Sustainable Development Optimization of a Plant Factory for Reducing Tip Burn Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Photosynthetic Physiology Experiment
2.1.1. Materials and Conditions
2.1.2. Test Methods
2.1.3. Plant Growth and Physiological Index Measurements
2.1.4. Data Processing and Analysis
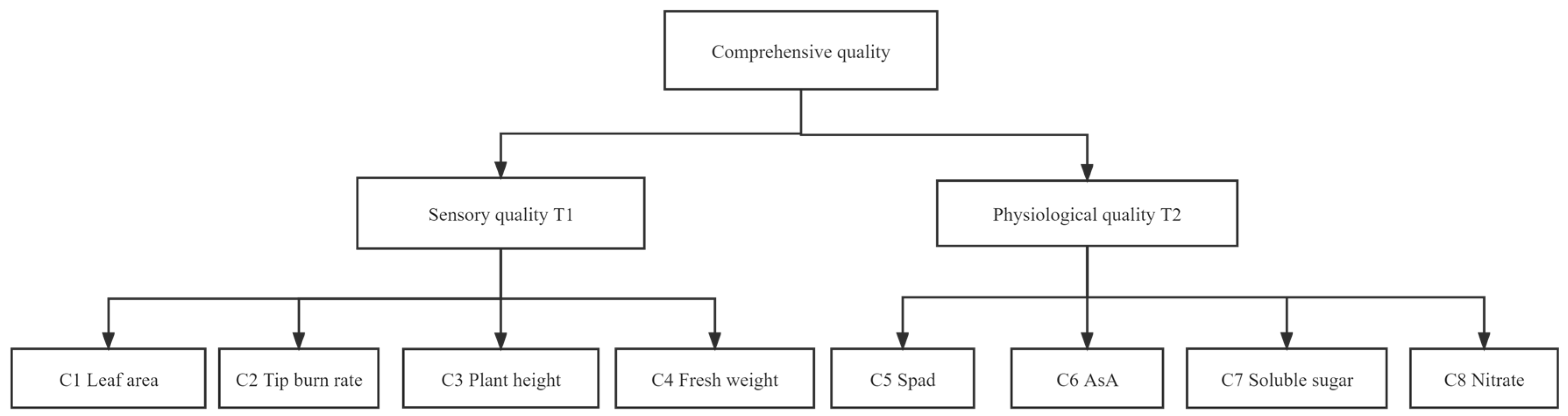
2.1.5. Analytic Hierarchy Process (Ahp)
2.2. Airflow Experiment
2.3. Porous Medium Model of Lettuce
2.4. CFD Simulation of Flow Field in the Plant Factory
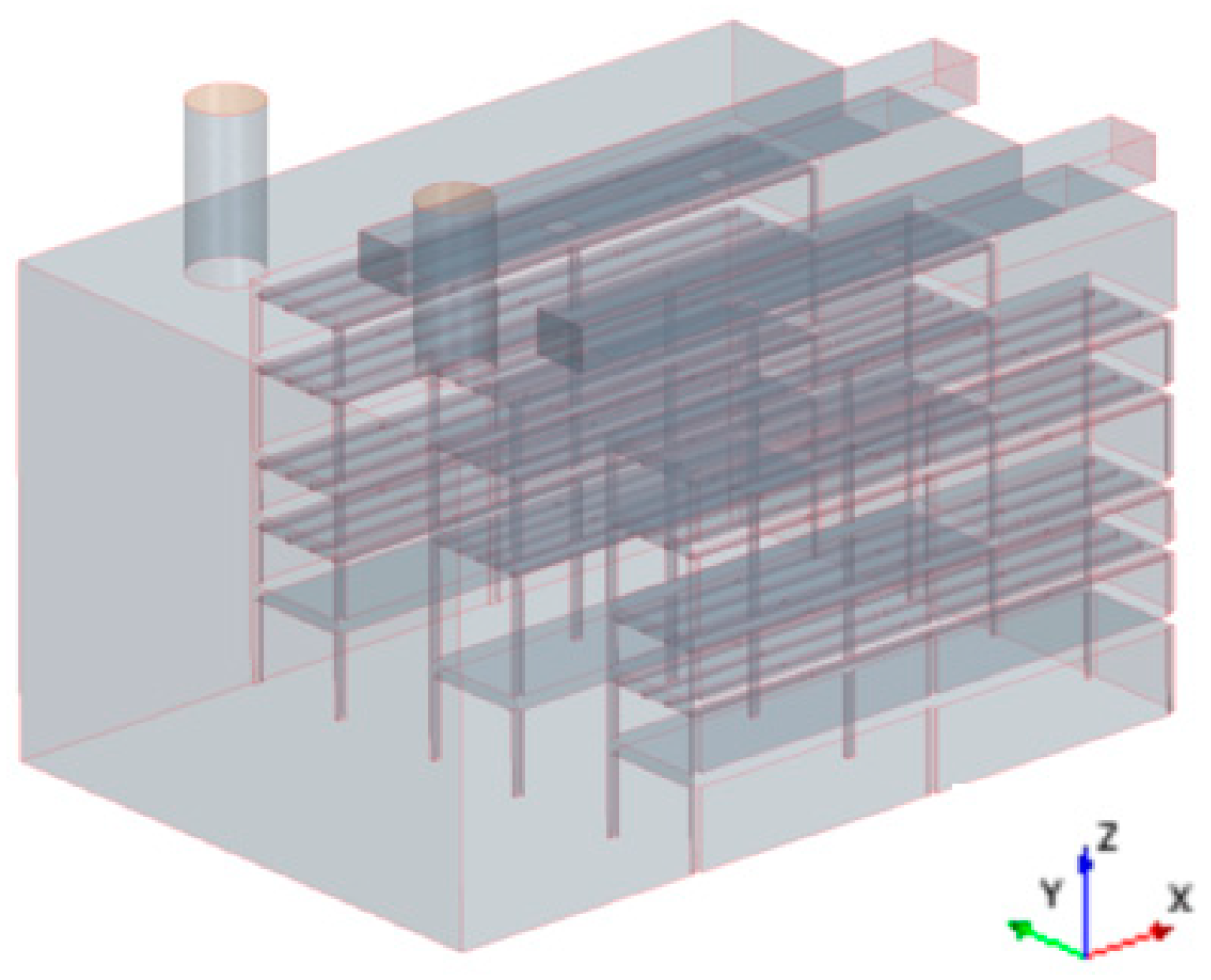
2.4.1. Geometric Model of the Plant Factory
2.4.2. Control Equation
2.4.3. Simulation Boundary Conditions of the Plant Factory
3. Results
3.1. Plant Physiological Indexes
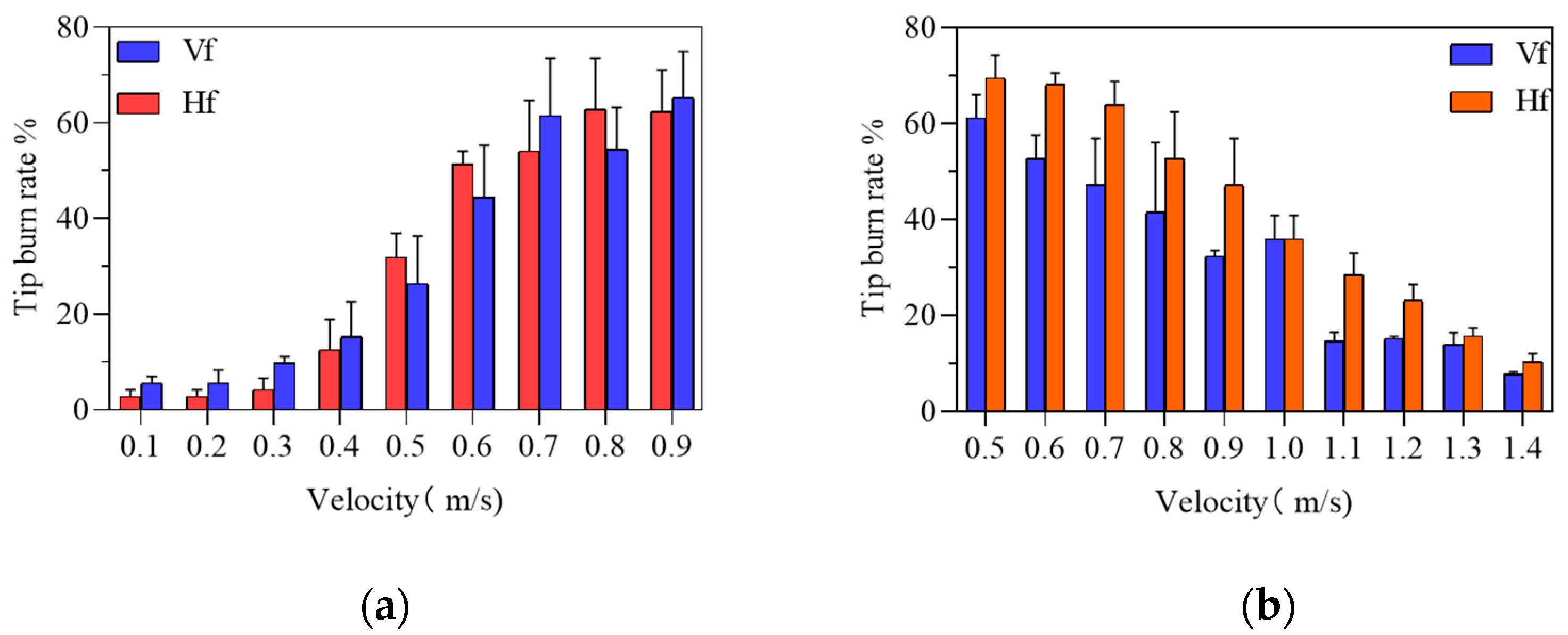
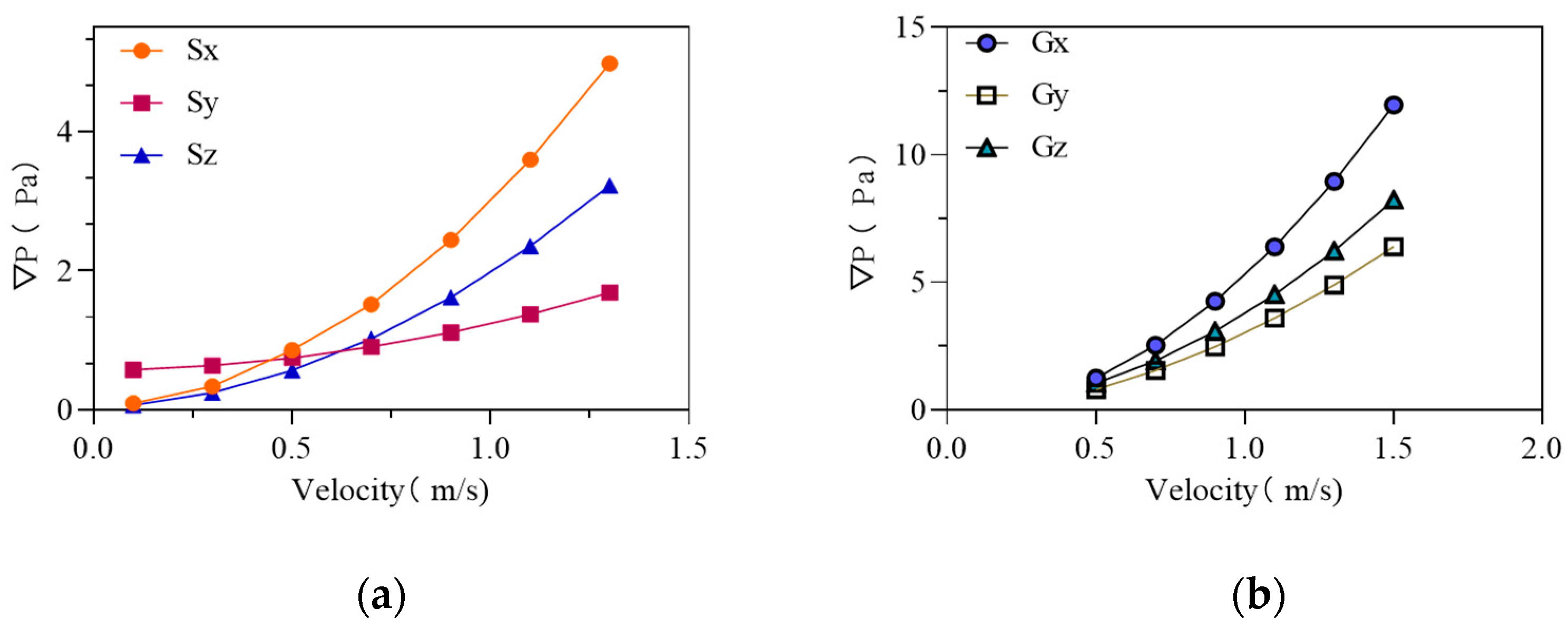
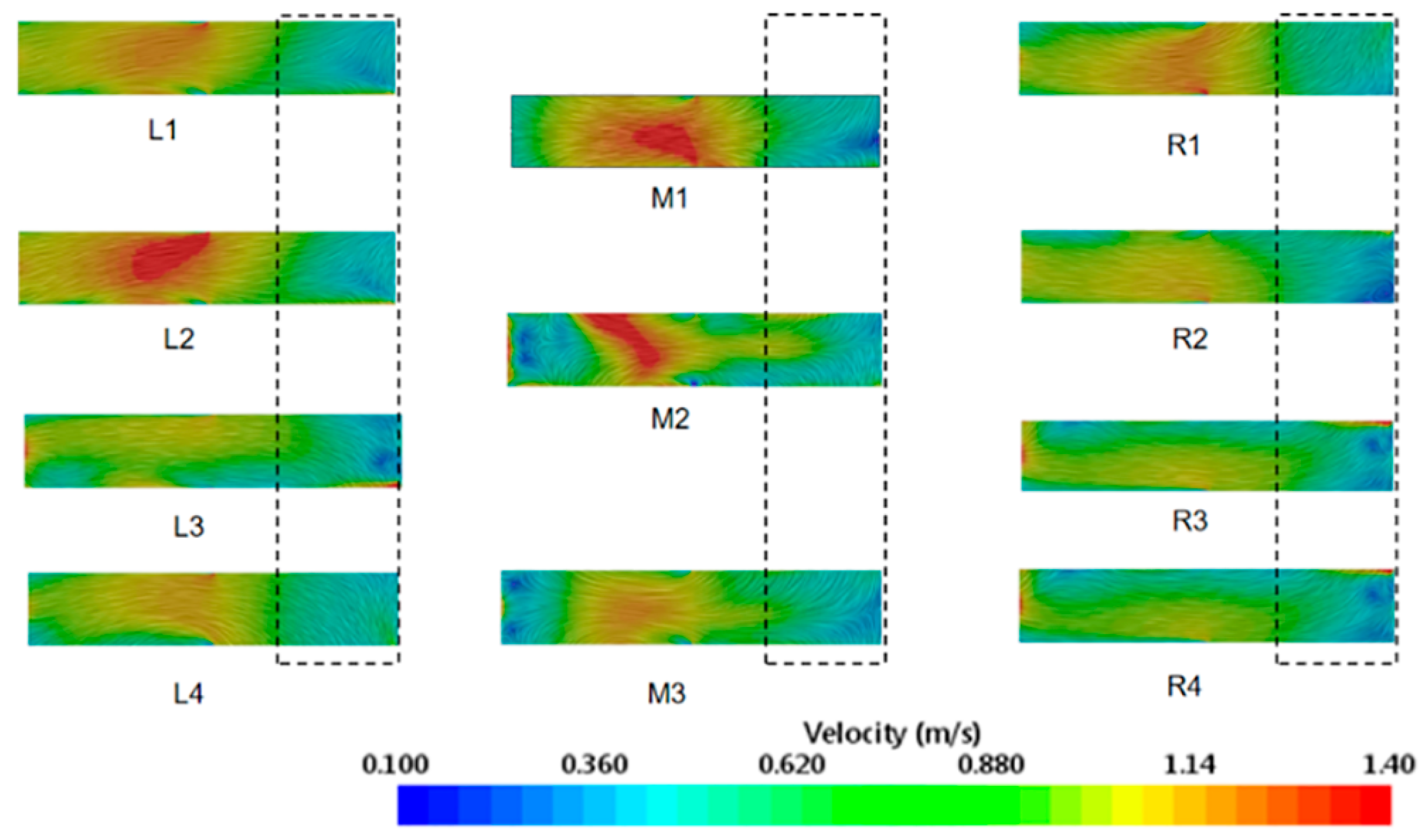
3.2. Airflow Experiment Results and Analysis
3.3. Porous Medium Parameters
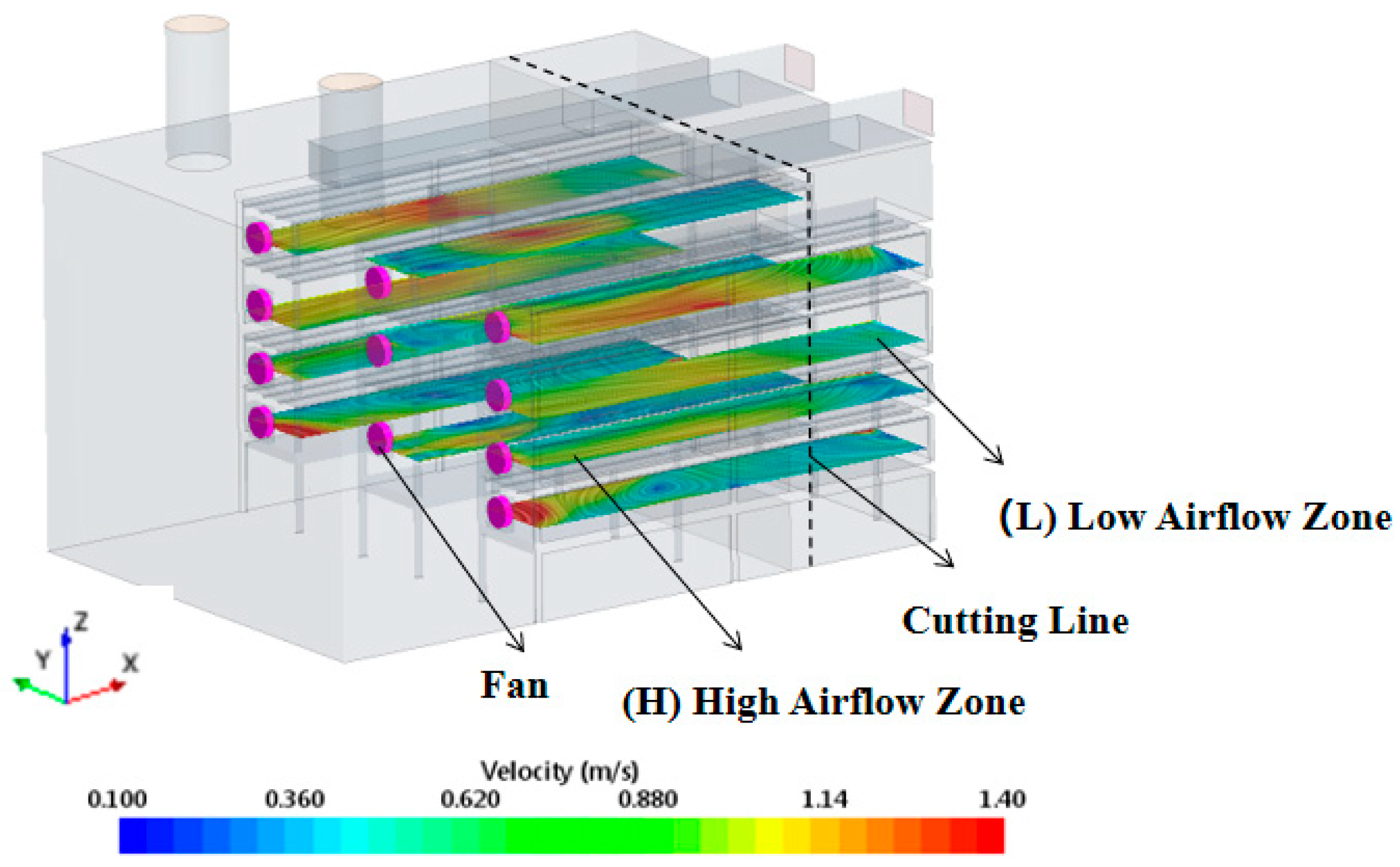
3.4. CFD Simulation and Analysis of Airflow Field
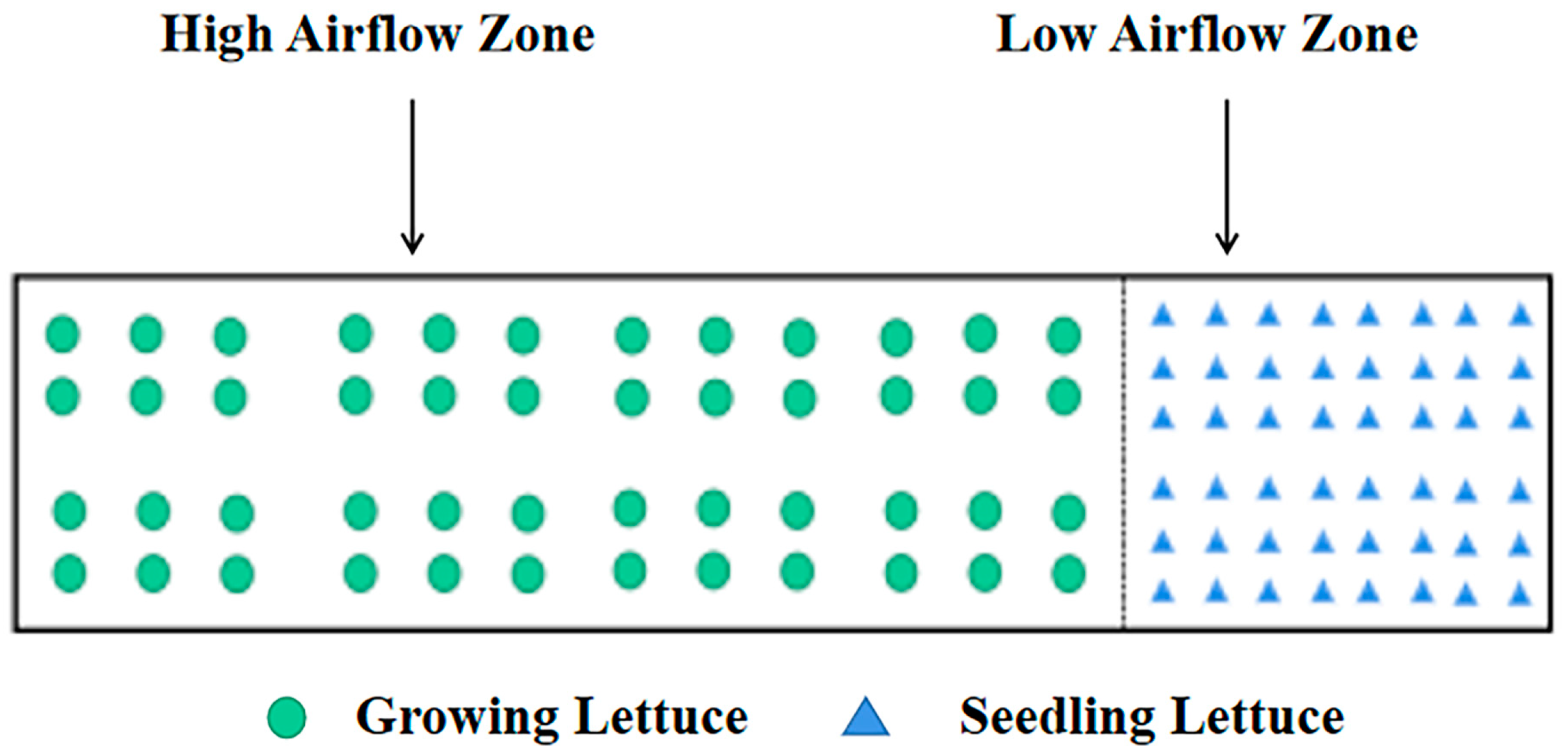
3.4.1. Optimization of Three-Dimensional Cultivation
3.4.2. Optimization of Airflow Field
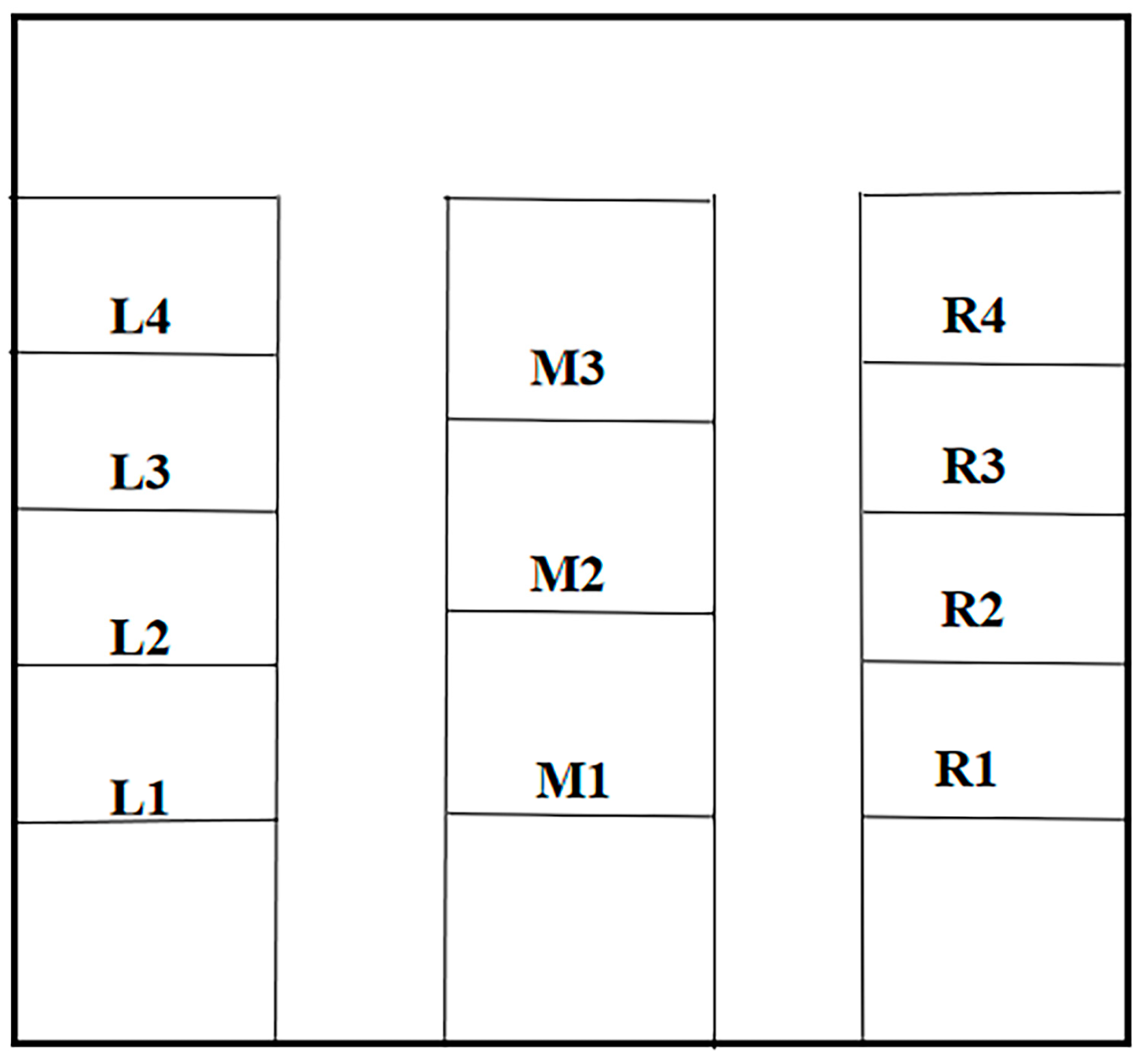
3.4.3. Verification of Simulation Results
3.5. Comparison of Tip Burn Rate of Plants before and after Optimization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Nomenclature | |
| WL | white full-spectrum LED lamp |
| RB | red and blue light source |
| S | seedling stage of lettuce |
| G | growth stage of lettuce |
| CR | consistency ratio |
| λmax | largest eigenvalue |
| Wi | weight coefficient of each index |
| D | viscous drag coefficient, dimensionless |
| C2 | inertial drag coefficient, dimensionless |
| l | thickness of the porous media domain, mm |
| air density, kg/m3 | |
| air viscosity, Pa.s | |
| Si | momentum sink |
| v | airflow velocity, m/s |
| velocity vector, m/s | |
| Γφ | generalized diffusion coefficient |
| Sφ | T source term |
| T | temperature, K |
| TS | best light LED lamp temperature in seedling stage, K |
| TG | best light LED lamp temperature in growth stage, K |
| Vf | top-to-bottom vertical airflow |
| Hf | horizontal airflow |
| ∇P | pressure drop, Pa |
| H | high airflow area |
| L | low airflow area |
