fuel 2011, 90, 2182–2197. [Google Scholar] [CrossRef]
- Yorulmaz, S.Y.; Atimtay, A.T. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process. Technol. 2009, 90, 939–946. [Google Scholar] [CrossRef]
- Ni, L.; Feng, Z.; Zhang, T.; Gao, Q.; Hou, Y.; He, Y.; Su, M.; Ren, H.; Hu, W.; Liu, Z. Effect of pyrolysis heating rates on fuel properties of molded charcoal: Imitating industrial pyrolysis process. Renew. Energy 2022, 197, 257–267. [Google Scholar] [CrossRef]
- Azam, M.; Ashraf, A.; Jahromy, S.S.; Raza, W.; Khalid, H.; Raza, N.; Winter, F. Isoconversional nonisothermal kinetic analysis of municipal solid waste, refuse-derived fuel, and coal. Energy Sci. Eng. 2020, 8, 3728–3739. [Google Scholar] [CrossRef]
- Ruyter, H.P. Coalification model. Fuel 1982, 61, 1182–1187. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, W.; Cheng, Y.; Liu, Z.; Zhang, Q.; Zhao, K. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification. Fuel 2019, 239, 559–572. [Google Scholar] [CrossRef]
- Chiodo, V.; Zafarana, G.; Maisano, S.; Freni, S.; Urbani, F. Pyrolysis of different biomass: Direct comparison among Posidonia Oceanica, Lacustrine Alga and White-Pine. Fuel 2016, 164, 220–227. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Z.; Liu, B.; Chen, Y.; Gong, M.; Li, S.; Chen, H. A new insight of lignin pyrolysis mechanism based on functional group evolutions of solid char. Fuel 2021, 288, 119–719. [Google Scholar] [CrossRef]
- Group, L.S. LIBERTY Steel UK Launches Ecoke—A New Method of Electric Steelmaking to Reduce CO2 Emissions; Liberty Steel Group: London, UK, 2022. [Google Scholar]
- Ma, Z.; Wang, J.; Yang, Y.; Zhang, Y.; Zhao, C.; Yu, Y.; Wang, S. Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J. Anal. Appl. Pyrolysis 2018, 134, 12–24. [Google Scholar] [CrossRef]
- Lu, J.-J.; Chen, W.-H. Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Srinivasan, M.P.; Balasubramanian, R. Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour. Technol. 2012, 123, 646–652. [Google Scholar] [CrossRef]
- Li, X.G.; Lv, Y.; Ma, B.G.; Jian, S.W.; Tan, H.B. Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Bioresour. Technol. 2011, 102, 9783–9787. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef] [Green Version]
- Luthfi, N.; Ohkoshi, T.; Tamaru, Y.; Fukushima, T.; Takisawa, K. Investigation into the combustion kinetics and spontaneous ignition of sweet sorghum as energy resource. Bioresour. Bioprocess. 2022, 9, 49. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70, 487–523. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- ASTM D7582-15; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM International: West Conshohocken, PA, USA, 2016. Available online: https://www.astm.org/d7582-15.html (accessed on 12 March 2023).
- ISO 17246:2010; Coal—Proximate Analysis. International Standards Organisation: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/55946.html (accessed on 12 March 2023).
- Di Gruttola, F.; Borello, D. Analysis of the EU Secondary Biomass Availability and Conversion Processes to Produce Advanced Biofuels: Use of Existing Databases for Assessing a Metric Evaluation for the 2025 Perspective. Sustainability 2021, 13, 7882. [Google Scholar] [CrossRef]
- LECO. ChromaTOF Software. Available online: https://www.leco.com/product/chromatof-software (accessed on 15 November 2022).