| SOPT | model of seedling optimization treatment |
| GOPT | model of growth period optimization treatment |
References
- Adnan, M.; Fahad, S.; Khan, I.A.; Saeed, M.; Ihsan, M.Z.; Saud, S.; Riaz, M.; Wang, D.; Wu, C. Integration of poultry manure and phosphate solubilizing bacteria improved availability of Ca bound P in calcareous soils. 3 Biotech 2019, 9, 368. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-C. Consumer Attitude, Concerns, and Brand Acceptance for the Vegetables Cultivated with Sustainable Plant Factory Production Systems. Sustainability 2019, 11, 4862. [Google Scholar] [CrossRef]
- Lezoche, M.; Hernandez, J.E.; Diaz, M.; Panetto, H.; Kacprzyk, J. Agri-food 4.0: A survey of the Supply Chains and Technologies for the Future Agriculture. Comput. Ind. 2020, 117, 103187. [Google Scholar] [CrossRef]
- Inthichack, P.; Nishimura, Y.; Fukumoto, Y. Effect of potassium sources and rates on plant growth, mineral absorption, and the incidence of tip burn in cabbage, celery, and lettuce. Hortic. Environ. Biotechnol. 2012, 53, 135–142. [Google Scholar] [CrossRef]
- Huang, L.-C.; Chen, Y.-H.; Chen, Y.-H.; Wang, C.-F.; Hu, M.-C. Food-Energy Interactive Tradeoff Analysis of Sustainable Urban Plant Factory Production Systems. Sustainability 2018, 10, 446. [Google Scholar] [CrossRef]
- Macias-González, M.; Truco, M.J.; Han, R.; Jenni, S.; Michelmore, R.W. High-resolution genetic dissection of the major QTL for tipburn resistance in lettuce, Lactuca sativa. G3 GenesGenomesGenetics 2021, 11, jkab097. [Google Scholar] [CrossRef]
- Holmes, S.C.; Wells, D.E.; Pickens, J.M.; Kemble, J.M. Selection of Heat Tolerant Lettuce (Lactuca sativa L.) Cultivars Grown in Deep Water Culture and Their Marketability. Horticulturae 2019, 5, 50. [Google Scholar] [CrossRef]
- Cox, E.F.; Mckee, J.M.T.; Dearman, A.S. The Effect of Growth Rate on Tipburn Occurrence in Lettuce. J. Hortic. Sci. 1976, 51, 297–309. [Google Scholar] [CrossRef]
- Borkowski, J.; Dyki, B.; Oskiera, M.; Machlańska, A.; Felczyńska, A. The Prevention of Tipburn on Chinese Cabbage (Brassica rapa L. var. pekinensis (Lour.) Olson) with Foliar Fertilizers and Biostimulators. J. Hortic. Res. 2016, 24, 47–56. [Google Scholar]
- Uno, Y.; Okubo, H.; Itoh, H.; Koyama, R. Reduction of leaf lettuce tipburn using an indicator cultivar. Sci. Hortic. 2016, 210, 14–18. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light Emitting Diodes (LEDs) as Agricultural Lighting: Impact and Its Potential on Improving Physiology, Flowering, and Secondary Metabolites of Crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review: Effects of light on vegetable phytochemicals. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhao, S.; Qian, Z.; Sun, Y.; Al-Salihi, M.; Deng, X. Influence of non-uniform electric field distribution on the atmospheric pressure air dielectric barrier discharge. Plasma Sci. Technol. 2021, 23, 75402. [Google Scholar] [CrossRef]
- Seungmi, M.; Kiyoun, C.; Sookyoun, K.; Kong, H.T.; Jaehyun, L. An Experimental Study on Environmental Elements and Airflow Distribution Characteristics in a Fully Artificial Plant Factory. In Proceedings of the International Conference on Convergence Technology, Seoul, Republic of Korea, 28–30 September 2011. [Google Scholar]
- Moon, S.-M.; Kwon, S.-Y.; Lim, J.-H. Improvement of Energy Efficiency of Plants Factory by Arranging Air Circulation Fan and Air Flow Control Based on CFD. J. Internet Comput. Serv. 2015, 16, 57–65. [Google Scholar] [CrossRef]
- Koyama, R.; Sanada, M.; Itoh, H.; Kanechi, M.; Inagaki, N.; Uno, Y. In vitro evaluation of tipburn resistance in lettuce (Lactuca sativa. L). Plant Cell Tissue Organ Cult. 2012, 108, 221–227. [Google Scholar] [CrossRef]
- Kitaya, Y.; Tsuruyama, J.; Kawai, M.; Shibuya, T.; Kiyota, M. Effects of Air Current on Transpiration and Net Photosynthetic Rates of Plants in a Closed Plant Production System. In Transplant Production in the 21st Century; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Zhuang, R.; Li, X.; Tu, J. CFD study of the effects of furniture layout on indoor air quality under typical office ventilation schemes. Build. Simul. 2014, 7, 263–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Kacira, M.; An, L. A CFD study on improving air flow uniformity in indoor plant factory system. Biosyst. Eng. 2016, 147, 193–205. [Google Scholar] [CrossRef]
- Naranjani, B.; Najafianashrafi, Z.; Pascual, C.; Agulto, I.; Chuang, P.-Y.A. Computational analysis of the environment in an indoor vertical farming system. Int. J. Heat Mass Transf. 2022, 186, 122460. [Google Scholar] [CrossRef]