- Demirbaş, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Jha, G.; Soren, S. Study on applicability of biomass in iron ore sintering process. Renew. Sustain. Energy Rev. 2017, 80, 399–407. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Cheng, M.; Liu, R.; Cen, K. Effect of flame-front speed on the pisolite-ore sintering process. Appl. Therm. Eng. 2015, 75, 307–314. [Google Scholar] [CrossRef]
- Gan, M.; Li, Q.; Ji, Z.Y.; Fan, X.H.; Lv, W. Influence of surface modification on combustion characteristics of charcoal and its performance on emissions reduction in iron ore sintering. ISIJ Int. 2017, 57, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Stewart, D.J.C.; Scrimshire, A.; Thomson, D.; Bingham, P.A.; Barron, A.R. The chemical suitability for recycling of zinc contaminated steelmaking by-product dusts: The case of the UK steel plant. Resour. Conserv. Recycl. Adv. 2022, 14, 200073. [Google Scholar] [CrossRef]




| Sample | Proximate Analysis (% by Mass) | Calorific Value (MJ/kg) | ||
|---|---|---|---|---|
| Fixed Carbon | Volatile Matter | Ash | ||
| ecoke®(A) | 78.7 | 13.3 | 8.9 | 27.9 |
| ecoke®(B) | 77.0 | 12.4 | 11.2 | 27.8 |
| PT Breeze | 83.4 | 7.4 | 9.2 | 26.5 |
| Coal A | 73.2 | 14.4 | 12.4 | 29.9 |
| ecoke®(A) | ecoke®(B) | PT Breeze | Coal A | |
|---|---|---|---|---|
| 5 °C/min | ||||
| Ti | 406 | 454 | 545 | 476 |
| T1 | 478 | 518 | 662 | 525 |
| Tb | 523 | 588 | 724 | 577 |
| Rmax | 0.42 | 0.28 | 0.25 | 0.46 |
| Rav | 0.10 | 0.08 | 0.09 | 0.12 |
| C (×10−6) | 2.55 | 1.36 | 0.84 | 2.03 |
| S (×10−10) | 5.02 | 1.78 | 1.02 | 4.29 |
| 10 °C/min | ||||
| Ti | 401 | 462 | 579 | 494 |
| T1 | 506 | 555 | 707 | 574 |
| Tb | 643 | 614 | 769 | 637 |
| Rmax | 0.49 | 0.44 | 0.45 | 0.71 |
| Rav | 0.14 | 0.13 | 0.20 | 0.29 |
| C (×10−6) | 3.05 | 2.06 | 1.34 | 2.91 |
| S (×10−10) | 6.82 | 4.30 | 3.49 | 13.02 |
| 15 °C/min | ||||
| Ti | 401 | 455 | 585 | 511 |
| T1 | 512 | 575 | 743 | 601 |
| Tb | 643 | 688 | 809 | 687 |
| Rmax | 0.53 | 0.5 | 0.53 | 0.77 |
| Rav | 0.20 | 0.16 | 0.22 | 0.32 |
| C (×10−6) | 3.30 | 2.42 | 1.55 | 2.95 |
| S (×10−10) | 10.41 | 5.51 | 4.21 | 13.74 |
| 20 °C/min | ||||
| Ti | 388 | 450 | 582 | 519 |
| T1 | 550 | 573 | 802 | 615 |
| Tb | 702 | 716 | 879 | 759 |
| Rmax | 0.59 | 0.53 | 0.58 | 0.8 |
| Rav | 0.37 | 0.30 | 0.16 | 0.23 |
| C (×10−6) | 3.92 | 2.62 | 1.71 | 2.97 |
| S (×10−10) | 20.82 | 10.86 | 3.08 | 8.88 |
| 25 °C/min | ||||
| Ti | 399 | 451 | 580 | 522 |
| T1 | 545 | 596 | 797 | 628 |
| Tb | 791 | 793 | 926 | 800 |
| Rmax | 0.61 | 0.54 | 0.59 | 0.85 |
| Rav | 0.36 | 0.26 | 0.32 | 0.48 |
| C (×10−6) | 3.83 | 2.65 | 1.75 | 3.12 |
| S (×10−10) | 17.24 | 8.84 | 6.14 | 18.76 |
| Parameter | ecoke®(A) | ecoke®(B) | PT Breeze | Coal (A) |
|---|---|---|---|---|
| EactF (kJ/mol) | 79.8 | 65.8/87.4 | 138.0 | 94.0 |
| AF | 6.6 | 4.0/6.4 | 13.9 | 6.9 |
| EactO | 80.2 | 64.0/84.6 | 141.5 | 91.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, S.; Holliman, P.J.; Martin, C.; Jones, E. Biomass–Coal Hybrid Fuel: A Route to Net-Zero Iron Ore Sintering. Sustainability 2023, 15, 5495. https://doi.org/10.3390/su15065495
Reis S, Holliman PJ, Martin C, Jones E. Biomass–Coal Hybrid Fuel: A Route to Net-Zero Iron Ore Sintering. Sustainability. 2023; 15(6):5495. https://doi.org/10.3390/su15065495
Chicago/Turabian StyleReis, Sam, Peter J. Holliman, Ciaran Martin, and Eurig Jones. 2023. "Biomass–Coal Hybrid Fuel: A Route to Net-Zero Iron Ore Sintering" Sustainability 15, no. 6: 5495. https://doi.org/10.3390/su15065495