- Chen, J.; Jin, L.; Yang, B.; Chen, Z.; Zhang, G. Influence of the Internal Structure Type of a Large-Area Lower Exhaust Workbench on Its Surface Air Distribution. Int. J. Environ. Res. Public Health 2022, 19, 11395. [Google Scholar] [CrossRef]
- Cheng, Q.; Wu, W.; Li, H.; Zhang, G.; Li, B. CFD study of the influence of laying hen geometry, distribution and weight on airflow resistance. Comput. Electron. Agric. 2018, 144, 181–189. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Li, K.; Wu, G.; Cheng, R.; Zhang, Y.; Yang, Q. A CFD analysis on improving lettuce canopy airflow distribution in a plant factory considering the crop resistance and LEDs heat dissipation. Biosyst. Eng. 2020, 200, 1–12. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, J.-Y.; Kwon, H.-J.; Han, J.-H.; Eek Son, J.; Nam, S.-W.; Giacomelli, G.A.; Lee, I.-B. 3-D CFD analysis of relative humidity distribution in greenhouse with a fog cooling system and refrigerative dehumidifiers. Biosyst. Eng. 2008, 100, 245–255. [Google Scholar] [CrossRef]
- Lim, T.-G.; Kim, Y.H. Analysis of Airflow Pattern in Plant Factory with Different Inlet and Outlet Locations using Computational Fluid Dynamics. J. Biosyst. Eng. 2014, 39, 310–317. [Google Scholar] [CrossRef]
- Baek, M.-S.; Kwon, S.-Y.; Lim, J.-H. Improvement of the Crop Growth Rate in Plant Factory by Promoting Air Flow inside the Cultivation Improvement of the Crop Growth Rate in Plant Factory by Promoting Air Flow inside the Cultivation. Int. J. Smart Home 2016, 10, 63–74. [Google Scholar] [CrossRef]
- Ahmed, Q.C. Lettuce plant growth and tipburn occurrence as affected by airflow using a multi-fan system in a plant factory with artificial light. J. Therm. Biol. 2020, 88, 102496. [Google Scholar] [CrossRef]
- Li, K.; Fang, H.; Zou, Z.; Cheng, R. Optimization of rhizosphere cooling airflow for microclimate regulation and its effects on lettuce growth in plant factory. J. Integr. Agric. 2021, 20, 2680–2695. [Google Scholar] [CrossRef]
- Ohara, H.; Hirai, T.; Kouno, K.; Nishiura, Y. Automatic Plant Cultivation System (Automated Plant Factory). Environ. Control Biol. 2015, 53, 93–99. [Google Scholar] [CrossRef]
- Kuno, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of Irradiation Patterns and Light Quality of Red and Blue Light-Emitting Diodes on Growth of Leaf Lettuce (Lactuca sativa L.“Greenwave”). Environ. Control Biol. 2017, 55, 129–135. [Google Scholar] [CrossRef]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C.B. The Effect of Supplemental Blue, Red and Far-Red Light on the Growth and the Nutritional Quality of Red and Green Leaf Lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef]
- Hytönen, T.; Pinho, P.; Rantanen, M.; Kariluoto, S.; Lampi, A.; Edelmann, M.; Joensuu, K.; Kauste, K.; Mouhu, K.; Piironen, V.; et al. Effects of LED light spectra on lettuce growth and nutritional composition. Light. Res. Technol. 2018, 50, 880–893. [Google Scholar] [CrossRef]
- Shalaka, S.; Cumming, J.R.; Collart, F.R.; Noirot, P.H.; Larsen, P.E. Pseudomonas fluorescens Transportome Is Linked to Strain-Specific Plant Growth Promotion in Aspen Seedlings under Nutrient Stress. Front. Plant Sci. 2017, 8, 348. [Google Scholar]
- Cagri Tolga, A.; Basar, M. The assessment of a smart system in hydroponic vertical farming via fuzzy MCDM methods. J. Intell. Fuzzy Syst. 2021, 42, 1–12. [Google Scholar] [CrossRef]
- Xiao, C.; Zou, H.; Fan, J.; Zhang, F.; Li, Y.; Sun, S.; Pulatov, A. Optimizing irrigation amount and fertilization rate of drip-fertigated spring maize in northwest China based on multi-level fuzzy comprehensive evaluation model. Agric. Water Manag. 2021, 257, 107157. [Google Scholar] [CrossRef]
- Han, L. Foliar application of zinc alleviates the heat stress of pakchoi (Brassica chinensis L.). J. Plant Nutr. 2020, 43, 194–213. [Google Scholar] [CrossRef]
- Bekraoui, A.; Chakir, S.; Fatnassi, H.; Mouqallid, M.; Majdoubi, H. Climate Behaviour and Plant Heat Activity of a Citrus Tunnel Greenhouse: A Computational Fluid Dynamic Study. AgriEngineering 2022, 4, 1095–1115. [Google Scholar] [CrossRef]
- Molina-Aiz, F.D.; Valera, D.L.; Álvarez, A.J.; Madueño, A. A Wind Tunnel Study of Airflow through Horticultural Crops: Determination of the Drag Coefficient. Biosyst. Eng. 2006, 93, 447–457. [Google Scholar] [CrossRef]
- Jazouli, M.E.; Lekouch, K.; Wifaya, A.; Gourdo, L.; Bouirden, L. CFD Study of Airflow and Microclimate Patterns Inside a Multispan Greenhouse. WSEAS Trans. Fluid Mech. 2021, 16, 102–108. [Google Scholar] [CrossRef]
- Haaab, C.; Tong, Y.; Yang, Q. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar]
- Xu, W.; Nguyen DT, P.; Sakaguchi, S.; Akiyama, T.; Tsukagoshi, S.; Feldman, A.; Lu, N. Relation between relative growth rate and tipburn occurrence of romaine lettuce under different light regulations in a plant factory with LED lighting. Eur. J. Hortic. Sci. 2020, 85, 351–361. [Google Scholar] [CrossRef]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Stanghellini, C. Plant Factories Are Heating Up: Hunting for the Best Combination of Light Intensity, Air Temperature and Root-Zone Temperature in Lettuce Production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef] [PubMed]
- Morgan Pattison, P.; Hansen, M.; Tsao, J.Y. LED lighting efficacy: Status and directions. Comptes Rendus Phys. 2018, 19, 134–145. [Google Scholar] [CrossRef]
- Graamans, L.; van den Dobbelsteen, A.; Meinen, E.; Stanghellini, C. Plant factories; crop transpiration and energy balance. Agric. Syst. 2017, 153, 138–147. [Google Scholar] [CrossRef]
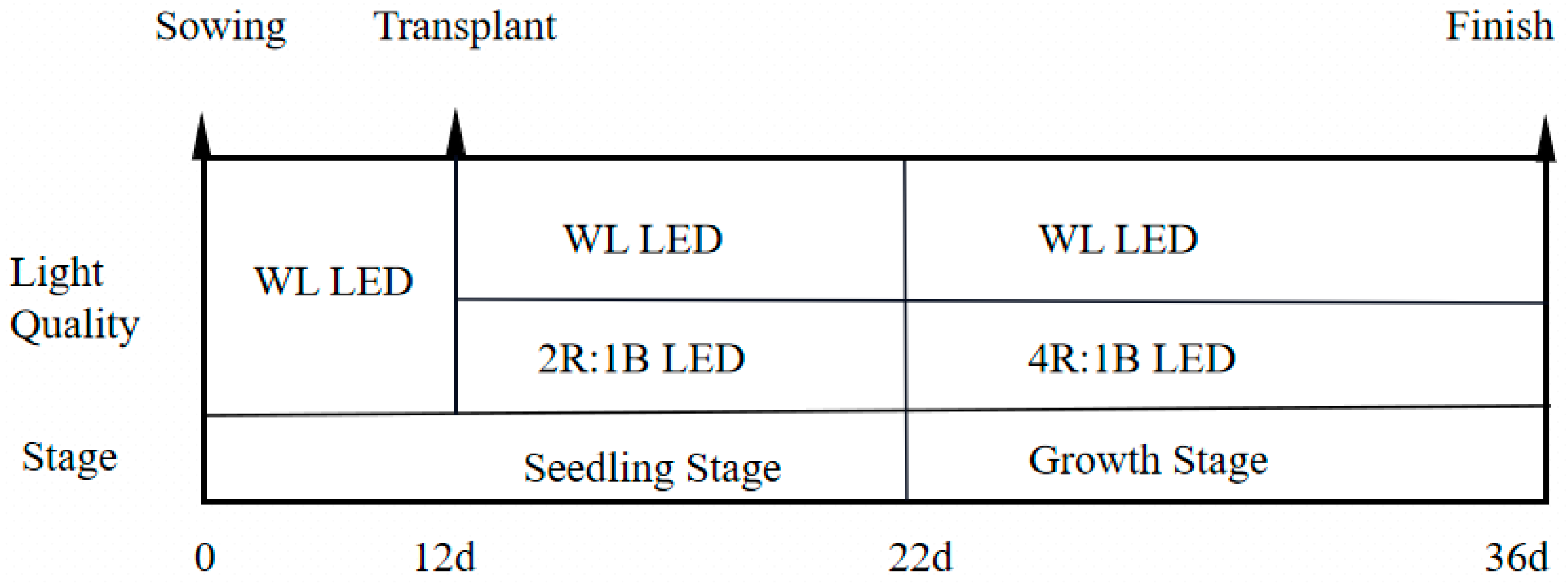



| Treatment | Light Quality | Light Intensity (μmol/m2·s) | Treatment | Light Quality | Light Intensity (μmol/m2·s) | ||
|---|---|---|---|---|---|---|---|
| CK1 | WL | 50 | CK2 | WL | 100 | ||
| T1 | WL | 100 | W1 | WL | 150 | ||
| Seedling | T2 | WL | 150 | Growth | W2 | WL | 200 |
| Stage | T3 | 2R:1B | 50 | Stage | W3 | 4R:1B | 100 |
| T4 | 2R:1B | 100 | W4 | 4R:1B | 150 | ||
| T5 | 2R:1B | 150 | W5 | 4R:1B | 200 |
| T1 | T2 | Wi | |||
|---|---|---|---|---|---|
| T1 | 1 | 1 | 0.5000 | ||
| T2 | 1 | 1 | 0.5000 | ||
| λmax = 4.1155 | CR = 0.0432 | ||||
| T1 | C1 | C2 | C3 | C4 | |
| C1 | 1 | 7 | 5 | 1 | 0.0789 |
| C2 | 1/7 | 1 | 5 | 1/3 | 0.1049 |
| C3 | 1/5 | 2 | 1 | 1/3 | 0.0534 |
| C4 | 1 | 3 | 3 | 1 | 0.2628 |
| λmax = 4.2654 | CR = 0.0994 | ||||
| T2 | C5 | C6 | C7 | C8 | |
| C5 | 1 | 3 | 3 | 1/2 | 0.2410 |
| C6 | 1/3 | 1 | 1 | 1/3 | 0.0839 |
| C7 | 1/3 | 1 | 1 | 1/3 | 0.0665 |
| C8 | 2 | 3 | 3 | 1 | 0.1087 |
| Fluid: Air | Turbulent Flow | |
|---|---|---|
| Inlet airflow temperature: 293.5 K | ||
| LED lamp | TS | TG |
| Viscous model | k-ε | |
| Boundary | Type | Settings |
| Inlet | Velocity Inlet | 8 m/s |
| Outlet | Pressure Outlet | Gauge pressure: 0 Pa |
| Plafond | Wall | Insulation |
| Wall | Wall | Insulation |
| Floor | Wall | Insulation |
| Lamp wall | Wall | Insulation |
| Treatment | Leaf Area (cm2) | Tip Burn Rate(%) | Plant Height (cm) | Fresh Weight (g) | Spad | AsA (mg/g) | Soluble Sugar (mg/g) | Nitrate (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| S | CK1 | 15.39 ± 2.95 d | 76.0 ± 9.0 b | 5.86 ± 0.30 c | 1.78 ± 0.87 c | 16.32 ± 3.17 c | 0.13 ± 0.03 d | 0.53 ± 0.12 b | 384.3 ± 11.1 a |
| T1 | 28.49 ± 4.70 b | 91.1 ± 4.5 a | 8.25 ± 0.32 a | 4.48 ± 1.35 a | 22.92 ± 2.54 b | 0.24 ± 0.08 b | 1.45 ± 0.16 a | 277.8 ± 8.3 d | |
| T2 | 21.34 ± 4.78 c | 92.2 ± 5.5 a | 7.51 ± 0.19 b | 4.36 ± 0.64a b | 23.93 ± 2.08 ab | 0.23 ± 0.02 b | 1.48 ± 0.18 a | 287.9 ± 5.8 c | |
| T3 | 16.73 ± 2.57 d | 75.1 ± 11.4 b | 5.59 ± 0.28 d | 2.28 ± 0.63 c | 15.47 ± 2.66 c | 0.16 ± 0.03 c | 0.55 ± 0.10 b | 373.1 ± 16.8 b | |
| T4 | 32.55 ± 7.48 a | 91.2 ± 6.4 a | 8.42 ± 0.26 a | 4.46 ± 0.92 a | 23.40 ± 2.56 b | 0.28 ± 0.02 a | 1.51 ± 0.15 a | 280.2 ± 13.1 cd | |
| T5 | 29.37 ± 4.34 ab | 92.5 ± 8.4 a | 7.48 ± 0.29 b | 3.67 ± 1.02 b | 25.40 ± 2.66 a | 0.29 ± 0.03 a | 1.51 ± 0.15 a | 280.3 ± 6.2 cd | |
| G | CK2 | 83.89 ± 2.73 e | 77.5 ± 3.5 b | 12.37 ± 0.54 d | 21.54 ± 1.75 f | 21.20 ± 3.41 c | 0.17 ± 0.03 d | 0.83 ± 0.15 f | 4634.7 ± 10.4 a |
| W1 | 116.72 ± 3.73 c | 91.2 ± 6.4 a | 14.60 ± 0.49 c | 26.60 ± 1.23 d | 30.26 ± 3.36 b | 0.29 ± 0.05 c | 1.38 ± 0.11 d | 3683.4 ± 11.9 c | |
| W2 | 126.13 ± 3.12 b | 93.3 ± 6.1 a | 14.87 ± 0.30 c | 33.38 ± 1.54 c | 31.58 ± 2.74 b | 0.34 ± 0.11 b | 1.63 ± 0.14 c | 388.7 ± 7.9 d | |
| W3 | 93.34 ± 2.01 d | 77.5 ± 7.1 b | 12.11 ± 0.49 d | 24.71 ± 1.15 e | 20.42 ± 3.51 c | 0.21 ± 0.04 d | 1.16 ± 0.16 e | 4445.4 ± 11.8 b | |
| W4 | 125.71 ± 4.26 b | 91.3 ± 8.9 a | 17.99 ± 0.36 a | 34.78 ± 1.39 b | 31.83 ± 3.28 b | 0.30 ± 0.04 c | 1.79 ± 0.17 b | 2965.3 ± 6.2 e | |
| W5 | 140.20 ± 4.81 a | 92.3 ± 8.3 a | 16.67 ± 0.41 b | 38.94 ± 1.62 a | 46.24 ± 3.49 a | 0.39 ± 0.04 a | 1.94 ± 0.17 a | 2649.7 ± 8.4 f |
| Seedling Stage | Growth Stage | |||||
|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |
| D | 1.454 × 104 | 0.97 × 104 | 5.66 × 104 | 0.6238 × 104 | 20.85 × 104 | 9.199 × 104 |
| C2 | 13.105 | 4.172 | 14.4 | 24.6 | 15.496 | 58.26 |
| L1 | L2 | L3 | L4 | M1 | M2 | M3 | R1 | R2 | R3 | R4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fan Speed m/s | 3.27 | 3.42 | 3.78 | 4.23 | 3.98 | 4.12 | 4.89 | 3.27 | 3.42 | 3.78 | 4.23 |
| L1 | L2 | L3 | L4 | M1 | M2 | M3 | R1 | R2 | R3 | R4 | Weighted Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | 89.7% | 92.1% | 78.6% | 84.3% | 92.4% | 75.6% | 72.1% | 88.6% | 91.9% | 81.2% | 82.5% | 84.5% |
| G | 98.7% | 97.7% | 92.4% | 93.1% | 94.2% | 85.4% | 89.7% | 96.5% | 97.4% | 96.9% | 92.6% | 94.1% |
| Leaf Area (cm2) | Tip Burn Rate (%) | Plant Height (mm) | Fresh Weight (g) | Spad | AsA (mg/g) | Soluble Sugar (mg/g) | Nitrate (mg/kg) | Ahp | |
|---|---|---|---|---|---|---|---|---|---|
| T4 | 32.55 ± 7.48 | 91.2 ± 6.4 | 8.42 ± 0.26 | 4.46 ± 0.92 | 23.40 ± 2.56 | 0.28 ± 0.02 | 1.51 ± 0.15 | 280.2 ± 13.1 | 0.983 |
| SOPT | 33.33 ± 0.77 | 26.9 ± 7.2 | 8.75 ± 0.25 | 4.86 ± 0.24 | 23.05 ± 0.97 | 0.26 ± 0.01 | 1.54 ± 0.06 | 254.3 ± 7.42 | 1.202 |
| W5 | 140.20 ± 4.81 | 92.3 ± 8.3 | 16.67 ± 0.41 | 38.94 ± 1.62 | 46.24 ± 3.49 | 0.39 ± 0.04 | 1.94 ± 0.17 | 2649.7 ± 8.4 | 1.077 |
| GOPT | 156.32 ± 1.38 | 23.3 ± 6.8 | 18.89 ± 0.48 | 40.26 ± 1.36 | 46.25 ± 0.93 | 0.39 ± 0.01 | 1.98 ± 0.05 | 2598 ± 10.25 | 1.354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haibo, Y.; Lei, Z.; Haiye, Y.; Yucheng, L.; Chunhui, L.; Yuanyuan, S. Sustainable Development Optimization of a Plant Factory for Reducing Tip Burn Disease. Sustainability 2023, 15, 5607. https://doi.org/10.3390/su15065607
Haibo Y, Lei Z, Haiye Y, Yucheng L, Chunhui L, Yuanyuan S. Sustainable Development Optimization of a Plant Factory for Reducing Tip Burn Disease. Sustainability. 2023; 15(6):5607. https://doi.org/10.3390/su15065607
Chicago/Turabian StyleHaibo, Yu, Zhang Lei, Yu Haiye, Liu Yucheng, Liu Chunhui, and Sui Yuanyuan. 2023. "Sustainable Development Optimization of a Plant Factory for Reducing Tip Burn Disease" Sustainability 15, no. 6: 5607. https://doi.org/10.3390/su15065607
APA StyleHaibo, Y., Lei, Z., Haiye, Y., Yucheng, L., Chunhui, L., & Yuanyuan, S. (2023). Sustainable Development Optimization of a Plant Factory for Reducing Tip Burn Disease. Sustainability, 15(6), 5607. https://doi.org/10.3390/su15065607







